Sustainable PVP-Capped Silver Nanoparticles as a Free-Standing Nanozyme Sensor for Visual and Spectrophotometric Detection of Hg2+ in Water Samples: A Green Analytical Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumental Devices
2.2. Reagents
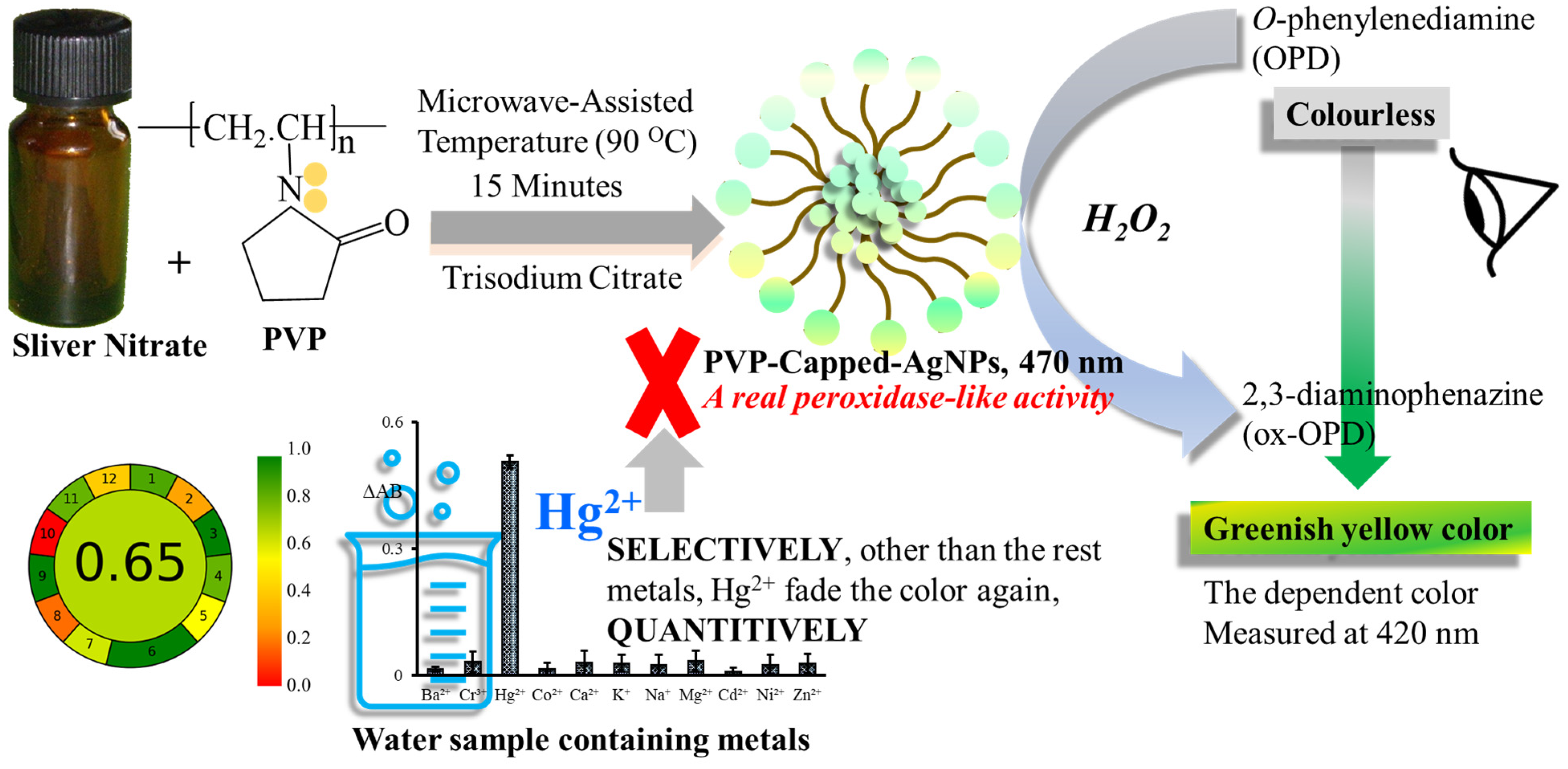
2.3. Synthesis and Stabilization of Polyvinylpyrrolidone-Capped Silver Nanoparticles (PVP-AgNPs)
2.4. General Analytical Procedures for Hg2+ Ions Detection
2.5. Detection of Hg2+ in Various Water Samples
3. Results and Discussion
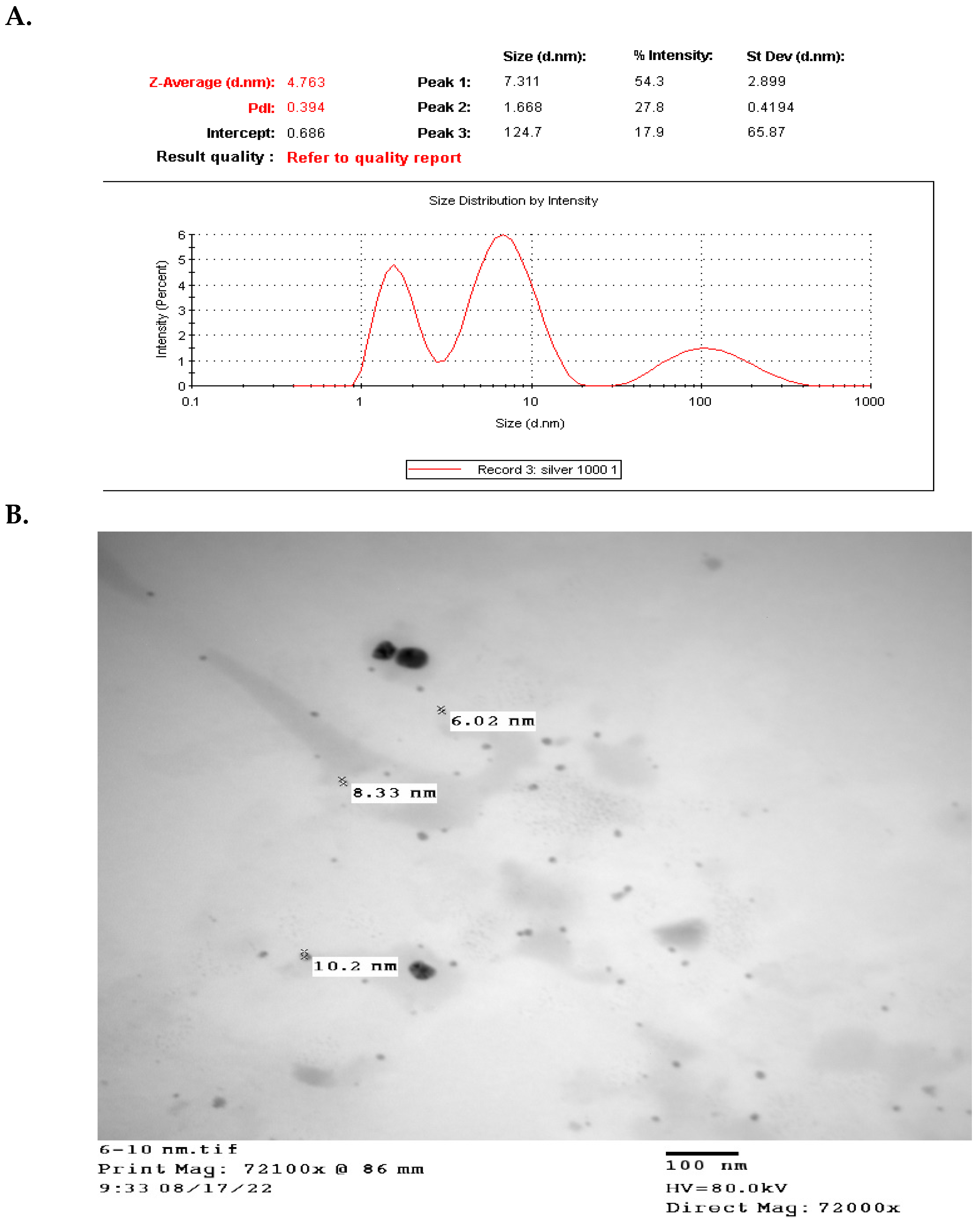
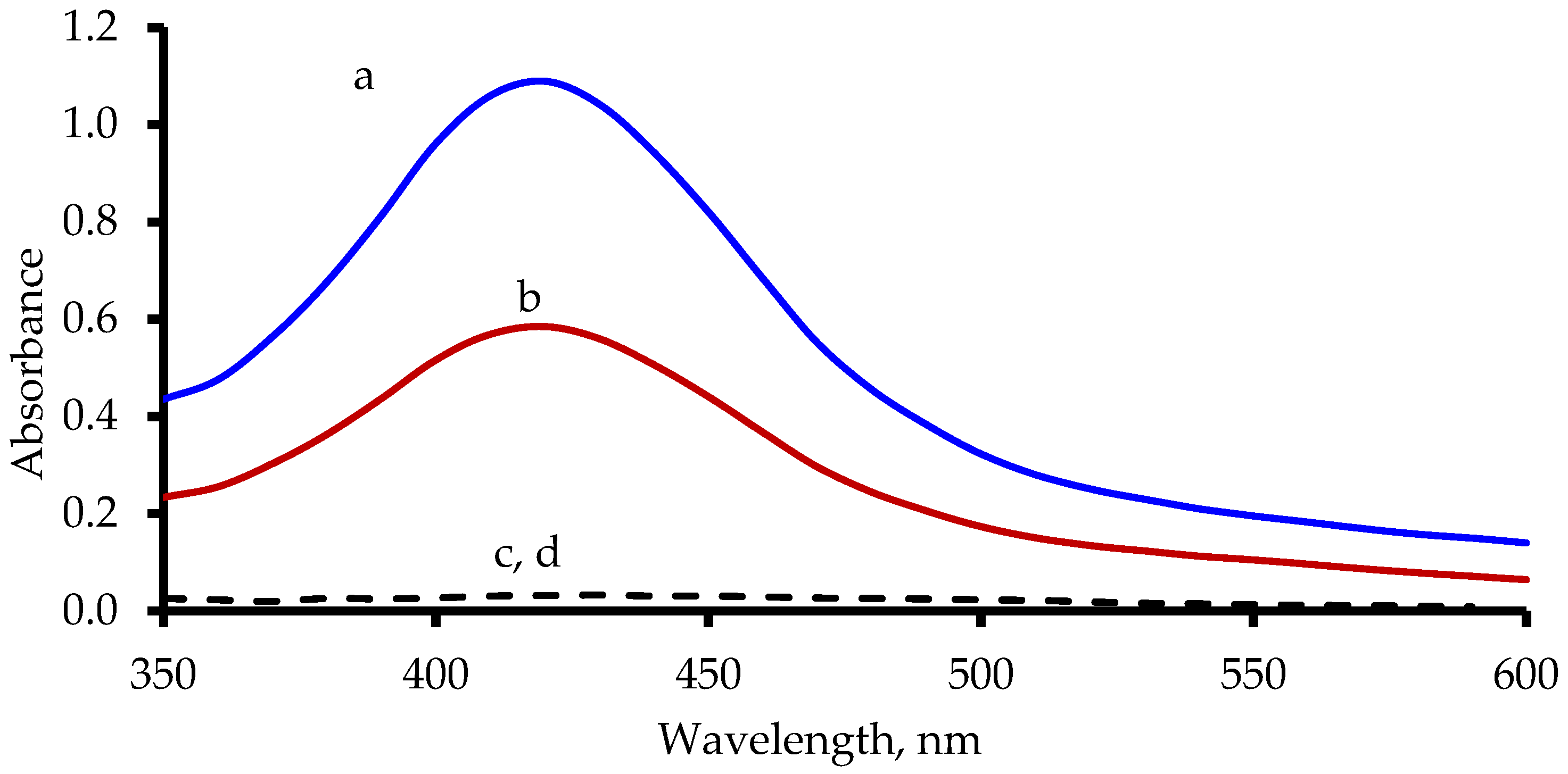
3.1. Characterization, Peroxidase Activity, and UV-Visible Spectrum of PVP-AgNPs
3.2. Sensing Mechanism and Factors Affecting the Colorimetric Detection of Hg2+
3.3. Selectivity of PVP-AgNPs/OPD/H2O2 System for Hg2+ Detection
3.4. Analytical Parameters for the Detection of Hg2+ in Different Matrixes
4. Evaluation of the Greenness Property
5. Comparison between the Performance of the Proposed Protocol and Reported Literature for Removal of Hg2+ from Waste Samples
5.1. Metals Nanoparticles Sensing Tools
5.1.1. AuNP-Based Colorimetric Assays
5.1.2. AgNP-Based Colorimetric Assays
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umapathi, R.; Venkateswara Raju, C.; Majid Ghoreishian, S.; Mohana Rani, G.; Kumar, K.; Oh, M.-H.; Pil Park, J.; Suk Huh, Y. Recent advances in the use of graphitic carbon nitride-based composites for the electrochemical detection of hazardous contaminants. Coord. Chem. Rev. 2022, 470, 214708. [Google Scholar] [CrossRef]
- Prosposito, P.; Burratti, L.; Venditti, I. Silver nanoparticles as colorimetric sensors for water pollutants. Chemosensors 2020, 8, 26. [Google Scholar] [CrossRef]
- Yu, L.; Li, N. Noble metal nanoparticles-based colorimetric biosensor for visual quantification: A mini review. Chemosensors 2019, 7, 53. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Vilian, A.E.; Ghoreishian, S.M.; Umapathi, R.; Hwang, S.-K.; Oh, C.W.; Huh, Y.S.; Han, Y.-K. Hybridized 1D–2D MnMoO4–MXene nanocomposites as high-performing electrochemical sensing platform for the sensitive detection of dihydroxybenzene isomers in wastewater samples. J. Hazard. Mater. 2022, 421, 126775. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, N.; Mehra, R.; Kumar, H.; Singh, V.P. Progress and challenges in the detection of residual pesticides using nanotechnology based colorimetric techniques. Trends Environ. Anal. Chem. 2020, 26, e00086. [Google Scholar] [CrossRef]
- Yan, Y.; Shin, W.I.; Chen, H.; Lee, S.-M.; Manickam, S.; Hanson, S.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. A Recent Trend: Application of Graphene in Catalysis. Carbon Lett. 2021, 31, 177–199. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Ranjith, K.S.; Lee, S.J.; Hwang, S.-K.; Umapathi, R.; Oh, C.W.; Huh, Y.S.; Han, Y.-K. Controllable synthesis of bottlebrush-like ZnO nanowires decorated on carbon nanofibers as an efficient electrocatalyst for the highly sensitive detection of silymarin in biological samples. Sens. Actuators B Chem. 2020, 321, 128544. [Google Scholar] [CrossRef]
- Rodelo, C.G.; Salinas, R.A.; JaimeArmenta, E.A.; Armenta, S.; Galdámez-Martínez, A.; Castillo-Blum, S.E.; Astudillo-de la Vega, H.; Grace, A.N.; Aguilar-Salinas, C.A.; Rodelo, J.G. Zinc associated nanomaterials and their intervention in emerging respiratory viruses: Journey to the field of biomedicine and biomaterials. Coord. Chem. Rev. 2022, 457, 214402. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alhathloul, S.S.; Aljohani, A.S.M.; Maswadeh, H.; Abdallah, E.M.; Hamid Musa, K.; El Hamd, M.A. Green Synthesis of Silver Nanoparticles Incorporated Aromatherapies Utilized for Their Antioxidant and Antimicrobial Activities against Some Clinical Bacterial Isolates. Bioinorg. Chem. Appl. 2022, 2022, 2432758. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Vo, D.-V.N. Critical review on hazardous pollutants in water environment: Occurrence, monitoring, fate, removal technologies and risk assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef]
- Giri, D.D.; Alhazmi, A.; Mohammad, A.; Haque, S.; Srivastava, N.; Thakur, V.K.; Gupta, V.K.; Pal, D.B. Lead removal from synthetic wastewater by biosorbents prepared from seeds of Artocarpus Heterophyllus and Syzygium Cumini. Chemosphere 2022, 287, 132016. [Google Scholar] [CrossRef] [PubMed]
- Chawla, P.; Kaushik, R.; Swaraj, V.J.S.; Kumar, N. Organophosphorus pesticides residues in food and their colorimetric detection. Environ. Nanotechnol. Monit. Manag. 2018, 10, 292–307. [Google Scholar] [CrossRef]
- Parra-Arroyo, L.; González-González, R.B.; Castillo-Zacarías, C.; Martínez, E.M.M.; Sosa-Hernández, J.E.; Bilal, M.; Iqbal, H.M.; Barceló, D.; Parra-Saldívar, R. Highly hazardous pesticides and related pollutants: Toxicological, regulatory, and analytical aspects. Sci. Total Environ. 2022, 807, 151879. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Jiang, L.; Shao, Q.; Liu, X.; Marks, R.S.; Ma, J.; Chen, X. Colorimetric detection of mercury ions based on plasmonic nanoparticles. Small 2013, 9, 1467–1481. [Google Scholar] [CrossRef]
- Miller, J.R.; Rowland, J.; Lechler, P.J.; Desilets, M.; Hsu, L.-C. Dispersal of mercury-contaminated sediments by geomorphic processes, Sixmile Canyon, Nevada, USA: Implications to site characterization and remediation of fluvial environments. Water Air Soil Pollut. 1996, 86, 373–388. [Google Scholar] [CrossRef]
- Moreno, F.N.; Anderson, C.W.N.; Stewart, R.B.; Robinson, B.H. Mercury volatilisation and phytoextraction from base-metal mine tailings. Environ. Pollut. 2005, 136, 341–352. [Google Scholar] [CrossRef]
- Renzoni, A.; Zino, F.; Franchi, E. Mercury levels along the food chain and risk for exposed populations. Environ. Res. 1998, 77, 68–72. [Google Scholar] [CrossRef]
- Wolfe, M.F.; Schwarzbach, S.; Sulaiman, R.A. Effects of mercury on wildlife: A comprehensive review. Environ. Toxicol. Chem. Int. J. 1998, 17, 146–160. [Google Scholar] [CrossRef]
- Amin-Zaki, L.; Elhassani, S.; Majeed, M.A.; Clarkson, T.W.; Doherty, R.A.; Greenwood, M. Intra-uterine methylmercury poisoning in Iraq. In Problems of Birth Defects; Springer: Berlin/Heidelberg, Germany, 1974; pp. 233–241. [Google Scholar]
- Weiss, B. Why methylmercury remains a conundrum 50 years after Minamata. Toxicol. Sci. 2007, 97, 223–225. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, X. Speciation analysis of mercury in water samples using dispersive liquid–liquid microextraction combined with high-performance liquid chromatography. Anal. Chim. Acta 2011, 702, 50–55. [Google Scholar] [CrossRef]
- Ma, S.; He, M.; Chen, B.; Deng, W.; Zheng, Q.; Hu, B. Magnetic solid phase extraction coupled with inductively coupled plasma mass spectrometry for the speciation of mercury in environmental water and human hair samples. Talanta 2016, 146, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.A.; dos Santos, L.O. A new method for preconcentration and determination of mercury in fish, shellfish and saliva by cold vapour atomic absorption spectrometry. Food Chem. 2014, 149, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Serafimovski, I.; Karadjova, I.; Stafilov, T.; Cvetković, J. Determination of inorganic and methylmercury in fish by cold vapor atomic absorption spectrometry and inductively coupled plasma atomic emission spectrometry. Microchem. J. 2008, 89, 42–47. [Google Scholar] [CrossRef]
- Badr, Z.; Abdel-Lateef, M.A.; Gomaa, H.; Abdelmottaleb, M.; Taher, M. Spectrofluorimetric determination of magnesium ions in water, ampoule, and suspension samples using a fluorescent azothiazol-benzenesulfonamide derivative. Luminescence 2022, 37, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Reddy, G. Engineering. Spectrophotometric Detection of Mercury Using Lignosulphonic Stabilized Silver Nanoparticles (AgNP). Iran. J. Mater. Sci. Eng. 2020, 17, 80–84. [Google Scholar]
- Sheoran, K.; Kaur, H.; Siwal, S.S.; Saini, A.K.; Vo, D.-V.N.; Thakur, V.K. Recent advances of carbon-based nanomaterials (CBNMs) for wastewater treatment: Synthesis and application. Chemosphere 2022, 299, 134364. [Google Scholar] [CrossRef]
- Rana, A.K.; Mishra, Y.K.; Gupta, V.K.; Thakur, V.K. Sustainable materials in the removal of pesticides from contaminated water: Perspective on macro to nanoscale cellulose. Sci. Total Environ. 2021, 797, 149129. [Google Scholar] [CrossRef]
- Li, F.; Ni, B.; Zheng, Y.; Huang, Y.; Li, G. A simple and efficient voltammetric sensor for dopamine determination based on ZnO nanorods/electro-reduced graphene oxide composite. Surf. Interfaces 2021, 26, 101375. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Xu, L.; Wan, X.; Liu, Y.; Chen, Y.; Li, Q. Ultrasensitive, label-free voltammetric determination of norfloxacin based on molecularly imprinted polymers and Au nanoparticle-functionalized black phosphorus nanosheet nanocomposite. J. Hazard. Mater. 2022, 436, 129107. [Google Scholar] [CrossRef]
- Gouda, A.A.; Alshehri, A.M.; El Sheikh, R.; Hassan, W.S.; Ibrahim, S.H. Development of green vortex-assisted supramolecular solvent-based liquid–liquid microextraction for preconcentration of mercury in environmental and biological samples prior to spectrophotometric determination. Microchem. J. 2020, 157, 105108. [Google Scholar] [CrossRef]
- Zezzi-Arruda, M.A.; Poppi, R.J. SPECTROPHOTOMETRY|Inorganic Compounds. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 351–358. [Google Scholar]
- Zhou, Y.; Ma, Z. Fluorescent and colorimetric dual detection of mercury (II) by H2O2 oxidation of o-phenylenediamine using Pt nanoparticles as the catalyst. Sens. Actuators B Chem. 2017, 249, 53–58. [Google Scholar] [CrossRef]
- Al-Onazi, W.A.; Abdel-Lateef, M.A. Catalytic oxidation of O-phenylenediamine by silver nanoparticles for resonance Rayleigh scattering detection of mercury (II) in water samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120258. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Qian, H.-L.; Zhao, X.; Yang, C.-X.; Yan, X.-P. Fabrication of a covalent organic framework and its gold nanoparticle hybrids as stable mimetic peroxidase for sensitive and selective colorimetric detection of mercury in water samples. Talanta 2019, 204, 224–228. [Google Scholar] [CrossRef]
- Borthakur, P.; Boruah, P.K.; Das, M.R. CuS and NiS Nanoparticle-Decorated Porous-Reduced Graphene Oxide Sheets as Efficient Peroxidase Nanozymes for Easy Colorimetric Detection of Hg (II) Ions in a Water Medium and Using a Paper Strip. ACS Sust. Chem. Eng. 2021, 9, 13245–13255. [Google Scholar] [CrossRef]
- Hasan, A.; Nanakali, N.M.Q.; Salihi, A.; Rasti, B.; Sharifi, M.; Attar, F.; Derakhshankhah, H.; Mustafa, I.A.; Abdulqadir, S.Z.; Falahati, M. Nanozyme-based sensing platforms for detection of toxic mercury ions: An alternative approach to conventional methods. Talanta 2020, 215, 120939. [Google Scholar] [CrossRef]
- Abdel-Lateef, M.A. Utilization of the peroxidase-like activity of silver nanoparticles nanozyme on O-phenylenediamine/H2O2 system for fluorescence detection of mercury (II) ions. Sci. Rep. 2022, 12, 6953. [Google Scholar] [CrossRef]
- Ragg, R.; Tahir, M.N.; Tremel, W. Solids go bio: Inorganic nanoparticles as enzyme mimics. Eur. J. Inorg. Chem. 2016, 2016, 1906–1915. [Google Scholar] [CrossRef]
- Breslow, R.; Overman, L.E. “Artificial enzyme” combining a metal catalytic group and a hydrophobic binding cavity. J. Am. Chem. Soc. 1970, 92, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.N.; Anderson, S.R.; Singh, S.; Ramanathan, R.; Bansal, V. Nanostructured silver fabric as a free-standing NanoZyme for colorimetric detection of glucose in urine. Biosens. Bioelectron. 2018, 110, 8–15. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Wei, H. Multifunctional nanozymes: Enzyme-like catalytic activity combined with magnetism and surface plasmon resonance. Nanoscale Horiz. 2018, 3, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2016, 3, 41–60. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Yan, X.; Fan, K. Structure and activity of nanozymes: Inspirations for de novo design of nanozymes. Mater. Today 2020, 41, 81–119. [Google Scholar] [CrossRef]
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.G.; Malinowska, E. Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8505–8513. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, Z.; Cao, H.; Huang, Y. Peroxidase-like activity of chitosan stabilized silver nanoparticles for visual and colorimetric detection of glucose. Analyst 2012, 137, 5560–5564. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, J.; Li, Z.; Luo, J.; Wang, J.; Sun, Y. Chitosan–gold nanoparticles as peroxidase mimic and their application in glucose detection in serum. RSC Adv. 2017, 7, 44463–44469. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-Dependent Toxicity of Silver Nanoparticles to Bacteria, Yeast, Algae, Crustaceans and Mammalian Cells In Vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef]
- Xu, R.; Wang, D.; Zhang, J.; Li, Y. Shape-dependent catalytic activity of silver nanoparticles for the oxidation of styrene. Chem. Asian J. 2006, 1, 888–893. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Yazgan, I.; Akgul, A.; Eshun, G.B.; Sakhaee, L.; Sadik, O.A. Size and shape-dependent antimicrobial activities of silver and gold nanoparticles: A model study as potential fungicides. Molecules 2020, 25, 2682. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Kovács, D.; Igaz, N.; Vágvölgyi, C.; Boros, I.M.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: A comprehensive study. Int. J. Nanomed. 2017, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Igaz, N.; Gopisetty, M.K.; Szerencsés, B.; Kovács, D.; Papp, C.; Vágvölgyi, C.; Boros, I.M.; Kónya, Z.; Kiricsi, M. Biosynthesized silver and gold nanoparticles are potent antimycotics against opportunistic pathogenic yeasts and dermatophytes. Int. J. Nanomed. 2018, 13, 695. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Bélteky, P.; Boka, E.; Zakupszky, D.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Kónya, Z.; Kiricsi, M. Polyvinyl-pyrrolidone-coated silver nanoparticles-the colloidal, chemical, and biological consequences of steric stabilization under biorelevant conditions. Int. J. Mol. Sci. 2021, 22, 8673. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S.; Jeon, H.-J.; Ahn, C.W. Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Prathna, T.C.; Chandrasekaran, N.; Mukherjee, A. Studies on aggregation behaviour of silver nanoparticles in aqueous matrices: Effect of surface functionalization and matrix composition. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 216–224. [Google Scholar] [CrossRef]
- Tejamaya, M.; Romer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Scheckel, K.G.; Suidan, M.; Tolaymat, T. The impact of stabilization mechanism on the aggregation kinetics of silver nanoparticles. Sci. Total Environ. 2012, 429, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Jini, D.; Sharmila, S. Green synthesis of silver nanoparticles from Allium cepa and its in vitro antidiabetic activity. Mater. Today: Proc. 2020, 22, 432–438. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Biosynthetic conversion of Ag+ to highly stable Ag0 nanoparticles by wild type and cell wall deficient strains of Chlamydomonas reinhardtii. Molecules 2019, 24, 98. [Google Scholar] [CrossRef]
- Jiang, B.; Duan, D.; Gao, L.; Zhou, M.; Fan, K.; Tang, Y.; Xi, J.; Bi, Y.; Tong, Z.; Gao, G.F. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat. Protoc. 2018, 13, 1506–1520. [Google Scholar] [CrossRef]
- Yang, W.; Yang, X.; Zhu, L.; Chu, H.; Li, X.; Xu, W. Nanozymes: Activity origin, catalytic mechanism, and biological application. Coord. Chem. Rev. 2021, 448, 214170. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size-and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef]
- Panacek, A.; Prucek, R.; Hrbac, J.; Nevečná, T.J.; Steffkova, J.; Zboril, R.; Kvítek, L. Polyacrylate-assisted size control of silver nanoparticles and their catalytic activity. Chem. Mater. 2014, 26, 1332–1339. [Google Scholar] [CrossRef]
- Lian, J.; Yin, D.; Zhao, S.; Zhu, X.; Liu, Q.; Zhang, X.; Zhang, X. Core-shell structured Ag-CoO nanoparticles with superior peroxidase-like activity for colorimetric sensing hydrogen peroxide and o-phenylenediamine. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125283. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Xu, X.; Ye, K.; Wang, L.; Zhu, H.; Wang, M.; Niu, X. Integrating peroxidase-mimicking activity with photoluminescence into one framework structure for high-performance ratiometric fluorescent pesticide sensing. Sens. Actuators B Chem. 2021, 328, 129024. [Google Scholar] [CrossRef]
- Fornera, S.; Walde, P. Spectrophotometric quantification of horseradish peroxidase with o-phenylenediamine. Anal. Biochem. 2010, 407, 293–295. [Google Scholar] [CrossRef]
- Farhadi, K.; Forough, M.; Molaei, R.; Hajizadeh, S.; Rafipour, A. Highly selective Hg2+ colorimetric sensor using green synthesized and unmodified silver nanoparticles. Sens. Actuators B Chem. 2012, 161, 880–885. [Google Scholar] [CrossRef]
- Jarujamrus, P.; Amatatongchai, M.; Thima, A.; Khongrangdee, T.; Mongkontong, C. Selective colorimetric sensors based on the monitoring of an unmodified silver nanoparticles (AgNPs) reduction for a simple and rapid determination of mercury. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 142, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Nidya, M.; Umadevi, M.; Rajkumar, B.J. Structural, morphological and optical studies of L-cysteine modified silver nanoparticles and its application as a probe for the selective colorimetric detection of Hg2+. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 133, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateef, M.A.; Almahri, A. Micellar sensitized Resonance Rayleigh Scattering and spectrofluorometric methods based on isoindole formation for determination of Eflornithine in cream and biological samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 258, 119806. [Google Scholar] [CrossRef]
- Abdel-Lateef, M.A.; Alzahrani, E.; Pashameah, R.A.; Almahri, A.; Abu-hassan, A.A.; El Hamd, M.A.; Mohammad, B.S. A specific turn-on fluorescence probe for determination of nitazoxanide based on feasible oxidation reaction with hypochlorite: Applying cobalt ferrite nanoparticles for pre-concentration and extraction of its metabolite from real urine samples. J. Pharm. Biomed. Anal. 2022, 219, 114941. [Google Scholar] [CrossRef]
- Ghonim, R.; El-Awady, M.I.; Tolba, M.M.; Ibrahim, F. Green quantitative spectrofluorometric analysis of rupatadine and montelukast at nanogram scale using direct and synchronous techniques. R. Soc. Open Sci. 2021, 8, 211196. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; Al-zahrani, M.A.; El Hamd, M.A.; El-Maghrabey, M.; Dahas, F.A.; El-Shaheny, R. High-temperature liquid chromatography for evaluation of the efficiency of multiwalled carbon nanotubes as nano extraction beds for removal of acidic drugs from wastewater. Greenness profiling and comprehensive kinetics and thermodynamics studies. J. Chromatogr. A 2021, 1639, 461891. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Duan, J.; Zhan, J. Recent developments on nanomaterials-based optical sensors for Hg2+ detection. Sci. China Mater. 2015, 58, 223–240. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, M. Nanomaterials in pollution trace detection and environmental improvement. Nano Today 2010, 5, 128–142. [Google Scholar] [CrossRef]
- Guo, S.; Wang, E. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264. [Google Scholar] [CrossRef]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef]
- Grasseschi, D.; Zamarion, V.M.; Araki, K.; Toma, H.E. Surface enhanced Raman scattering spot tests: A new insight on Feigl’s analysis using gold nanoparticles. Anal. Chem. 2010, 82, 9146–9149. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Chiang, C.-K.; Lin, Z.-H.; Lee, K.-H.; Chang, H.-T. Bioconjugated gold nanodots and nanoparticles for protein assays based on photoluminescence quenching. Anal. Chem. 2008, 80, 1497–1504. [Google Scholar] [CrossRef]
- Du, J.; Sun, Y.; Jiang, L.; Cao, X.; Qi, D.; Yin, S.; Ma, J.; Boey, F.Y.C.; Chen, X. Flexible colorimetric detection of mercuric ion by simply mixing nanoparticles and oligopeptides. Small 2011, 7, 1407–1411. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.; Xu, H.; Zhang, Z.; Zhong, X. Highly selective and sensitive visualizable detection of Hg2+ based on anti-aggregation of gold nanoparticles. Talanta 2011, 84, 508–512. [Google Scholar] [CrossRef]
- Lou, T.; Chen, Z.; Wang, Y.; Chen, L. Blue-to-red colorimetric sensing strategy for Hg2+ and Ag+ via redox-regulated surface chemistry of gold nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 1568–1573. [Google Scholar] [CrossRef]
- Lee, J.S.; Han, M.S.; Mirkin, C.A. Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 4093–4096. [Google Scholar] [CrossRef]
- Xue, X.; Wang, F.; Liu, X. One-step, room temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J. Am. Chem. Soc. 2008, 130, 3244–3245. [Google Scholar] [CrossRef]
- Kim, Y.; Johnson, R.C.; Hupp, J.T. Gold nanoparticle-based sensing of “spectroscopically silent” heavy metal ions. Nano Lett. 2001, 1, 165–167. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chang, H.-T. Parameters for selective colorimetric sensing of mercury (II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem. Commun. 2007, 12, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-R.; Mahajan, R.K.; Kim, J.S.; Kim, H. Highly sensitive gold nanoparticle-based colorimetric sensing of mercury (II) through simple ligand exchange reaction in aqueous media. ACS Appl. Mater. Interfaces 2010, 2, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.-Q.; Liu, J.-F.; Liu, R.; Yin, Y.-G.; Jiang, G.-B. Visual and colorimetric detection of Hg2+ by cloud point extraction with functionalized gold nanoparticles as a probe. Chem. Commun. 2009, 45, 7030–7032. [Google Scholar] [CrossRef]
- Chen, L.; Lou, T.; Yu, C.; Kang, Q.; Chen, L. N-1-(2-mercaptoethyl) thymine modification of gold nanoparticles: A highly selective and sensitive colorimetric chemosensor for Hg2+. Analyst 2011, 136, 4770–4773. [Google Scholar] [CrossRef]
- Liu, C.-W.; Hsieh, Y.-T.; Huang, C.-C.; Lin, Z.-H.; Chang, H.-T. Detection of mercury (II) based on Hg2+–DNA complexes inducing the aggregation of gold nanoparticles. Chem. Commun. 2008, 19, 2242–2244. [Google Scholar] [CrossRef]
- Li, D.; Wieckowska, A.; Willner, I. Optical analysis of Hg2+ ions by oligonucleotide–gold-nanoparticle hybrids and DNA-based machines. Angew. Chem. 2008, 120, 3991–3995. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Jiao, K.; Yang, X. Colorimetric detection of mercury ion (Hg2+) based on DNA oligonucleotides and unmodified gold nanoparticles sensing system with a tunable detection range. Biosens. Bioelectron. 2009, 24, 3153–3158. [Google Scholar] [CrossRef]
- Liu, D.; Qu, W.; Chen, W.; Zhang, W.; Wang, Z.; Jiang, X. Highly sensitive, colorimetric detection of mercury (II) in aqueous media by quaternary ammonium group-capped gold nanoparticles at room temperature. Anal. Chem. 2010, 82, 9606–9610. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Yu, C.-J.; Lin, Y.-H.; Tseng, W.-L. Colorimetric sensing of silver (I) and mercury (II) ions based on an assembly of tween 20-stabilized gold nanoparticles. Anal. Chem. 2010, 82, 6830–6837. [Google Scholar] [CrossRef]
- Chen, X.; Zu, Y.; Xie, H.; Kemas, A.M.; Gao, Z. Coordination of mercury (II) to gold nanoparticle associated nitrotriazole towards sensitive colorimetric detection of mercuric ion with a tunable dynamic range. Analyst 2011, 136, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Deng, L.; Wang, H.; Ouyang, X.; Zheng, J.; Li, J.; Yang, R. Metal-induced aggregation of mononucleotides-stabilized gold nanoparticles: An efficient approach for simple and rapid colorimetric detection of Hg (II). Chem. Commun. 2011, 47, 6039–6041. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-L.; Hsiung, T.-M.; Chen, Y.-Y.; Huang, Y.-F.; Huang, C.-C. Colorimetric detection of heavy metal ions using label-free gold nanoparticles and alkanethiols. J. Phys. Chem. C 2010, 114, 16329–16334. [Google Scholar] [CrossRef]
- Yang, X.; Liu, H.; Xu, J.; Tang, X.; Huang, H.; Tian, D. A simple and cost-effective sensing strategy of mercury (II) based on analyte-inhibited aggregation of gold nanoparticles. Nanotechnology 2011, 22, 275503. [Google Scholar] [CrossRef]
- Lou, T.; Chen, L.; Zhang, C.; Kang, Q.; You, H.; Shen, D.; Chen, L. A simple and sensitive colorimetric method for detection of mercury ions based on anti-aggregation of gold nanoparticles. Anal. Methods 2012, 4, 488–491. [Google Scholar] [CrossRef]
- Ding, N.; Zhao, H.; Peng, W.; He, Y.; Zhou, Y.; Yuan, L.; Zhang, Y. A simple colorimetric sensor based on anti-aggregation of gold nanoparticles for Hg2+ detection. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 161–167. [Google Scholar] [CrossRef]
- You, J.; Hu, H.; Zhou, J.; Zhang, L.; Zhang, Y.; Kondo, T. Novel cellulose polyampholyte–gold nanoparticle-based colorimetric competition assay for the detection of cysteine and mercury (II). Langmuir 2013, 29, 5085–5092. [Google Scholar] [CrossRef]
- Henglein, A.; Brancewicz, C. Absorption spectra and reactions of colloidal bimetallic nanoparticles containing mercury. Chem. Mater. 1997, 9, 2164–2167. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Z.; Zhan, J. Synthesis of starch-stabilized Ag nanoparticles and Hg2+ recognition in aqueous media. Nanoscale Res. Lett. 2009, 4, 1230–1235. [Google Scholar] [CrossRef]
- Bera, R.K.; Das, A.K.; Raj, C.R. Enzyme-cofactor-assisted photochemical synthesis of Ag nanostructures and shape-dependent optical sensing of Hg (II) ions. Chem. Mater. 2010, 22, 4505–4511. [Google Scholar] [CrossRef]
- Ramesh, G.V.; Radhakrishnan, T.P. A universal sensor for mercury (Hg, HgI, HgII) based on silver nanoparticle-embedded polymer thin film. ACS Appl. Mater. Interfaces 2011, 3, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, F.; Yang, X. Colorimetric detection of mercury (II) ion using unmodified silver nanoparticles and mercury-specific oligonucleotides. ACS Appl. Mater. Interfaces 2010, 2, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-L.; Zhu, X.-Y.; Jiao, H.-J.; Dong, Y.-M.; Li, Z.-J. Ultrasensitive and dual functional colorimetric sensors for mercury (II) ions and hydrogen peroxide based on catalytic reduction property of silver nanoparticles. Biosens. Bioelectron. 2012, 31, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yin, H.; Wei, R.; Wang, W. Facile colorimetric detection of Hg2+ based on anti-aggregation of silver nanoparticles. Biosens. Bioelectron. 2014, 57, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Lytton-Jean, A.K.R.; Hurst, S.J.; Mirkin, C.A. Silver nanoparticle− oligonucleotide conjugates based on DNA with triple cyclic disulfide moieties. Nano Lett. 2007, 7, 2112–2115. [Google Scholar] [CrossRef] [Green Version]





| Parameter | Ultra-Pure Water | Bottled Water | River Water |
|---|---|---|---|
| The Linear range (nM) | 0.090–0.10 | 0.10–0.80 | 0.150–8.0 |
| The standard error (SE) | 0.0095 | 0.0105 | 0.008 |
| The Intercept | 0.0516 | 0.0085 | 0.059 |
| The SE of intercept | 0.0073 | 0.0089 | 0.0073 |
| The slope | 7.6 × 10−4 | 8.8 × 10−4 | 5.9 × 10−4 |
| The SE of the slope | 1.2 × 10−5 | 1.8 × 10−5 | 1.3 × 10−5 |
| R2 | 0.9989 | 0.998 | 0.9983 |
| The LOQ (nM) | 31.90 | 33.40 | 40.90 |
| The LOD (nM) | 96.80 | 101.20 | 124.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Lateef, M.A.; Almahri, A.; Alzahrani, E.; Pashameah, R.A.; Abu-Hassan, A.A.; El Hamd, M.A. Sustainable PVP-Capped Silver Nanoparticles as a Free-Standing Nanozyme Sensor for Visual and Spectrophotometric Detection of Hg2+ in Water Samples: A Green Analytical Method. Chemosensors 2022, 10, 358. https://doi.org/10.3390/chemosensors10090358
Abdel-Lateef MA, Almahri A, Alzahrani E, Pashameah RA, Abu-Hassan AA, El Hamd MA. Sustainable PVP-Capped Silver Nanoparticles as a Free-Standing Nanozyme Sensor for Visual and Spectrophotometric Detection of Hg2+ in Water Samples: A Green Analytical Method. Chemosensors. 2022; 10(9):358. https://doi.org/10.3390/chemosensors10090358
Chicago/Turabian StyleAbdel-Lateef, Mohamed A., Albandary Almahri, Eman Alzahrani, Rami Adel Pashameah, Ahmed A. Abu-Hassan, and Mohamed A. El Hamd. 2022. "Sustainable PVP-Capped Silver Nanoparticles as a Free-Standing Nanozyme Sensor for Visual and Spectrophotometric Detection of Hg2+ in Water Samples: A Green Analytical Method" Chemosensors 10, no. 9: 358. https://doi.org/10.3390/chemosensors10090358
APA StyleAbdel-Lateef, M. A., Almahri, A., Alzahrani, E., Pashameah, R. A., Abu-Hassan, A. A., & El Hamd, M. A. (2022). Sustainable PVP-Capped Silver Nanoparticles as a Free-Standing Nanozyme Sensor for Visual and Spectrophotometric Detection of Hg2+ in Water Samples: A Green Analytical Method. Chemosensors, 10(9), 358. https://doi.org/10.3390/chemosensors10090358






