Abstract
The recent increase in demand for Okinawan pineapples has necessitated the development of new varieties with attractive aromas. This study aimed to evaluate the volatile characteristics of five Okinawan pineapple breeding lines, i.e., ‘No. 22’, ‘No. 25’, ‘No. 26’, ‘No. 27’, and ‘No. 28’. The total volatiles in the cryopulverized fruit flesh were examined using headspace gas-chromatography–mass-spectrometry-based electronic nose analysis. The total ion masses of the volatiles were visualized using principal component analysis, and three replicates of each line with comparable volatile characteristics were selected. Furthermore, the composition of the volatile components in these replicates was assessed, and the odor activity values (OAVs) were calculated. The breeding lines varied in the quantity and composition of their volatile compounds, which were predominantly esters, ketones, terpenes, and alcohols. The ‘No. 22’ fruit contained a greater content of volatiles than the other lines. Moreover, 14 volatiles with OAV > 1 were accounted as aroma-active compounds, and their variations were distinguished as follows: the highest OAV (786.96) was recorded for methyl 2-methylbutanoate of the ‘No. 26’ line; 2,5-dimethyl-4-methoxy-3(2H)-furanone was superior in the ‘No. 26’ and ‘No. 27’ lines; and δ-decalactone was only present in the ‘No. 22’ and ‘No. 27’ fruits, suggesting different potent practical uses for these new breeding lines.
1. Introduction
Pineapple (Ananas comosus), an evergreen perennial plant of the Bromeliaceae family, is a popular fruit worldwide because of its unique flavor characteristics [1,2,3]. In Japan, the pineapple is one of the few horticultural trees that can be grown in the southernmost prefecture, Okinawa, where the subtropical soil is poor in organic matter and is strongly acidic [4,5]. The most cultivated variety in Okinawa is a Smooth Cayenne cultivar, ‘N67-10’, known for its large size, yellowish-white flesh, and high sugar content [5,6]. The fruits from the ‘N67-10’ cultivar have been consumed as fresh fruit and used as main ingredients in the pineapple-canning industry in the prefecture for many years; however, this variety has a relatively weak scent due to its low concentration of volatile compounds [5,7]. Moreover, the production ratio increases with the raw consumption of pineapple, and, simultaneously, the incremental demand for pineapple-based product diversification from the local food and agrotourism industries is also increasing in Japan [7,8]. Through selective breeding, scientists and breeders can introduce the desired characteristics of different pineapple varieties into a single plant to create hybrids that exhibit improved quality traits, particularly volatile profiles, and can adapt to the climate and growing conditions of Okinawa [4,7].
Electronic nose (e-nose) profiling is used for the selection and quality assessment of various horticultural products, including fruits [9,10,11]. Generally, an e-nose device is equipped with an array of gas sensors that can detect and analyze volatile compounds in fruits [10,11,12]. Gas sensor-type e-noses, such as metal oxide semiconductors, can differentiate the volatiles emitted from minimally processed fruit cuts and brewed pineapple beverages due to their chemical sensitivities [12,13]. These instruments measure the unique chemical fingerprints of the volatile compounds released from fruits and provide valuable information on their ripeness, aroma characteristics, and flavor quality [9,11,12,13]. Among the many types of e-nose systems, the combination of gas chromatography–mass spectrometry (GC–MS) and the e-nose profiling concept, viz., the GC–MS–e-nose, could potentially enhance the capabilities of aroma analysis by providing a higher resolution and sensitivity of the ion masses captured from samples, allowing for more comprehensive fingerprints of the volatiles without component separation. This profiling technique does not require compounds’ identification, but it is based on the calculation of the total ion masses needed for multivariate statistical analysis [14,15,16]. Moreover, the GC–MS–e-nose may be equipped with autosampler systems, such as a headspace, and, thus, can be applied to evaluate large sample quantities [10,14,15]. The GC–MS–e-nose is employed in flavor studies, including the flavor authentication of different food products, the evaluation of the volatile profiles of fruits during ripening, and the assessment of flavor changes due to processing or storage conditions [10,14,15,16,17]. Thus, the MS-based e-nose is as a powerful tool for food technologists, aiding in product development, quality control, and optimization of flavor profiles in the food and beverage industry as well as in monitoring horticultural product qualities [15,16,17,18].
The flavor of fruits is influenced by the composition and concentration of the volatile compounds that contribute to their aroma [1,3,17]. Chemical analysis techniques such as GC–MS and the MS–e-nose can provide objective measurements of volatile compounds and contribute to understanding the flavor quality of fruits, including pineapples [5,17]. Odor activity values (OAVs) are used to assess the sensory impact of individual volatile compounds on the overall aroma perception of a material [2,19]. An OAV is calculated by dividing the concentration of a specific volatile compound in a food material by its odor threshold concentration, the minimum concentration at which the human olfactory system can detect the compound [19,20]. A high OAV suggests that the concentration of a particular compound is above its odor threshold and is, thus, likely to significantly contribute to the aroma profile of foods [3,19]. When the OAV of a compound is greater than 1, the compound is present at concentrations above its sensory threshold and is likely to significantly contribute to the overall aroma of the food [2,19,21]. Thus, these tools offer a useful approach for identifying aroma-contributing compounds and enable the development of horticultural and food products with appealing flavor profiles that enhance consumer satisfaction [17,21,22].
In this study, we examined the volatile profiles of five new breeding lines of Okinawan pineapple and compared them with that of ’N67-10’ as a control cultivar. This study aimed to assess the aroma characteristics of new pineapple breeding lines from Okinawa using GC–MS–e-nose profiling and OAV calculations. This is the first report on the use of the MS–e-nose technique for the selection of pineapple samples with comparable volatile profiles via unsupervised multivariate analyses, such as principal component analysis (PCA) and hierarchical cluster analysis (HCA). The data from the MS–e-nose profiling were used as a basis for the sample selection for the chemical composition measurements. The volatile components of the selected fruits were examined using GC–flame ionization detection (FID)/MS analysis, and aroma-active compounds with OAV greater than 1 were compared.
2. Materials and Methods
2.1. Standards and Reagents
2-Methyl-1-pentanol was purchased from Tokyo Chemical Industry (Tokyo, Japan), and NaCl was obtained from Merck (Darmstadt, Germany). n-Alkanes (C7–C30) mixture solution was purchased from Sigma-Aldrich (St. Louis, MO, USA). Authentic standards for identifying volatile components were obtained from Sigma-Aldrich and Tokyo Chemical Industry. All the other reagents were of analytical grade.
2.2. Pineapple Samples
Pineapple fruits of ‘N67-10’ cultivar and five new breeding lines, namely, ‘Okinawa No. 22’, ‘Okinawa No. 25’, ‘Okinawa No. 26’, ‘Okinawa No. 27’, and ‘Okinawa No. 28’ (shortened accession names: ‘No. 22’, ‘No. 25’, ‘No. 26’, ‘No. 27’, and ‘No. 28’, respectively) were harvested from the Okinawa Prefectural Agricultural Research Center, Nago, Okinawa, Japan, from July to October 2019. The accession and parentage of the ‘N67-10’ cultivar and Okinawan breeding lines used in the study are shown in Table 1. Six biological replicates of fruits from each cultivar or breeding line were collected at the mature ripening stage (the weight and total soluble solids of the fruits ranged from approximately 1100 to 1600 g and from 15% to 17% Brix, respectively). The fruits were immediately peeled and cut into eight pieces. Then, the fruit flesh was cryopulverized using a multi-bead shocker (Yasui Kikai, Osaka, Japan), and the homogenized puree was stored in sealed tubes at −30 °C prior to analysis.

Table 1.
Accessions and parentages of Okinawan pineapple cultivar and breeding lines.
2.3. GC-MS-e-Nose Analysis
Volatile profiles of six pineapple fruits of the ‘N67-10’ cultivar and new breeding lines were examined using GC–MS–e-nose analysis [10]. Briefly, pineapple puree (2 g) and NaCl (0.2 g) were placed in a 10 mL glass headspace vial, and the mixture was then sonicated at 25 °C for 10 min. The volatiles in the mixture were analyzed using a GERSTEL ChemSensor (GERSTEL, Mülheim, Germany) in an Agilent G1888 HSS-7890A GC-5975C MS system (Agilent Technologies, Santa Clara, CA, USA). The volatiles were extracted at 40 °C for 5 min and subsequently pressurized at 11 psi for 0.3 min into the injection port. The headspace loop and transfer line were set at 80 and 130 °C, respectively, and helium was used as the carrier gas. The injector was programmed to operate at 250 °C at a split ratio of 10:1. The volatiles were passed through an HP-5MS column (30 m × 0.25 mm inner diameter, 0.25 μm film thickness; Agilent Technologies) for 10 min with the following temperature arrangement: initially held at 40 °C for 1 min, raised to 250 °C at a rate of 30 °C/min, and then maintained at 250 °C for 2 min. The volatile profiles were also obtained using a shorter capillary column (HP-5MS 30 m × 0.25 mm, 0.25 μm; Agilent Technologies) for a 5 min analysis run. The oven program for the HP-5MS 30 m column was set as follows: initially held at 40 °C for 0.5 min, raised to 250 °C at a rate of 60 °C/min, and then maintained at 250 °C for 1 min. Typical chromatograms with different column dimensions are shown in Figure 1. The total mass spectrum intensities of m/z 30–300 from the volatiles were obtained in the electron ionization mode (70 eV). The ion source and transfer line temperatures were both set to 250 °C. The acquired ion mass data were converted into a multivariate analysis dataset and normalized to standard deviation units using SIMCA Version 17 (Sartorius, Göttingen, Germany).
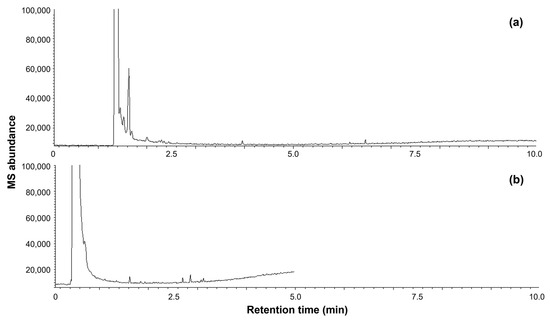
Figure 1.
Typical chromatograms of GC–MS–e-nose analysis of Okinawan pineapple with different HP-5MS column dimensions (length × i.d. and film thickness): (a) 30 m × 0.25 mm and 0.25 μm; (b) 15 m × 0.25 mm and 0.25 μm.
2.4. Volatile Component Analysis and OAV Calculation
The volatile components of the selected fruits were examined using solid-phase microextraction (SPME) Arrow–GC–FID/MS [5]. Briefly, pineapple puree (2 g), 2-methyl-1-pentanol as internal standard (3 µg/mL in H2O, 20 µL), and NaCl (0.2 g) were placed into a 10 mL glass vial. Afterward, the closed vial was sonicated at 25 °C for 10 min and heated at 40 °C for 7.5 min. The volatiles in the mixture were then extracted using SPME Arrow fiber (120 µm divinylbenzene/polydimethylsiloxane; Restek, PA, USA) while heating at 40 °C for 30 min. GC–FID analysis was performed using an Agilent 7890 B GC system equipped with a DB-Wax column (60 m × 0.25 mm, 0.25 µm, Agilent Technologies). The injection temperature was set to 250 °C at a split ratio of 10:1. The column temperature was set at 40 °C for 2 min and raised to 200 °C at a rate of 2 °C/min without a final hold time. The helium carrier gas flow rate was programmed at 32 cm/s, and the FID was set at 250 °C. Volatile components were identified, based on linear RIs, upon measurement of the homologous series of n-alkanes (C7–C30), followed by the comparison of the MS patterns of the peaks with the MS data obtained from the NIST MS Library Version 2008 and with the MS data of co-injected authentic standards. GC–MS was performed using an Agilent 7890A GC-5975C MS system (Agilent Technologies). SPME Arrow extraction and GC conditions were set as described above. For MS detection, the ion source and interface were both set to 230 °C. The ionization energy was 70 eV, and the mass acquisition range was m/z 33–450. All analyses were performed in triplicates.
The weight Intensities of the peaks were calibrated using the FID response (peak area) of the internal standard; the normalized intensity of the peak was equal to its peak area divided by the peak area of the internal standard. The relative concentration was determined by dividing the peak area of the compound by the total peak area of the sample and multiplying by 100 and was expressed as a percentage (%). The compound content was assessed by calibrating the correlation factor corresponding to the FID response of the internal standard (2-methyl-1-pentanol, 3 µg/mL, 20 µL; or equal to 0.06 µg) and was expressed as micrograms of volatile compound per kilogram fruit puree (µg/kg). OAV was determined by dividing the concentration of the compound by its odor threshold. The odor threshold concentrations of volatile compounds in water were retrieved from the latest published reports [23]. The aroma-active volatiles were set for compounds with an OAV greater than 1, and the value was calculated as log10.
2.5. Statistical Analysis
Each result was expressed as the mean value and standard deviation, and statistical differences between samples were examined using the Tukey–Kramer honest significant difference test (JMP Version 13, SAS Institute, Cary, NC, USA). Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were performed using SIMCA Version 17 (Sartorius).
3. Results
3.1. GC–MS–e-Nose Profiles of Okinawan Pineapple
The multivariate analysis presented GC–MS–e-nose profiles of the ‘N67-10’ cultivar and five Okinawan pineapple breeding lines through PCA plots as well as an HCA dendrogram for selecting fruits with comparable volatile qualities within the cultivar or breeding line (Figure 2 and Supplementary Materials, Figure S1). The three-dimensional score plots of GC–MS–e-nose of the HP-5MS 30 m column displayed the outlier plots as separated by a Hotelling ellipse at the 95% confidence level, i.e., one fruit of the ‘No. 26’ line, two fruits of the ‘No. 27’ line, and one fruit of the ‘No. 28’ line, and, therefore, they could be omitted from the selection (Figure 2a). Outlier fruits were confirmed using the HCA dendrogram (Supplementary Materials, Figure S1). Furthermore, the first two principal component (PC) factors in the two-dimensional score plots showed similarities or dissimilarities among the biological replicates of the evaluated fruits due to their volatile qualities (Figure 2b). Three fruits from cultivars or breeding lines with closed score plots were selected for further volatile composition examination because these replicates could have comparable volatile constituents. The volatile profiles of these fruits were associated with the MS intensities of the ions of their volatiles captured from the scanned ion masses (m/z 30–300). The separation of the outlier fruits could be affected by several ions plotted at the farthest positions on the two positive axes of the factor loadings (Figure 2c). The distant ions included m/z 42, 71, 56, 58, 85, 88, 89, 90, and 130, which were outlined in the positive direction of PC1, whereas m/z 67, 79, 81, 96, and 97 were plotted the farthest, in the positive direction of PC2. Moreover, the PCA computation also displayed two-dimensional score plots and factor loadings of the selected fruits from the combined data of two GC columns, i.e., the 15 m and 30 m HP-5MS columns (Figure 2d,e). The distribution patterns of score plots of the two columns in the first two PCs were similar in that fruits of the ‘No. 22’ breeding line were well scattered compared with fruits of the ‘N67-10’ cultivar and other lines (Figure 2d). These patterns agreed with the affiliation arrangements among selected fruits of the 15 m and 30 m HP-5MS columns’ data in the HCA dendrogram (Supplementary Materials, Figure S2). The score plots also showed a more influential separation of fruits from the GC–MS–e-nose analysis with the 15 m column than with the 30 m column. This arrangement was strongly associated with factor loadings, wherein many ions were outlined in the positive direction of PC1 (Figure 2e).
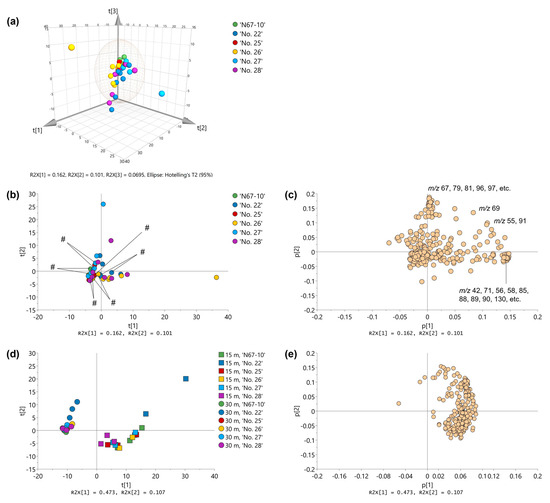
Figure 2.
PCA plots of volatile profiles of Okinawan pineapple generated by GC–MS–e-nose analysis on HP-5MS columns: (a) three-dimensional score plots of screened fruits, obtained by 30 m column (PC1 = 16.2%; PC2 = 10.1%; PC3 = 6.95%); (b) two-dimensional (2D) score plots of screened fruits, obtained by 30 m column (PC1 = 16.2%; PC2 = 10.1%; selected fruits are marked with #); (c) 2D factor loadings of screened fruits, obtained by 30 m column; (d) 2D score plots of selected fruits from the combined 15 m and 30 m columns’ data (PC1 = 47.3%; PC2 = 10.7%); (e) 2D factor loadings of selected fruits from the combined 15 m and 30 m columns’ data.
3.2. Volatile Composition of Okinawan Pineapple
The selected pineapple fruits of the ‘N67-10’ cultivar and five breeding lines had different intensities and compositions of volatile components (Figure 3a,b). The ‘No. 22’ breeding line had significantly higher amounts of total volatiles (normalized intensity, 96.51; p < 0.05), followed by the ‘No. 26’, ‘No. 28’, and ‘No. 27’ lines (Figure 3a). In addition, the ‘No. 25’ breeding line had comparable levels of volatiles to those in the ‘N67-10’ cultivar (normalized intensities, 14.48 and 10.75, respectively). The predominant volatile compounds in these fruits were esters, followed by ketones, terpenes, and alcohols (Figure 3b; Table 2). The relative concentrations of the total esters in the newly bred lines varied from 39.93% to 72.20%; these percentages were higher than in the ‘N67-10’ cultivar (28.54%). Moreover, the ‘No. 27’ breeding line had a high proportion of ketones that were accumulated at 26.28%, compared with 6.32% and 7.24% in the ‘No. 22’ and ‘No. 26’ lines, respectively, while a lower percentage of ketones, i.e., approximately 3.84%, were detected in ‘N67-10’ and the other breeding lines. Conversely, compared with the new breeding lines, the ‘N67-10’ cultivar comprised higher portions of terpenes and alcohols (13.16% and 10.61%, respectively). The moderate volatile components in all pineapple fruits included aldehydes and carboxylic acids that had ranges of 0.97–7.18% and 0.95–4.06%, respectively. In addition, a hydrocarbon component was detected in the fruits of the ‘No. 22’, ‘No. 25’, and ‘No. 28’ lines. The remaining portions responsible for summating the relative concentrations to 100% in the ‘N67-10’ cultivar and five breeding lines included unknown compounds and artifact chromatographic peaks (Figure 3b).
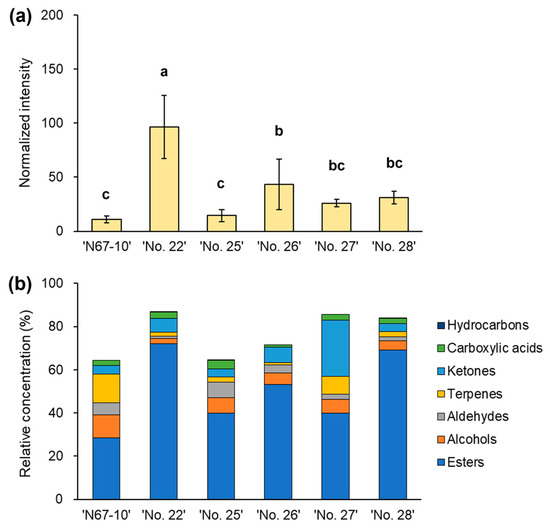
Figure 3.
(a) Normalized intensity of total chromatographic peaks and (b) relative concentration (%) of volatile components of Okinawan pineapple, obtained by SPME Arrow–GC–FID/MS analysis on DB-Wax column. Each value is expressed as the mean ± standard deviation of three replicates. Different letters indicate significant differences at p < 0.05.

Table 2.
Relative concentration (%) of volatile components of Okinawan pineapple, obtained by SPME Arrow–GC–FID/MS analysis on DB-Wax column.
Forty ester components were identified in the Okinawan pineapple, and there was variation in the composition of these compounds among the ‘N67-10’ cultivar and five breeding lines (Table 2). Compared with the newly bred lines, the ‘N67-10’ cultivar had significantly more ethyl acetate (p < 0.05) and solely contained tetrahydrofuranyl acetate. The relative concentration of the predominant methyl hexanoate in the ‘No. 22’ breeding line was significantly greater than in the ‘N67-10’ cultivar and other lines (39.53%). Furthermore, it had a significantly higher amount of methyl (E)-3-hexanoate and exclusively contained methyl (E)-3-octenoate and methyl (Z)-4-decenoate. In contrast, moderate-to-major ester components, which comprised more than 1% of the relative concentrations in the other breeding lines, included methyl 3-(methylthio)propanoate, methyl hexanoate, methyl butanoate, methyl 2-methylbutanoate, ethyl acetate, and ethyl 3-(methylthio)propanoate. Moreover, the ‘No. 25’, ‘No. 26’, and ‘No. 27’ lines significantly contained more 3-(methylthio)propyl acetate, methyl 3-hydroxydecanoate, and ethyl hexanoate, respectively.
The other volatile groups are also presented in Table 2 based on the order of their RIs in the GC chromatogram. In the alcohols group, seven compounds were identified, but 2-methyl-3-buten-2-ol was only present in the ‘N67-10’ cultivar. Moreover, this control cultivar contained higher amounts of other alcohols, such as 1-heptanol, 2-ethyl-1-hexanol, 1-octanol, and 2-furanmethanol. In the aldehyde group, nonanal was the main element of the ‘No. 25’ breeding line, and the amount was significantly higher than in other fruits. Other noteworthy aldehydes were hexanal and trans-2-decenal, ranging from 0.29% to 2.05% and from 0.36% to 2.07%, respectively. Furthermore, in the terpenes, the control cultivar, ‘N67-10’, had significantly greater amounts of β-elemene, α-muurolene, γ-muurolene, menthol, and 4-terpineol and solely contained copaene and δ-cadinene. In contrast, β-ocimene substances in cis and trans isomeric forms were found in significantly higher amounts in the ‘No. 27’ line (0.74% and 4.97%, respectively). As for the ketones, the ‘No. 22’ line comprised all 11 identified compounds, wherein the line had a significantly higher amount of γ-octalactone than other fruits. Conversely, fewer ketone components were observed in the fruits of the ‘N67-10’ cultivar and other breeding lines. In addition, the ‘No. 27’ breeding line had a very high proportion of 2,5-dimethyl-4-methoxy-3(2H)-furanone in its fruit, followed by the ‘No. 26’ and ‘No. 28’ lines (20.52, 3.47, and 1.12, respectively). Except for the ‘No. 26’ line, the other fruits contained moderate amounts of hexanoic acid (1.75–2.95%) in the carboxylic acid group, while the ‘No. 25’ line had 1.11% octanoic acid. Additionally, the ‘No. 22’, ‘No. 25’, and ‘No. 28’ lines contained a small portion of hydrocarbon, 1,3,5,8-undecatetraene, ranging from 0.25% to 0.31%.
3.3. OAVs of Okinawan Pineapple
The OAV of the volatile compounds of the ‘N67-10’ cultivar and five breeding lines were assessed, wherein the odor threshold concentrations of the compounds were from the latest reported literature [23]. Among the identified volatiles, 14 compounds were considered to be aroma-active components (OAV > 1), consisting of nine esters, three ketones, one aldehyde, and one terpene (Table 3; Figure 4). The concentration of the compounds was assessed using an internal standard (2-methyl-1-pentanol) that may cause imprecise approximations; however, the single use of an internal standard is widely accepted in volatiles quantification [2,3,17,19]. These volatiles had different odor thresholds, wherein a low threshold concentration was detected for ethyl octanoate, whereas methyl hexanoate and methyl 3-(methylthio)propanoate had much higher thresholds (0.1 vs. 75 and 150 µg/kg, respectively) (Table 3). Accordingly, the OAVs of ethyl octanoate in fruits of the ‘N67-10’ cultivar and ‘No. 27’ line were 10 times higher than their concentrations, while the OAVs of the other two predominant esters were depressed due to their high odor threshold concentrations. Nevertheless, methyl hexanoate was accounted for as one of contributing aroma-active compounds in the ‘No. 22’ and ‘No. 28’ lines, while it was methyl 3-(methylthio)propanoate in the ‘No. 22’, ‘No. 26’, and ‘No. 28’ lines. Hence, the heat map visualization of the OAVs in log10 shows the aroma-active compounds of the ‘N67-10’ cultivar and five breeding lines in white-to-red color plots (OAV > 1 or log10 OAV > 0) (Figure 4). The fruits of newly bred lines noticeably presented higher OAVs of methyl 2-methylbutanoate than those of ‘N67-10’ cultivar, with the following order: ‘No. 26’ (OAV = 786.96 or log10 OAV = 2.90) > ‘No. 28’ (337.72, 2.53) > ‘No. 22’ (235.92, 2.37) > ‘No. 27’ (121.11, 2.08) > ‘No. 25’ (70.40, 1.85) > ‘N67-10’ (4.72, 0.67). Furthermore, the ‘No. 22’, ‘No. 26’, and ‘No. 28’ breeding lines displayed higher OAVs for methyl butanoate, methyl 3-methylbutanoate, and ethyl butanoate (11.52–20.94, 16.21–22.89, and 7.01–12.49, respectively). The ‘No. 22’ line also had stronger odor activity for methyl 3-(methylthio)propanoate, while the ‘No. 26’ and ‘No. 27’ lines contained higher OAVs for 3-methylbutyl acetate. Besides esters, nonanal was accounted for as an aroma-active compound in all pineapple fruits, in which the highest OAV was found in the ‘No. 26’ line. In contrast, except for ‘No. 25’, the other fruits were complemented by the strong odor activity of linalool, but the ‘No. 22’ line had the most. Moreover, the fruits of the ‘No. 27’ line as well as the ‘No. 26’ line were complemented by 2,5-dimethyl-4-methoxy-3(2H)-furanone, whereas ‘No. 22’ and ‘No. 28’ presented higher activity of 2,5-dimethyl-4-hydroxy-3(2H)-furanone. Additionally, δ-decalactone, only present in the ‘No. 22’ and ‘No. 27’ fruits, displayed strong activities in both lines.

Table 3.
Aroma description, odor threshold, and content of aroma-active volatile components of Okinawan pineapple.
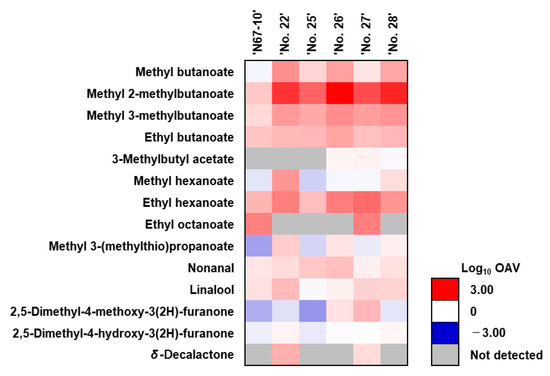
Figure 4.
Heat map visualization of odor activity values (OAVs) of aroma-active volatile components of Okinawan pineapple. OAV is calculated by dividing the concentration of volatile compounds by their odor threshold.
4. Discussion
The GC–MS–e-nose distinguished the volatile profiles of Okinawan pineapple fruits by comparing the intensities of each ion mass of their volatile components (Figure 1 and Figure 2). Captured MS data from pineapple fruits could be obtained in short periods, 5–10 min per sample (Figure 1), which compared volatile organic compounds as aggregated olfaction signal, similar to the human nose [16,17]. MS detection using this technique captures and measures the specific volatile organic compounds released by each evaluated sample [14,16,17,18]. MS–e-nose analysis does not require chromatographic peaks separation; therefore, compounds’ identification is not necessitated [10,14]. The measured ion masses were processed and analyzed using pattern recognition algorithms to generate a profile or fingerprint of the volatile characteristics of each fruit. Accordingly, the differences between the ‘N67-10’ cultivar and five breeding lines (six biological replicates for each cultivar or line) could be assessed via multivariate analysis such as PCA and HCA, indicating the separation of the chemical characteristics of the fruits (Figure 2a,b and Supplementary Materials, Figure S1). Multivariate plots provided a systematic proposal for selecting representative replicates of each cultivar or line for further compositional analysis (Figure 2d and Supplementary Materials, Figure S2). Conversely, omitted replicates may have irregular volatile qualities due to different degrees of ripeness and possible fruit defects. This agrees with previous studies on the sensing capability of e-nose technology for assessing the volatile profiles of horticultural products during fruit development and maturity [10,17].
Although the primary purpose of GC–MS–e-nose was to obtain MS data as a chemical fingerprint of the volatiles rather than for compound identification [15,17], analysis may also provide indications of the important ion masses in the PCA loading plots that are responsible for the separation of biological replicates of the fruits (Figure 2b,c). For instance, the outlier score plot of one of the fruits of the ‘No. 26’ line at the remotest positive direction of PC1 could be affected by a greater intensity of ion mass m/z 88, which might be derived from ethyl acetate or methyl propanoate (retrieved from https://webbook.nist.gov/, accessed on 7 August 2023). The other possible sources of volatiles for the outlying replicate were 2-methylbutyl acetate, which generated ions m/z 85 and 130, and 2-methylpropyl-acetate, which fragmented specific ion masses at m/z 71, 88, and 89. In addition, the ‘No. 27’ outlier at the distant positive direction of PC1 might have high amounts of ocimene isomers for displaying their characteristic ion masses, such as m/z 67 and 79. By measuring the unique chemical fingerprints of these ion masses, the GC–MS–e-nose technique can provide valuable information on the volatile characteristics of food materials and horticultural products [14,15,17]. The current study demonstrated the sensing capability for pineapple fruits. Moreover, because the score plot distributions of both dimensions of the HP-5MS capillary column were highly comparable, the GC–MS–e-nose measurement could be performed within 5 min, resulting in a shorter analytical time (Figure 2d). Therefore, GC–MS–e-nose profiling coupled with multivariate analysis could be a valuable tool for fruit selection and quality assessment of Okinawan pineapples.
The pineapple fruits of the newly bred lines varied in their content and composition of volatile organic compounds; however, among the five evaluated breeds, the ‘No. 25’ line accounted for the lowest volatile content compared with the ‘N67-10’ cultivar, indicating that this line had less potential for the development of a new pineapple variety (Figure 3 and Table 2). Nonetheless, volatile variations were observed in the largest component group: ester compounds. Esters are one of the most important volatiles in many fruits, as they typically provide pleasant odors and are often responsible for the characteristic aroma of fruits, including pineapple [19,24,25]. Various ester compounds primarily emit fruity, floral, and sweet odors, along with other specific likable scents that contribute to the overall aroma profile of fruits and may play crucial roles in determining consumers’ overall liking [1,24]. The ‘No. 22’ line, with high amounts of predominant esters such as methyl hexanoate and methyl 3-(methylthio)propanoate, could clearly distinguish its fruits from the other lines (Table 3). These two esters produce distinct fruity–ethereal and sweet–sulfureous odors and were reported as key volatiles in numerous pineapple varieties worldwide [1,3,25]. Fruits of the ‘No. 26’, ‘No. 27’, and ‘No. 28’ breeding lines might also be affected by the two esters mentioned above as well as by the distinctive fruity aromas from other esters that make up a significant proportion of their volatile constituents, including the typical fruity–sweet pineapple aroma (methyl butanoate), unique fruity–ethereal and green scents (methyl 2-methylbutanoate), strong fruity–ethereal and solvent-like odors (ethyl acetate), and fresh fruity–sulfureous odors (ethyl 3-(methylthio)propanoate) [3,24,25].
Compared with the control cultivar, ‘N67-10’, the new breeding lines might lack various alcoholic or solvent-like odors from their alcohol components (Table 2), i.e., herbal (2-methyl-3-buten-2-ol), green (1-heptanol), oily–citrus (2-ethyl-1-hexanol), waxy (1-octanol), and musty–sweet (2-furanmethanol) (the aroma description of the volatiles was retrieved from http://www.thegoodscentscompany.com/, accessed on 5 April 2023). On the other hand, the fruit of the ‘No. 25’ line could have greater fatty and citrus-like odors from nonanal than that of other lines [27]. Then again, most newly bred lines might have fewer fresh–terpenic aromas due to having fewer terpene components than the fruit of the ‘N67-10’ cultivar [26,28]. However, the fruit of the ‘No. 27’ breeding line might comprise a stronger herbal aroma from monoterpene isomers cis-β-ocimene and trans-β-ocimene [28]. The high proportion of 2,5-dimethyl-4-methoxy-3(2H)-furanone in the ‘No. 27’ line might also supplement its fruit with fruity and sweet–caramel scents [24,25]. The ‘No. 22’ line could be distinguished for comprising the greater lactone compounds that release distinct tropical fruit aromas such as sweet–creamy, peach-like, and coconut-like scents [24]. When selecting a pineapple, consumers are often drawn to its aroma, and the variation in aroma characteristics in the pineapple fruits of newly bred lines could potentially contribute to the uniqueness of their sensory traits [1,2,3,25].
Assuming that the cultivation method and environment of the evaluated pineapples were the same, the variation in the concentration and composition of volatile organic compounds in the fruits could have been caused by genetic differences (Figure 3 and Table 1 and Table 2). Although genetic identity evaluation, such as DNA profiling, in relation to parents was performed, the specific molecular mechanisms underlying the variations in the biosynthesis of the volatile compounds in these breeding lines remain unknown [4]. Biogenesis involves the synthesis of esters from acyl-CoA and alcohol precursors via reactions catalyzed by alcohol acetyltransferases [29,30]. Alcohols are derived from fatty acids via the lipoxygenase oxidation pathway or from degraded amino acids [30]. Aldehyde components such as hexanal and nonanal are synthesized from unsaturated fatty acids via the lipoxygenase pathway [30,31]. Terpene biosynthesis is mainly regulated by terpene synthases from two common five-carbon precursors: isopentenyl diphosphate and its allylic isomer, dimethylallyl diphosphate. The formation of the C5-isoprene building units is controlled by either the mevalonate or methylerythritol phosphate pathways [32,33]. Furthermore, furanones may be derived from phosphorylated carbohydrate precursors in the ketone group, whereas lactones are formed from various fatty acids [33]. Therefore, measuring the generation of VOCs in Okinawan pineapples of different breeding lines could be a challenging task in the future, particularly for synthesizing the key ester compounds involved in the overall aroma characteristics of the fruits.
The variation in the overall flavor quality of Okinawan pineapple fruits from different breeding lines was also altered by their aroma-active compound profiles, as shown in the OAVs heat map chart (Figure 4). The OAVs were calculated by dividing the concentration of a particular volatile compound by its sensory threshold value, which plays a crucial role in assessing the aromatic effects of specific volatile compounds in newly bred lines [2,19,27]. When the OAV of a compound is greater than 1 (log10 OAV > 0), the compound is present at concentrations above its sensory threshold and is likely to significantly contribute to the overall aroma of the fruit [19,21,24]. In contrast, a low OAV indicates that the compound is present below its odor threshold and may have less influence on the overall aroma perception. Accordingly, except for ethyl octanoate, the active-aroma esters contributed more in the new breeds than in the control cultivar, ‘N67-10’, indicating that the fruits of these lines had notably stronger pleasant fruity aromas [3,24,25]. Since nonanal is considered as an aroma-active substance in all breeding lines, the aroma of the fruits could be supplemented by this waxy–fatty odor-producing aldehyde [27]. Moreover, the ‘No. 22’, ‘No. 26’, ‘No. 27’, and ‘No. 28’ fruits might also be complemented by aroma-active linalool that promotes floral, sweet, and citrus-like scents [26,28]. Then again, the aroma of the ‘No. 26’ and ‘No. 27’ lines could be much sweeter due to the high OAVs in their 2,5-dimethyl-4-methoxy-3(2H)-furanone, whereas the ‘No. 22’ and ‘No. 27’ fruits might have strong peach-like or coconut-like scents from aroma-active δ-decalactone [24]. These OAVs data not only provide information on the aroma impact of the volatile components in the fruits of newly bred lines but also can help identify and prioritize the key aroma-active compounds that contribute the most to the overall aroma when the fruits are processed into various food and beverage products [2,19,21]. By quantifying the relative importance of individual compounds, OAVs can guide flavorists and food scientists in ingredient selection, formulation optimization, and flavor enhancement strategies [19,21,22]. They provided insights into the aroma impact of the volatile compounds in pineapples, allowing for targeted flavor modifications and the creation of products with the desired sensory profiles.
Altogether, research on new pineapple breeding lines is important for the pineapple industry in Okinawa Prefecture, Japan, and the characterization of their volatile components is required to provide key information on the flavor quality of these fruits. Furthermore, the volatile compound characterization of these newly bred lines revealed a unique composition of aroma-active compounds that can be used as a basis for developing new pineapple varieties for food production. The GC–MS–e-nose profiling and odor activity data obtained in this study provide an important basis for the practical use of newly developed breeding lines by farmers and the agribusiness industry. For example, the ‘No. 22’ breeding line, with a superior volatile content with aroma-active compounds such as esters and lactones emitting fruity and coconut-like aromas, can be used to produce pineapple jam and juice. On the other hand, the ‘No. 26’, ‘No. 27’, and ‘No. 28’ lines, which are rich in aroma-active compounds with pleasant fruity and sweet aromas, are potential candidates for fresh raw consumption or processing into various food and beverage products, including canned pineapple. Therefore, further investigations into the correlation between taste- and aroma-responsible components and the sensory profile of fruits and their derivatives need to be carried out in the future, and the results of the current study can be used as a basis for conducting further research. Moreover, the assessment of the volatile compounds of interest in the fruits and derived products using external standard calibrations as well as their release characteristics in the human olfactory system could be the next appealing studies on pineapple aroma.
5. Conclusions
GC–MS–e-nose profiling and multivariate analysis via PCA provided valuable technical guidance for selecting biological replicates of Okinawan pineapples from different breeding lines with comparable volatile characteristics. The PCA loading plots that displayed the captured scanned ion masses displayed some ion masses of the volatiles as hints of similarity or dissimilarity of the screened fruit replicates in the PCA score plots. Moreover, the GC–MS–e-nose analysis time could be reduced from 10 min to 5 min for similar PCA results, indicating its beneficial use in monitoring fruit selection and assessing the primary volatile profile of horticultural products, such as pineapple. The compositional analysis showed variations in the amount and composition of volatile components in the ‘N67-10’ cultivar and five new breeding lines. The proportion of volatiles predominantly included esters (40 compounds), followed by ketones (furanones and lactones), terpenes, and alcohols. The ‘No. 22’ breeding line contained a significantly higher volatile content than the ‘N67-10’ cultivar and other lines, indicating that its fruit could emit greater overall volatile potentials. Fourteen components with an OAV greater than 1, which could significantly contribute to the aroma perception of the fruits, were identified, including nine ester components. Most newly bred lines had higher OAVs of those esters than the ‘N67-10’ cultivar, suggesting they could be rich in pleasant fruity aromas. The compound with the highest OAVs was methyl 2-methylbutanoate (aroma characteristics: fruity, ethereal, and green), which accounted for 786.96, 337.72, and 235.92 in the ‘No. 26’, ‘No. 28’, and ‘No. 22’ lines, respectively. In contrast, the superior OAVs of 2,5-dimethyl-4-methoxy-3(2H)-furanone, which may be responsible for producing sweet, caramel, and musty fruits, were observed in the ‘No. 26’ and ‘No. 27’ lines. Additionally, the impact of aroma-active δ-decalactone with tropical creamy fruit aromas, such as peach-like or coconut-like, could be an exclusive complement for the ‘No. 22’ and ‘No. 27’ fruits. Taken together, with distinct volatile characteristics and, thus, attractive aroma qualities, the ‘No. 22’ breeding line has the potential to be used in the processed food and beverage industry, such as in pineapple jam and juice, whereas the fruits of the ‘No. 26’, ‘No. 27’, and ‘No. 28’ lines can be suitable for fresh consumption or canned pineapple production. Thus, the new Okinawan pineapple breeding lines have potential uses in agro-industries, including by breeders and farmers. Thus, the data from the current study could provide a valuable basis for further sensory evaluation studies for practical applications when the fruits of these newly bred lines are used in food and beverage production.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemosensors11100512/s1. Figure S1: HCA dendrogram of volatile profiles of screened Okinawan pineapple fruits, generated by GC–MS–e-nose analysis on HP-5MS 30 m column (selected fruits are marked with #); Figure S2: HCA dendrogram of volatile profiles of selected Okinawan pineapple fruits, generated by GC–MS–e-nose analysis on HP-5MS 15 m and 30 m columns.
Author Contributions
Conceptualization, Y.A., M.T. and K.W.; methodology, Y.A. and K.W.; software, Y.A. and K.W.; validation, Y.A., M.K., K.T. and K.W.; formal analysis, Y.A. and M.K.; investigation, Y.A., S.K. and K.W.; resources, R.M., Y.O. and M.T.; data curation, Y.A., M.K., S.K., R.M. and Y.O.; writing—original draft preparation, Y.A., M.K. and S.K.; writing—review and editing, Y.A. and K.W.; visualization, Y.A. and M.K.; supervision, M.T., K.T. and K.W.; project administration, R.M., Y.O., M.T. and K.W.; funding acquisition, M.T. and K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the research program on development of innovative technology grants from the Project of the Bio-oriented Technology Research Advancement Institution (BRAIN), grant number JPJ007097.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Eriko Arakaki (University of the Ryukyus) and Masahiro Hirouchi (Takata Koryo Co., Ltd.) for their technical assistance with the volatile component analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spence, C. Are pineapples really delicious? The history of the pineapple’s taste/flavour and the role of varietal and terroir. Int. J. Gastron. Food Sci. 2023, 31, 100682. [Google Scholar] [CrossRef]
- Wei, C.-B.; Liu, S.-H.; Liu, Y.-G.; Lv, L.-L.; Yang, W.-X.; Sun, G.-M. Characteristic aroma compounds from different pineapple parts. Molecules 2011, 16, 5104–5112. [Google Scholar] [CrossRef]
- Pino, J.A. Odour-active compounds in pineapple (Ananas comosus [L.] Merril cv. Red Spanish). Int. J. Food Sci. Technol. 2013, 48, 564–570. [Google Scholar] [CrossRef]
- Shoda, M.; Urasaki, N.; Sakiyama, S.; Terakami, S.; Hosaka, F.; Shigeta, N.; Nishitani, C.; Yamamoto, T. DNA profiling of pineapple cultivars in Japan discriminated by SSR markers. Breed. Sci. 2012, 62, 352–359. [Google Scholar] [CrossRef]
- Asikin, Y.; Shimoda, K.; Takeuchi, M.; Maekawa, R.; Kamiyoshihara, Y.; Takara, K.; Wada, K. Free and glycosidically bound volatile compounds in Okinawan pineapple (Ananas comosus). Appl. Sci. 2022, 12, 9522. [Google Scholar] [CrossRef]
- Sugawara, T.; Nishiba, Y.; Takeuchi, M.; Moromizato, C. Carotenoid content in different varieties of pineapple (Ananas comosus L.) cultivated in Okinawa Prefecture. Nippon. Shokuhin Kagaku Kogaku Kaishi 2019, 66, 100–107. [Google Scholar] [CrossRef]
- Ogata, T.; Yamanaka, S.; Shoda, M.; Urasaki, N.; Yamamoto, T. Current status of tropical fruit breeding and genetics for three tropical fruit species cultivated in Japan: Pineapple, mango, and papaya. Breed. Sci. 2016, 66, 69–81. [Google Scholar] [CrossRef]
- Santo, S.; Uchiyama, T. Analysis of importers’ behavior in the context of pineapple distribution in Japan: A case study of Dole Japan and a survey of consumers’ attitudes. J. Food Syst. Res. 2014, 21, 2–16. [Google Scholar] [CrossRef]
- Kim, C.; Kim, S.J.; Lee, Y.; Nguyen, T.M.; Lee, J.M.; Moon, J.S.; Han, D.W.; Oh, J.W. A phage-and colorimetric sensor-based artificial nose model for banana ripening analysis. Sens. Actuators B Chem. 2022, 362, 131763. [Google Scholar] [CrossRef]
- Asikin, Y.; Kusumiyati; Shikanai, T.; Wada, K. Volatile aroma components and MS-based electronic nose profiles of dogfruit (Pithecellobium jiringa) and stink bean (Parkia speciosa). J. Adv. Res. 2018, 9, 79–85. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, X.; Zhang, C.; Li, X.; Yue, N.; Shao, H.; Wang, J.; Jin, F. Discrimination and characterization of volatile flavor compounds in fresh oriental melon after forchlorfenuron application using electronic nose (e-nose) and headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). Foods 2023, 12, 1272. [Google Scholar] [CrossRef] [PubMed]
- Torri, L.; Sinelli, N.; Limbo, S. Shelf life evaluation of fresh-cut pineapple by using an electronic nose. Postharvest Biol. Technol. 2010, 56, 239–245. [Google Scholar] [CrossRef]
- Yang, Q.; Gong, X.; Chen, M.; Tu, J.; Zheng, X.; Yuan, Y. Comparative analysis of the aroma profile of pineapple beers brewed with juice added at different times. J. Inst. Brew. 2023, 129, 151–163. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Asikin, Y.; Kamchonemenukool, S.; Tamaki, H.; Takara, K.; Wada, K. Physicochemical, antioxidant, volatile component, and mass spectrometry-based electronic nose analyses differentiated unrefined non-centrifugal cane, palm, and coconut sugars. Food Meas. 2021, 15, 1563–1577. [Google Scholar] [CrossRef]
- Majcher, M.A.; Kaczmarek, A.; Klensporf-Pawlik, D.; Pikul, J.; Jelen, H.H. SPME-MS-based electronic nose as a tool for determination of authenticity of PDO cheese, Oscypek. Food Anal. Methods 2015, 8, 2211–2217. [Google Scholar] [CrossRef]
- Sung, J.; Kim, B.K.; Kim, B.S.; Kim, Y. Mass spectrometry-based electric nose system for assessing rice quality during storage at different temperatures. J. Stored Prod. Res. 2014, 59, 204–208. [Google Scholar] [CrossRef]
- Chaparro-Torres, L.A.; Bueso, M.C.; Fernández-Trujillo, J.P. Aroma volatiles obtained at harvest by HS-SPME/GC-MS and INDEX/MS-E-nose fingerprint discriminate climacteric behaviour in melon fruit. J. Sci. Food Agric. 2016, 96, 2352–2365. [Google Scholar] [CrossRef]
- Cervellieri, S.; Lippolis, V.; Mancini, E.; Pascale, M.; Logrieco, A.F.; De Girolamo, A. Mass spectrometry-based electronic nose to authenticate 100% Italian durum wheat pasta and characterization of volatile compounds. Food Chem. 2022, 383, 132548. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Zhang, R.; Liu, S.; Yang, S.; Li, Y.; Li, J. Flavoromics approach in critical aroma compounds exploration of peach: Correlation to origin based on OAV combined with chemometrics. Foods 2023, 12, 837. [Google Scholar] [CrossRef]
- Williams, J.; Ringsdorf, A. Human odour thresholds are tuned to atmospheric chemical lifetimes. Philos. Trans. R. Soc. Lond B Biol. Sci. 2020, 375, 20190274. [Google Scholar] [CrossRef]
- Qian, Y.L.; Zhang, D.; An, Y.; Zhou, Q.; Qian, M.C. Characterization of aroma-active compounds in northern highbush blueberries “Bluecrop” (Vaccinium corymbosum “Bluecrop”) and “Elliott” (Vaccinium corymbosum “Elliott”) by gas chromatography-olfactometry dilution analysis and odor activity value. J. Agric. Food Chem. 2021, 69, 5691–5701. [Google Scholar] [CrossRef]
- Liang, S.; Liu, Y.; Yuan, S.; Liu, Y.; Zhu, B.; Zhang, M. Study of consumer liking of six Chinese vinegar products and the correlation between these likings and the volatile profile. Foods 2022, 11, 2224. [Google Scholar] [CrossRef] [PubMed]
- van Gemert, L.J. Flavour Thresholds: Compilations of Flavour Threshold Values in Water and Other Media, Second Enlarged and Revised, ed.; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Tokitomo, Y.; Steinhaus, M.; Büttner, A.; Schieberle, P. Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation. Biosci. Biotechnol. Biochem. 2005, 69, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Arakawa, N.; Takamura, A.; Morimitsu, Y.; Kubota, K. Potent odorants in Sweetio pineapple (Ananas comosus [L.] Merr. var. Marian-gold (MG-3)). Nippon. Shokuhin Kagaku Kogaku Kaishi 2006, 53, 121–129. [Google Scholar] [CrossRef]
- Asikin, Y.; Kawahira, S.; Goki, M.; Hirose, N.; Kyoda, S.; Wada, K. Extended aroma extract dilution analysis profile of Shiikuwasha (Citrus depressa Hayata) pulp essential oil. J. Food Drug Anal. 2018, 26, 268–276. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Sun, M.; Liu, J.; Bai, Y.; Liu, X.; Guo, Y. Characterization of key aroma compounds in fermented bamboo shoots using gas chromatography-olfactometry-mass spectrometry, odor activity values, and aroma recombination experiments. Foods 2022, 11, 2106. [Google Scholar] [CrossRef]
- Cai, X.; Mai, R.Z.; Zou, J.J.; Zhang, H.Y.; Zeng, X.L.; Zheng, R.R.; Wang, C.Y. Analysis of aroma-active compounds in three sweet osmanthus (Osmanthus fragrans) cultivars by GC-olfactometry and GC-MS. J. Zhejiang Univ. Sci. B 2014, 15, 638–648. [Google Scholar] [CrossRef]
- Qian, X.; Liu, Y.; Zhang, G.; Yan, A.; Wang, H.; Wang, X.; Pan, Q.; Xu, H.; Sun, L.; Zhu, B. Alcohol acyltransferase gene and ester precursors differentiate composition of volatile esters in three interspecific hybrids of Vitis labrusca × V. vinifera during berry development period. Food Chem. 2019, 295, 234–246. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Hashem, C.; Hochrinner, J.; Bürgler, M.B.; Rinnofner, C.; Pichler, H.; Winkler, M. From linoleic acid to hexanal and hexanol by whole cell catalysis with a lipoxygenase, hydroperoxide lyase and reductase cascade in Komagataella phaffii. Front. Mol. Biosci. 2022, 9, 965315. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, J.Y.; Choi, Y.I.; Hu, L.; Yang, C.; Jin, Z.; Park, Y.J.; Kim, S.U.; Kim, S.M. Enhanced metabolic flux of methylerythritol phosphate (MEP) pathway by overexpression of Ginkgo biloba 1-Hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate Reductase 1 (GbHDR1) gene in poplar. Appl. Biol. Chem. 2022, 65, 50. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).