Abstract
Flexible and stretchable strain sensors can be applied for human health monitoring and disease diagnoses via the output of multiple biophysical signals. However, it is still a challenge to fabricate short-peptide-based strain sensors. Here, we prepared a novel polymer-dipeptide double-network hydrogel with excellent stretchability, responsiveness, and stability. The poly(acrylic acid) (PAA) gel, by cross-linking, maintains mechanical and flexible properties, and the fluorenyl methoxycarbonyl-diphenylalanine (Fmoc-FF) network, by non-covalent interactions, is helpful for energy dissipation. With increasing tensile or compression strains, the PAA/Fmoc-FF hydrogel exhibited a high mechanical strength and fast recovery. Moreover, as the presence of KCl improves the electronic conductivity, the hybrid gel exhibited a cyclic strain-stress performance, which is the foundation of a strain sensor. Based on that, its application as a motion sensor was demonstrated by monitoring the movements of human joints, such as the forefinger, wrist, elbow, and knee. Consequently, the hybrid polymer-peptide gel could be an ideal candidate for wearable sensors in the future.
1. Introduction
Flexible sensors have attracted tremendous attention due to their low cost, low modulus, high flexibility, and inexpensive substrate materials [1,2,3]. However, flexible sensors face challenges in practical applications where they go through large deformations of bending, twisting, and stretching or directly integrate with curvilinear substances, leading to the requirement of stretchable electronics [4]. Recently, flexible and stretchable electrical sensors attached to the human skin have been used more commonly, as they can perceive external stimuli, thus exhibiting reliable physical signals for health monitoring [5,6,7]. Carbon-based nanomaterials, conductive polymers, metal nanowires, and nanoparticles are also popular materials for stretchable sensors [8,9,10,11,12]. Further, strain sensors are ideal for the realization of joint angle estimation, gait recognition, fall detection, and activity recognition [13,14,15,16,17]. This kind of sensor usually integrates slight changes in current, resistance, capacity, or other relative parameters to fabricate strain-based modules to output multi-dimensional motion monitoring [18].
As an important soft matter, hydrogels contain three-dimensional porous network structures and a large amount of water trapped inside [19]. Hydrogels based on peptides with fibrillar networks have attracted significant attention as they possess excellent and unique biomimetic and biocompatible properties [20]. However, peptide hydrogels are prepared through non-covalent interactions, leading to a poor gelation stability and weak mechanical strength. Therefore, significant efforts have been made to produce highly durable peptide hydrogels for domestic and industrial applications, including tough double-network hydrogels (DN gels) [21,22,23]. DN gels consist of two interpenetrating networks: a relatively brittle first network with a low concentration and a sparsely cross-linked ductile second network with a high concentration [24,25,26]. Polymer gels are quite superior and chosen as a member of DN gels due to their excellent mechanical properties via their covalent chains [27,28]. Therefore, peptide and polymer DN gels with a good flexibility, stretchability and resilience are potential candidates for strain sensors.
Diphenylalanine (FF) and its derivatives, as popular dipeptides with a subtle biocompatibility and powerful self-assembly capabilities, have experienced great development in the past few decades [29,30]. Among them, fluorenyl methoxycarbonyl-diphenylalanine (Fmoc-FF), reported as an efficient hydrogelator due to π-stacking, has been revealed to successfully form fibrillar aggregates and gels with a perfect biocompatibility [31]. However, due to the poor mechanical property of FF hydrogels, it is still challenging to use them as a stretchable strain sensor. Herein, we design and prepare a novel polymer-supramolecular DN gel with a combination of poly(acrylic acid) (PAA) and Fmoc-FF. We hypothesize that the break of the Fmoc-FF network could dissipate energy and that the reassembly could help with the recovery. Therefore, in addition to the PAA gel with a high mechanical toughness, we use the PAA/Fmoc-FF hydrogel (PAA/FF gel) as a stretchable gel, which could be applied as a strain sensor in the future.
2. Materials and Methods
2.1. Materials
Fluorenyl methoxycarbonyl-diphenylalanine (Fmoc-FF, >98%) was purchased from Leyan Compony, Shanghai, China. Dimethylsulfoxide (DMSO, >99.9%), acrylic acid (AA, >99%), and ammonium persulfate (APS, >98%) were purchased from Aladdin Reagent, Shanghai, China. N,N′-Methylenebis-(acrylamide) (MBAA, >98%) and potassium chloride (KCl) were purchased from J&K Scientific Ltd., Beijing, China.
2.2. Preparation of the Gels
Fmoc-FF hydrogel: Fmoc-FF was diluted in DMSO to a final Fmoc-FF concentration of 0.167 g/mL. Then, deionized water was added into it to obtain a certain Fmoc-FF concentration.
Poly(acrylic acid) (PAA) gel: 0.1 mol% MBAA was diluted in 500 μL AA and then mixed with 550 μL 5 wt% APS aqueous solution to initiate polymerization aging in a 60 °C oven for 30 min.
PAA/FF gel: Fmoc-FF was diluted in DMSO to a final Fmoc-FF concentration of 0.167 g/mL, and 0.1 mol% MBAA was diluted in 500 μL AA. After mixing the above two solutions, 550 μL 5 wt% APS aqueous solution was added to initiate polymerization aging in a 60 °C oven for 30 min.
PAA/FF/KCl gel: KCl was added into the APS aqueous solution just before the mixture. Fmoc-FF was diluted in DMSO to a final Fmoc-FF concentration of 0.167 g/mL, and 0.1 mol% MBAA was diluted in 500 μL AA. After mixing the above two solutions, 550 μL 5 wt% APS and KCl aqueous solution were added to initiate polymerization aging in a 60 °C oven for 30 min.
2.3. Measurements and Characterizations
Scanning electron microscope (SEM) measurements were taken on JEOL JSM6700F or Hitachi S-4800 field-emission scanning electron microscopes (Hitachi, Tokyo, Japan). The freeze-dried samples with gold sputtering were transferred onto the microscope stage and examined at 10 kV.
Attenuated total reflection (ATR)–Fourier transform infrared (FTIR) measurements were performed on a Bruker Tensor II spectrometer with a spectral range of 4000–400 cm–1 and a resolution of 4 cm–1 (Bruker, Bremen, Germany).
The mechanical strength of gel was obtained by Texture Analyzers (FTC, TMS-PRO, Irvine, CA, USA). For tensile experiments, samples were prepared in a cuboid (length = 15 mm, width = 4 mm, height = 2 mm) and tested at a speed of 20 mm/min; and for compression tests, samples were shaped into a cylinder (height = 8 mm, radius = 6 mm) and tested at a speed of 10 mm/min.
The real-time relative resistance changes of the gel in response to stretching were performed on a VersaSTAT four electrochemical workstation at a frequency range of 1 × 105 Hz and amplitude of 5 mV, and the data was analyzed using Z-view software to obtain the resistance. The gel (15 mm × 4 mm) is glued with copper foil on both ends to link the alligator clips. The relative resistance changes were calculated from (R − R0)/R0 × 100%, where R and R0 are the dynamic resistance under different strains and the resistance at the initial state without strain. To investigate the performance of the strain sensor, the gel was attached to the body part that needed to be tested in the same way.
3. Results and Discussion
3.1. Characterization of the PAA/FF Gel
As illustrated in Scheme 1, we prepared a DN gel (PAA/FF gel), which consisted of two networks. These are the first Fmoc-FF network and the second PAA network, created by the heat-induced polymerization of acrylic acid (AA), N,N′-methylenebis-acrylamide (MBAA), and ammonium persulfate (APS). The insert images in Figure 1a,b show that the PAA gel is transparent, while in the presence of Fmoc-FF, the PAA/FF gel turns white and semitransparent, indicating the uniformity of the PAA/FF gel. Figure 1a,b show the morphology images by scanning electron microscope (SEM) observation. It is found that because of the addition of Fmoc-FF, the gels changed from a smooth and homogeneous surface to more compact structures, showing the successful fabrication of double networks.
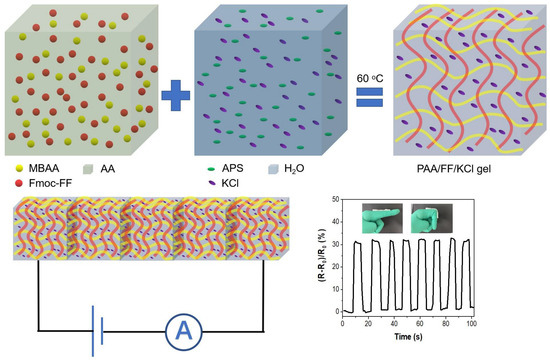
Scheme 1.
Schematic illustration of poly(acrylic acid)-dipeptide double-network hydrogel for high stretchable strain sensor.

Figure 1.
SEM images of (a) PAA gel, (b) PAA/FF gel; (c) the FTIR spectra of Fmoc-FF, PAA, and PAA/FF gels.
To illustrate the interactions in the secondary structures of the gels, Fourier- transformed infrared (FTIR) tests were performed (Figure 1c). Compared with the Fmoc-FF hydrogel and PAA hydrogel, the observed bands of PAA/FF gel generally correspond to the characteristic peaks of the individual components. The broad absorption bands around 3050–3700 cm–1 caused by the stretching vibration of –NH and –OH groups appear in all three gels. They are attributed to the hydrogen bonding interaction between –COOH groups in the PAA chains and/or to Fmoc-FF molecules [32,33,34]. The appearance of a broad peak around 2950 cm–1 in the PAA/FF gel is assigned to more hydrogen bonding between PAA and Fmoc-FF [35]. The peak at 1698 cm–1 of the PAA/FF gel is ascribed to the characteristic peak at 1688 cm–1 in the β-sheet structure in the Fmoc-FF gel and to the peak at 1702 cm–1 from the symmetrical stretching vibration of C=O groups in the PAA gel [36,37,38]. The characteristic peak at 1530 cm–1 based on Fmoc-FF is also assigned to the C=O stretching vibration [39]. PAA/FF performs the peak at 1652 cm–1, demonstrating that antiparallel β sheet structures remain among the Fmoc-FF molecules. One can clearly see that the combination of PAA and Fmoc-FF does not significantly change with the individual arrangement but only improves the formation of hydrogen bonds.
3.2. Mechanical Properties of PAA/FF Gel
It is worth mentioning that more hydrogen bonding, due to the addition of Fmoc-FF, plays a key role in this DN gel. When the amount of Fmoc-FF was increased, the mechanical properties were further explored. The results in Figure 2 show the tensile and compressive stress-strain curves of PAA/FF with different amounts of Fmoc-FF. The PAA/FF gels were stretched under a rising strain (Figure 2a,b). As the weight ratio (wt%) of Fmoc-FF was changed from 0 to 4 wt%, the stress first increased and then decreased. The tensile strength and stiffness were optimal at 1 wt% Fmoc-FF, with a high stretchability with a 570% elongation at the break. The variation of Fmoc-FF could result in different interactions in double networks. As the Fmoc-FF gel is not strong enough to ensure any stretch, the initial improvement is because of the extra hydrogen bonding between Fmoc-FF and PAA. However, the excess Fmoc-FF (1 wt%) in the PAA/FF gel could restrain radical polymerization, damaging the strength of PAA networks. Moreover, the compressive stress-strain curves in Figure 2c,d indicate that within a 90% compressive strain (the maximum range of the instrument), the amount of Fmoc-FF has little impact on the stress of PAA/FF gels.
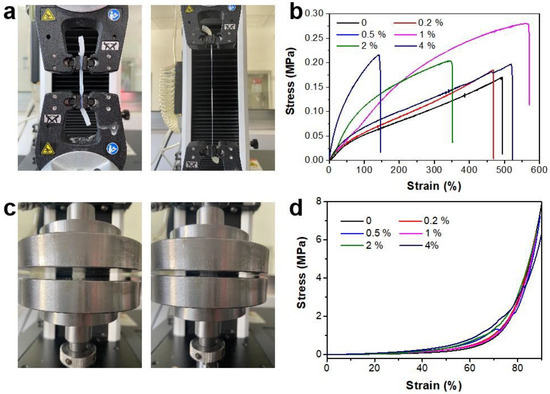
Figure 2.
Photograph and stress-strain curves of the PAA/FF gels: (a,b) tensile and (c,d) compression with different amounts of Fmoc-FF (wt%).
To further investigate the structural stability of the PAA/FF gel, FTIR was performed after the final stretched break (Figure 3a). The disappearance of the peak at 1531 cm–1 suggests damage of the C=O stretching vibration in Fmoc-FF, proving that Fmoc-FF was sacrificed first. In addition, the broad band at 2950 cm–1 is less weak, demonstrating that the hydrogen bonds are a little damaged. These results suggest that the Fmoc-FF network can easily dissipate energy during the stretching and that the reassembly of the Fmoc-FF network allows for the flexible and tough mechanical property to recovery quickly, as demonstrated for other polymer-supermolecoular double-network hydrogels [22]. The non-covalent interactions within Fmoc-FF molecules, such as hydrogen bonds and π-π interactions, dominate during the stretching process.
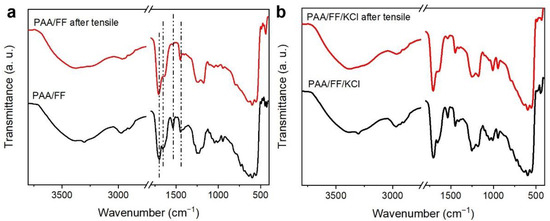
Figure 3.
The FTIR spectra of (a) the original PAA/FF gel and of that after the final stretch break, and those of (b) the PAA/FF and PAA/FF/KCl gels.
As the tensile mechanical performance of the PAA/FF gel is highly stretchable and mechanically compliant under different strains, it shows promise for the fabrication of flexible electronic devices. To increase the electrical conductivity of the PAA/FF gel, inorganic salt KCl was introduced into the gels. The molecular interactions of the PAA/FF/KCl gel are displayed by FTIR in Figure 3b, demonstrating the same interactions as with the PAA/FF gel. The addition of K+ and Cl− improved the stretchability a bit, and it was with 6 wt% KCl that the best stretchability was achieved, with a 760% elongation (Figure 4a). The antifatigue property of the PAA/FF/KCl gel was evaluated by cyclic tests (Figure 4b). Within 10 cycles, especially after the first cycle, the maximum stress gradually decreased due to damage to the networks. However, the effective energy dissipation of hydrogen bonds helped maintain the maximum stress from the third to tenth cycle, thus showing a good antifatigue ability. As its performances are not repeatable in the first two cycles, an intial cycling of three cycles might be needed before sensor use.
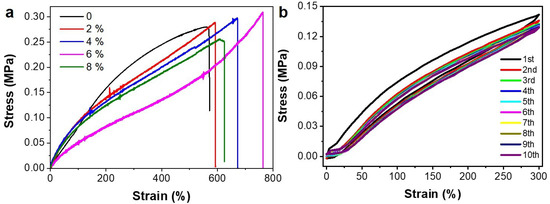
Figure 4.
(a) The tensile stress-strain curves of the PAA/FF/KCl gels with different KCl (wt%). (b) The cyclic tensile stress-strain curves of the PAA/FF/KCl gel with 6 wt% KCl.
3.3. Performance as a Strain Sensor
Given the good recovery and reliability of the PAA/FF/KCl gel, it was further investigated as an applied strain sensor. The water in the gel provides a conductive pathway for K+ and Cl− transport [40,41,42]. When the gel is stretched, it becomes long and narrow. The incremental strain of the gel led to an increased ion transport distance, leading to a change in the sensor’s current. Therefore, the gel strain sensor can translate the stretching force into electrical signals. As Figure 5a proves, the strain sensor maintained uniform output signals with the repeated stretching force, and the relative resistance change also increased as the strain increased in the range of 0–150% for a period of time (10 s). Figure S1 shows the linear relationship between the ratio of the relative resistance change and the applied strain (0–100%), demonstrating a good sensitive stretchability as a potential detector. Figure S2 shows the resistance results after 200 cycles with a 50% strain. It can be observed that with repeatable stretching, the relative resistance was a bit higher than that in the beginning. This is because the mechanical structure of the gel may not completely recover under such highly frequent and repeated stretching.
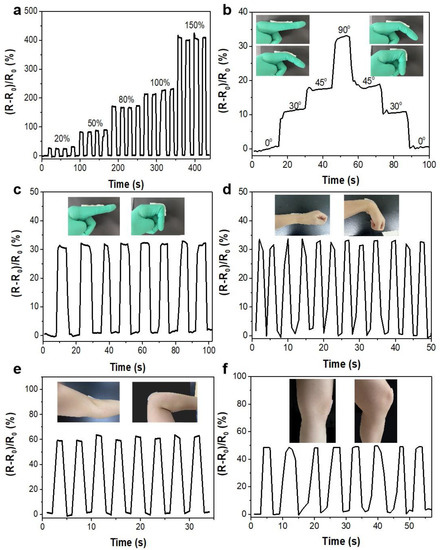
Figure 5.
Relative resistance change vs. time for the gel-based strain sensor under various cyclic strains: (a) different elongations (20, 50, 80, 100, 150%); (b) different bending angles of the forefinger; with different joint movements of the human body: (c) forefinger, (d) wrist, (e) elbow, (f) knee.
The PAA/FF/KCl gel-based strain sensors performed with quite a reliable and stable response to the applied stretched strain. These strain sensors were attached to the finger, wrist, elbow, and knee joints to monitor body motions. The strain sensor could recognize different bending degrees of the forefinger (Figure 5b). Moreover, the bending movements of joints could be detected with repeatable signals (Figure 5c–f), suggesting a favorable responsiveness and stability. Although the light hysteresis of the response time and a small vibration of the relative resistance were the results of long-time damage to the structures, the strain sensor exhibited a good performance.
4. Conclusions
In summary, we fabricated a stretchable strain sensor based on a polymer-peptide double network hydrogel (PAA/FF gel). It had Fmoc-FF as the first network and cross-linked PAA as the second network. The presence of Fmoc-FF strengthened the hydrogen bond interaction in the system, and then increased the stretched strain. However, the excess Fmoc-FF partly damaged the PAA network and decreased the strains in reverse. Therefore, the optimal amount of Fmoc-FF was chosen to be 1 wt%. PAA/FF displayed a good performance with the cyclic, stretched strains because of the energy dissipation and recoverability from non-covalent interactions of Fmoc-FF. In addition, the water in the gel provided a conductive pathway for K+ and Cl− transport, helping the PAA/FF/KCl gel successfully translate the stretching strains into electrical signals. Then, the sensitivity and reliability of this gel-based strain sensor were demonstrated by monitoring different human motions such as the bending of the forefinger, wrist, elbow, and knee. We anticipate that this work could expand the application of polymer-peptide hybrid gels as multifunctional, wearable sensors for human health or disease diagnosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors10090360/s1, Figure S1: Relative resistance change of gels as a function of the applied strain; Figure S2: Cyclic stability of gels after repeated stretching with a strain of 50% for 200 cycles.
Author Contributions
X.L. (Xingcen Liu) conceived and designed the experiments. X.L. (Xin Luo) and B.D. performed most of the experiments and analyzed the results. X.L. (Xingcen Liu) contributed to various aspects of the scientific analysis and provided invaluable financial and organizational support. X.L. (Xin Luo) and X.L. (Xingcen Liu) wrote the manuscript and revised it. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 21902089 & 22002072), the Natural Science Foundation of Shandong Province (ZR2018BB044) and the Young Scholars Program of Shandong University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yao, S.S.; Zhu, Y. Nanomaterial-enabled stretchable conductors: Strategies, materials and devices. Adv. Mater. 2015, 27, 1480–1511. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Takahashi, T.; Ho, J.C.; Ko, H.; Gillies, A.G.; Leu, P.W.; Fearing, R.S.; Javey, A. Nanowire active-matrix circuitry for low-voltage macroscale artificial skin. Nat. Mater. 2010, 9, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Qin, Y.; Xu, C.; Wei, Y.; Yang, R.; Wang, Z.L. Self-powered nanowire devices. Nat. Nanotechnol. 2010, 5, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.F.; Li, J.H.; Cao, D.X.; Zhang, G.P.; Li, J.; Li, K.; Yang, Y.; Wang, W.; Jin, Y.F.; Sun, R.; et al. Recent advancements in flexible and stretchable electrodes for electromechanical sensors: Strategies, materials, and features. ACS Appl. Mater. Interfaces 2017, 9, 12147–12164. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, skin mountable, and wearable strain sensors and their potential applications: A review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Chen, K.; Gao, W.; Emaminejad, S.; Kiriya, D.; Ota, H.; Nyein, H.Y.Y.; Takei, K.; Javey, A. Printed carbon nanotube electronics and sensor systems. Adv. Mater. 2016, 28, 4397–4414. [Google Scholar] [CrossRef]
- Wang, S.H. A comprehensive review of wearable applications and material construction. Open J. Appl. Sci. 2020, 10, 364–408. [Google Scholar] [CrossRef]
- Wang, C.Y.; Xia, K.L.; Wang, H.M.; Liang, X.P.; Yin, Z.; Zhang, Y.Y. Advanced carbon for flexible and wearable electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef]
- Park, M.; Im, J.; Shin, M.; Min, Y.; Park, J.; Cho, H.; Park, S.; Shim, M.B.; Jeon, S.; Chung, D.Y.; et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotech. 2012, 7, 803–809. [Google Scholar] [CrossRef]
- Lipomi, D.; Vosgueritchian, M.; Tee, B.K.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z.N. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotech. 2011, 6, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.L.; Niu, X.F.; Zhao, R.; Pei, Q.B. Elastomeric transparent capacitive sensors based on an interpenetrating composite of silver nanowires and polyurethane. Appl. Phys. Lett. 2013, 102, 083303. [Google Scholar]
- Poitras, I.; Dupuis, F.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J.S. Validity and reliability of wearable sensors for joint angle estimation: A systematic review. Sensors 2019, 19, 1555. [Google Scholar] [CrossRef] [PubMed]
- Pang, I.; Okubo, Y.; Sturnieks, D.; Lord, S.R.; Brodie, M.A. Detection of near falls using wearable devices: A systematic review. J. Geriatr. Phys. Ther. 2019, 42, 48–56. [Google Scholar] [CrossRef]
- Chen, L.; Nugent, C.D. Sensor-based activity recognition review. In Human Activity Recognition and Behaviour Analysis; Springer: Berlin/Heidelberg, Germany, 2019; pp. 23–47. [Google Scholar]
- Aroganam, G.; Manivannan, N.; Harrison, D. Review on wearable technology sensors used in consumer sport applications. Sensors 2019, 19, 1983. [Google Scholar] [CrossRef]
- Yang, G.; Tan, W.; Jin, H.; Zhao, T.; Tu, L. Review wearable sensing system for gait recognition. Cluster Comput. 2019, 22, 3021–3029. [Google Scholar] [CrossRef]
- Cheng, H.W.; Yan, S.; Shang, G.J.; Wang, S.; Zhong, C.J. Strain sensors fabricated by surface assembly of nanoparticles. Biosens. Bioelectron. 2021, 186, 113268. [Google Scholar] [CrossRef]
- Sun, X.; Yao, F.L.; Li, J.J. Nanocomposite hydrogel-based strain and pressure sensors: A review. J. Mater. Chem. A 2020, 8, 18605–18623. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef]
- Cui, K.P.; Sun, T.L.; Liang, X.B.; Nakajima, K.; Ye, Y.N.; Chen, L.; Kurokawa, T.; Gong, J.P. Multiscale energy dissipation mechanism in tough and self-healing hydrogels. Phys. Rev. Lett. 2018, 121, 185501. [Google Scholar] [CrossRef]
- Sun, W.X.; Xue, B.; Li, Y.; Qin, M.; Wu, J.Y.; Lu, K.; Wu, J.H.; Cao, Y.; Jiang, Q.; Wang, W. Polymer-supramolecular polymer double-network hydrogel. Adv. Funct. Mater. 2016, 26, 9044–9052. [Google Scholar] [CrossRef]
- Wang, R.; Yao, M.J.; Huang, S.; Tian, J.L.; Niu, Z.Q. An anti-freezing and anti-drying multifunctional gel electrolyte for flexible aqueous zinc-ion batteries. Sci. China Mater. 2022, 65, 2189–2196. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakajima, T.; Yang, J.J.; Kurokawa, T.; Liu, J.; Lu, J.S.; Mizumoto, S.J.; Sugahara, K.; Kitamura, N.; Yasuda, K.; et al. Proteoglycans and glycosaminoglycans improve toughness of biocompatible double network hydrogels. Adv. Mater. 2014, 26, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583. [Google Scholar] [CrossRef]
- Voorhaar, L.; Hoogenboom, R. Supramolecular polymer networks: Hydrogels and bulk materials. Chem. Soc. Rev. 2016, 45, 4013–4031. [Google Scholar] [CrossRef]
- Chen, H.; Yang, F.Y.; Hu, R.D.; Zhang, M.Z.; Ren, B.P.; Gong, X.; Ma, J.; Jiang, B.B.; Chen, Q.; Zheng, J. A comparative study of the mechanical properties of hybrid double-network hydrogels in swollen and as-prepared states. J. Mater. Chem. B 2016, 4, 5814–5824. [Google Scholar] [CrossRef]
- Fei, J.B.; Zhang, H.; Wang, A.H.; Qin, C.C.; Xue, H.M.; Li, J.B. Biofluid-triggered burst release from an adaptive covalently assembled dipeptide nanocontainer for emergency treatment. Adv. Healthcare Mater. 2017, 6, 1601198. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Xing, R.R.; Yan, X.H. Peptide self-assembly: Thermodynamics and kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, M.; Miravet, J.F.; Ulijn, R.V.; Escuder, B. Peptide-based molecular hydrogels as supramolecular protein mimics. Chem. Eur. J. 2017, 23, 981–993. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Zhang, F.; Xu, Z.; Chen, F.; Shi, Y.; Hou, C.; Huang, Y.; Lin, C.; Yu, R.; et al. Highly stretchable, adhesive, and self-healing silk fibroin-dopted hydrogels for wearable sensors. Adv. Healthcare Mater. 2021, 10, 2002083. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lou, D.; Wang, H.; Sun, X.; Li, J.; Liu, Y.N. Flexible supercapacitor based on organohydrogel electrolyte with long-term anti-freezing and anti-drying property. Adv. Sci. 2020, 7, 1902740. [Google Scholar] [CrossRef]
- Hu, R.F.; Ji, G.C.; Zhao, J.; Gu, X.L.; Zhou, L.W.; Zheng, J.P. The preparation of dual cross-linked high strain composite gel with manifold excellent properties and its application as a strain sensor. Compos. Sci. Technol. 2022, 217, 109110. [Google Scholar] [CrossRef]
- Laksmono, J.A.; Sudibandriyo, M.; Saputra, A.H.; Haryono, A. Development of porous structured polyvinyl alcohol/zeolite/carbon composites as adsorbent. IOP Conf. Ser. Mater. Sci. Eng. 2017, 201, 012006. [Google Scholar] [CrossRef]
- Smith, A.M.; Williams, R.J.; Tang, C.; Coppo, P.; Collins, R.F.; Turner, M.L.; Saiani, A.; Ulijn, R.V. Fmoc-diphenylalanine self assembles to a hydrogel via a novel architecture based on π-π Interlocked β-Sheets. Adv. Mater. 2008, 20, 37–41. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Z.; Yan, H.; Peng, Q.; Wang, R.; Barkey, M.E.; Jeon, J.; Wujcik, E.K. Ultrastretchable conductive polymer complex as a strain sensor with a repeatable autonomous self-healing ability. ACS Appl. Mater. Interfaces 2019, 11, 20453–20464. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Zhang, H.; Wang, G.; Wei, H.; Zhang, X.; Ma, N. Bioinspired conductive hydrogel with ultrahigh toughness and stable antiswelling properties for articular cartilage replacement. ACS Mater. Lett. 2021, 3, 807–814. [Google Scholar] [CrossRef]
- Han, Y.H.; Jian, L.; Yao, Y.M.; Wang, X.L.; Han, L.J.; Liu, X. Insight into rapid DNA-specific identification of animal origin based on FTIR analysis: A case study. Molecules 2018, 23, 2842. [Google Scholar] [CrossRef]
- Wang, C.; Hu, K.; Zhao, C.; Zou, Y.; Liu, Y.; Qu, X.; Jiang, D.; Li, Z.; Zhang, M.R.; Li, Z. Customization of conductive elastomer based on PVA/PEI for stretchable sensors. Small 2020, 16, 1904758. [Google Scholar] [CrossRef]
- Hang, C.Z.; Zhao, X.F.; Xi, S.Y.; Shang, Y.H.; Yuan, K.P.; Yang, F.; Wang, Q.G.; Wang, J.C.; Zhang, D.W.; Lu, H.L. Highly stretchable and self-healing strain sensors for motion detection in wireless human-machine interface. Nano Energy 2020, 76, 105064. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, G.; Wang, F.; Xu, Y.; Wang, C.; Zhu, Y.; Jiang, W. Design of flexible strain sensor with both ultralow detection limit and wide sensing range via the multiple sensing mechanisms. Compos. Sci. Technol. 2021, 213, 108932. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).