Smartphone-Based Quantitative Analysis of Protein Array Signals for Biomarker Detection in Lupus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Antigens, Antibodies, and Reagents
2.1.2. CD14 Detection on an Antibody Array
2.1.3. Antigen Array
2.2. Instruments and Software
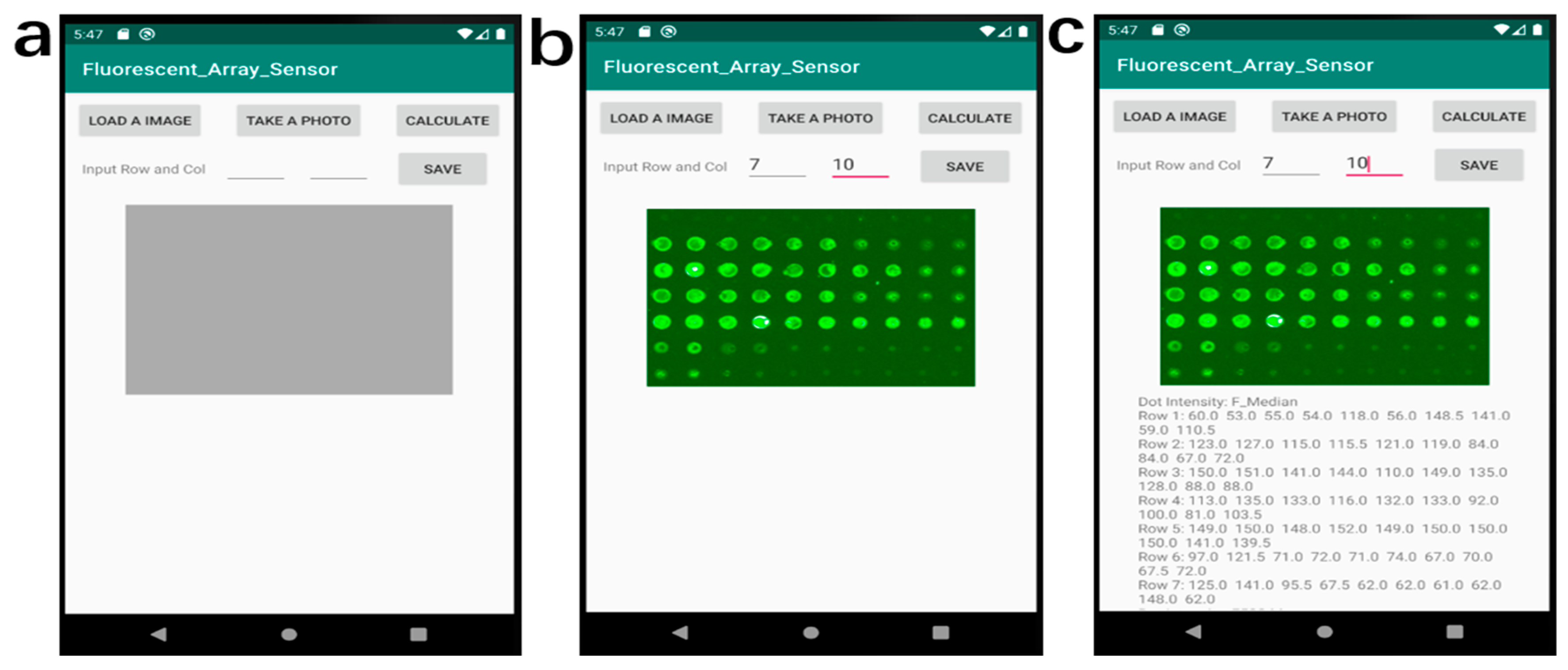
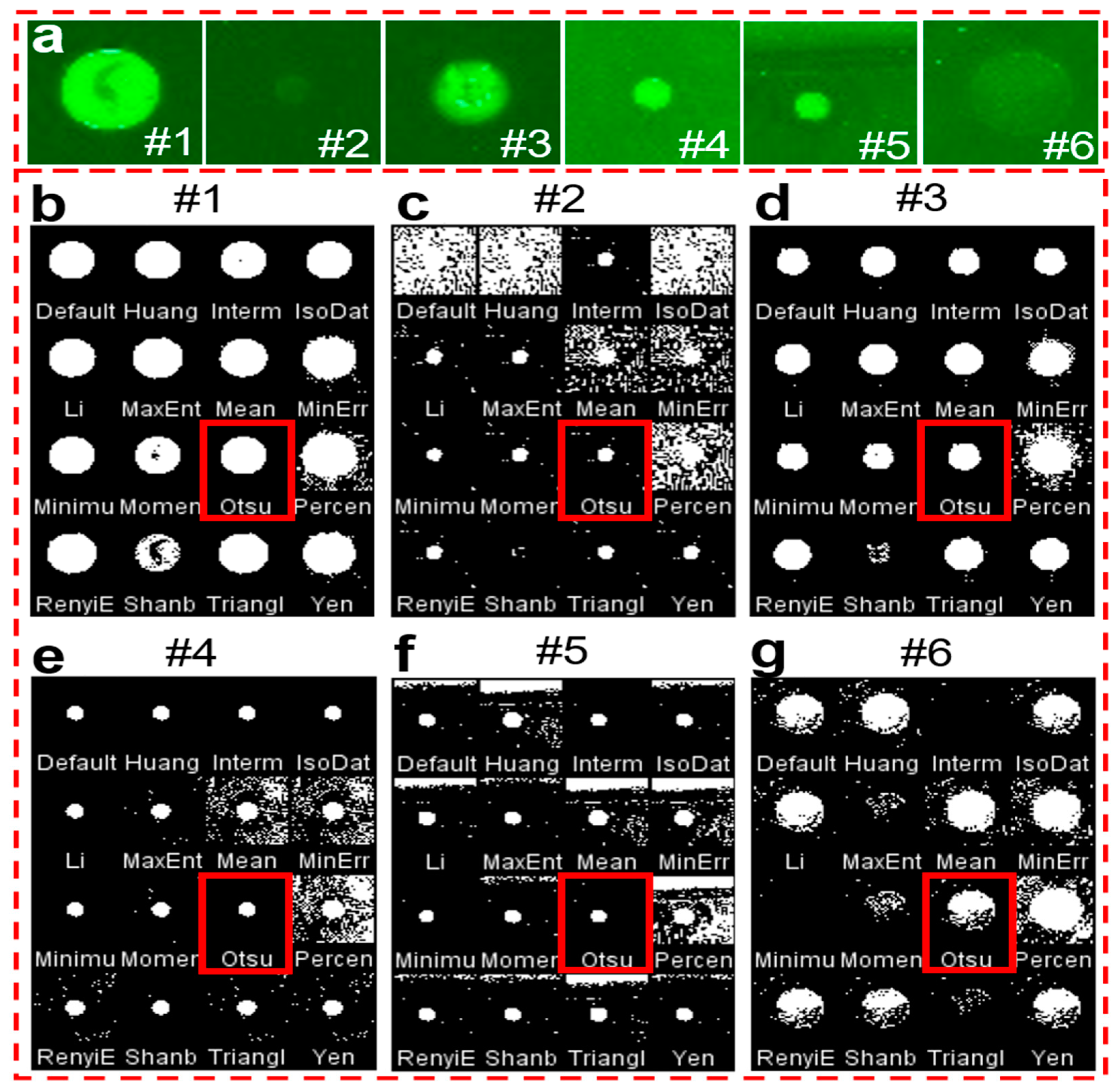
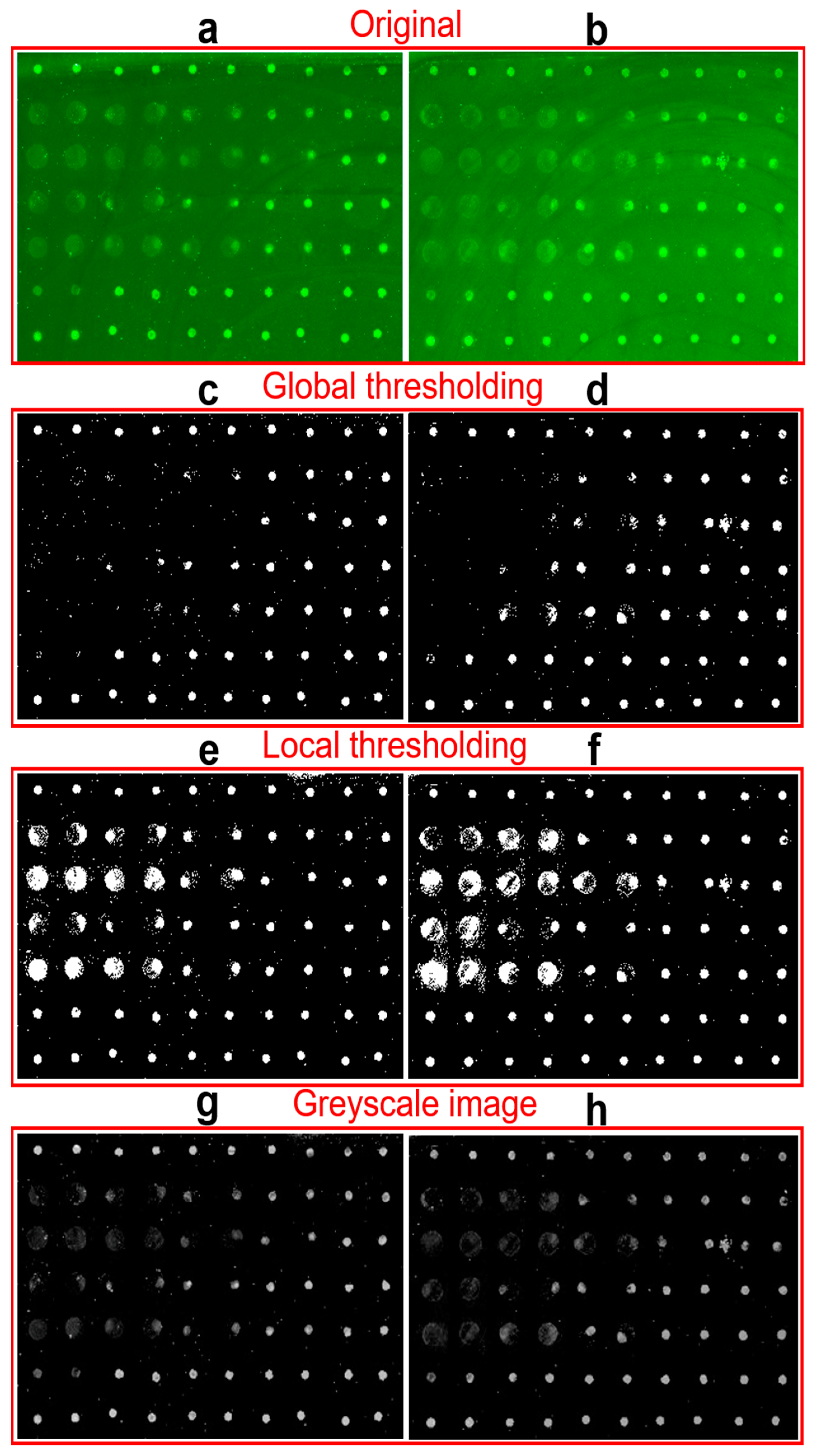
2.3. Image Analysis
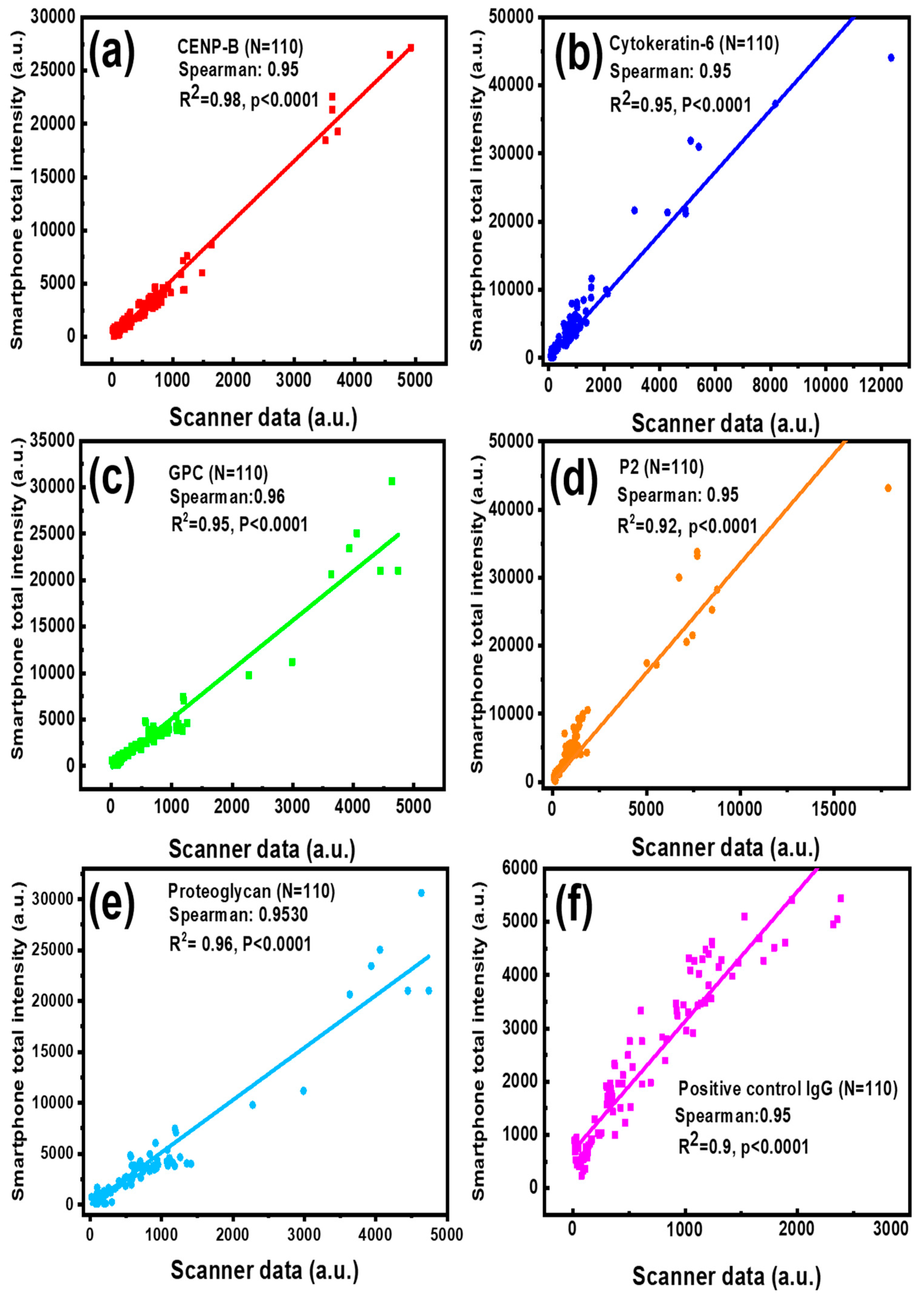
2.4. Statistical Analysis
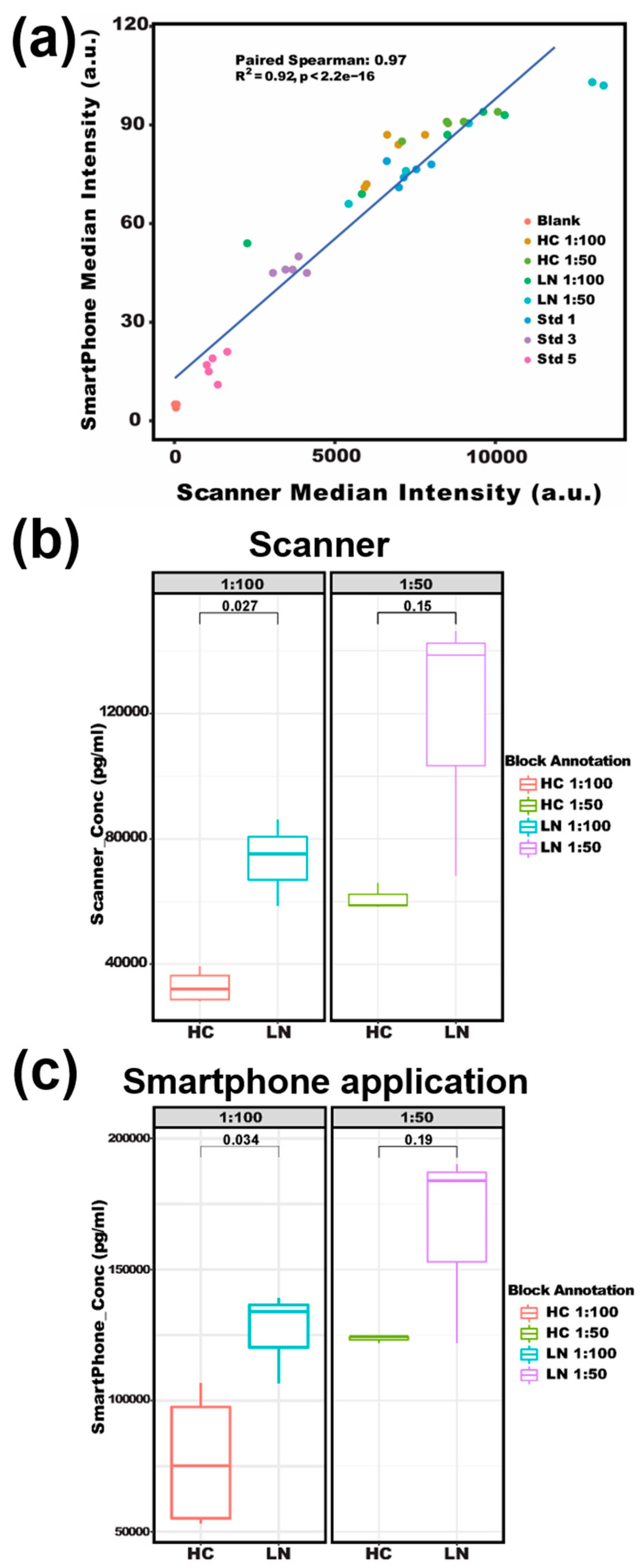
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alebiosu, C.; Ayodele, O. The global burden of chronic kidney disease and the way forward. Ethn. Dis. 2005, 15, 418–423. [Google Scholar]
- Zou, Y.; Liu, F.; Cooper, M.E.; Chai, Z. Advances in clinical research in chronic kidney disease. J. Transl. Med. 2021, 9, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Culcuoglu, M.U.; Wang, S.; Powers, C.; Hillman, M. Patient Centered Medical Home Transformation from Systems Engineering Perspective. In Proceedings of the IIE Annual Conference and Expo: 2012 Conference Proceedings, Orlando, FL, USA, 19–23 May 2012; Institute of Industrial Engineers: Peachtree Corners, GA, USA; p. 1. [Google Scholar]
- Tüdős, A.J.; Besselink, G.A.J.; Schasfoort, R.B.M. Trends in miniaturized total analysis systems for point-of-care testing in clinical chemistry. Lab A Chip 2001, 1, 83–95. [Google Scholar] [CrossRef]
- Newman, J.D.; Turner, A.P. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef] [PubMed]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.K.; Tokarski, C.; Lang, S.N.; van Ginkel, L.A.; Zhu, H.; Ozcan, A.; Nielen, M.W. Calling Biomarkers in Milk Using a Protein Microarray on Your Smartphone. PLoS ONE 2015, 10, e0134360. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; Cortazar, B.; Tseng, D.; Ozkan, H.; Feng, S.; Wei, Q.; Chan, R.Y.; Burbano, J.; Farooqui, Q.; Lewinski, M.; et al. Cellphone-Based Hand-Held Microplate Reader for Point-of-Care Testing of Enzyme-Linked Immunosorbent Assays. ACS Nano 2015, 9, 7857–7866. [Google Scholar] [CrossRef]
- Lu, M.Y.; Kao, W.C.; Belkin, S.; Cheng, J.Y. A Smartphone-Based Whole-Cell Array Sensor for Detection of Antibiotics in Milk. Sensors 2019, 19, 3882. [Google Scholar] [CrossRef]
- Hedde, P.N.; Abram, T.J.; Jain, A.; Nakajima, R.; Ramiro de Assis, R.; Pearce, T.; Jasinskas, A.; Toosky, M.N.; Khan, S.; Felgner, P.L.; et al. A modular microarray imaging system for highly specific COVID-19 antibody testing. Lab A Chip 2020, 20, 3302–3309. [Google Scholar] [CrossRef]
- Qiu, J.; Yuan, Y.; Li, Y.; Haley, C.; Mui, U.N.; Swali, R.; Mohan, C.; Tyring, S.K.; Wu, T. Discovery of IgG4 anti-gliadin autoantibody as a potential biomarker of psoriasis using an autoantigen array. Proteom. Clin. Appl. 2020, 14, 1800114. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Z.; Li, Y.; Xiao, M.; Tan, G.; Wang, W.; Wang, Y.; Fang, M.; Zhang, S.; Tang, C. An Aptamer-Array-Based Sample-to-Answer Biosensor for Ochratoxin A Detection via Fluorescence Resonance Energy Transfer. Chemosensors 2021, 9, 309. [Google Scholar] [CrossRef]
- Ludwig, S.K.J.; Zhu, H.; Phillips, S.; Shiledar, A.; Feng, S.; Tseng, D.; van Ginkel, L.A.; Nielen, M.W.F.; Ozcan, A. Cellphone-based detection platform for rbST biomarker analysis in milk extracts using a microsphere fluorescence immunoassay. Anal. Bioanal. Chem. 2014, 406, 6857–6866. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.; Poljak, M. Smartphones as mobile microbiological laboratories. Clin. Microbiol. Infect. 2020, 26, 421–424. [Google Scholar] [CrossRef]
- Coskun, A.F.; Wong, J.; Khodadadi, D.; Nagi, R.; Tey, A.; Ozcan, A. A personalized food allergen testing platform on a cellphone. Lab A Chip 2013, 13, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhao, Z.; Lin, Y.; Zhao, Y.; Liu, X.Y.; Lin, C.; Wu, C. A nanoneedle-based reactional wettability variation sensor array for on-site detection of metal ions with a smartphone. J. Colloid Interface Sci. 2019, 547, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Nagi, R.; Sadeghi, K.; Feng, S.; Yan, E.; Ki, S.J.; Caire, R.; Tseng, D.; Ozcan, A. Detection and spatial mapping of mercury contamination in water samples using a smart phone. ACS Nano 2014, 8, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. Transforming medicine via digital innovation. Sci. Transl. Med. 2010, 2, 16cm14. [Google Scholar] [CrossRef]
- Chen, H.; Li, Z.; Zhang, L.; Sawaya, P.; Shi, J.; Wang, P. Quantitation of Femtomolar-Level Protein Biomarkers Using a Simple Microbubbling Digital Assay and Bright-Field Smartphone Imaging. Angew Chem. Int. Ed. 2019, 58, 13922–13928. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Qi, H.; Luo, W.; Tseng, D.; Ki, S.J.; Wan, Z.; Göröcs, Z.N.; Bentolila, L.A.; Wu, T.-T.; Sun, R. Fluorescent imaging of single nanoparticles and viruses on a smart phone. ACS Nano 2013, 7, 9147–9155. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Sun, R.; Vasile, T.; Chang, Y.C.; Li, L. High-Throughput Optical Sensing Immunoassays on Smartphone. Anal. Chem. 2016, 88, 8302–8308. [Google Scholar] [CrossRef]
- Wang, L.J.; Chang, Y.C.; Sun, R.; Li, L. A multichannel smartphone optical biosensor for high-throughput point-of-care diagnostics. Biosens. Bioelectron. 2017, 87, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Balsam, J.; Bruck, H.A.; Rasooly, A. Capillary Array Waveguide Amplified Fluorescence Detector for mHealth. Sens. Actuators B Chem. 2013, 186, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.H.; Chen, P.H.; Lin, C.; Chen, C.F.; Lee, I.R.; Yeh, Y.C. Determination of Gold Ions in Human Urine Using Genetically Engineered Microorganisms on a Paper Device. ACS Sens. 2018, 3, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, S.; Yu, T.; Dai, Z.; Wei, Q. Aptamer-Based Fluorescent Sensor Array for Multiplexed Detection of Cyanotoxins on a Smartphone. Anal. Chem. 2019, 91, 10448–10457. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lin, Z.T.; Wang, H.; Hong, X.; Heon, M.; Wu, T. Protein arrays I: Antibody arrays. In Functional Genomics; Humana Press: New York, NY, USA, 2017; pp. 261–269. [Google Scholar]
- Yuan, Y.; Lin, Z.T.; Wang, H.; Hong, X.; Heon, M.; Wu, T. Protein Arrays II: Antibody Arrays. In Functional Genomics; Humana Press: New York, NY, USA, 2017; Volume 1654, pp. 271–277. [Google Scholar]
- Yuan, Y.; Lin, Z.T.; Wang, H.; Hong, X.; Heon, M.; Wu, T. Protein arrays III: Reverse-phase protein arrays. In Functional Genomics; Humana Press: New York, NY, USA, 2017; Volume 1654, pp. 279–289. [Google Scholar]
- Li, Y.; Wu, T. Proteomic approaches for novel systemic lupus erythematosus (SLE) drug discovery. Expert Opin. Drug Discov. 2018, 13, 765–777. [Google Scholar] [CrossRef]
- Yuan, Y.; Qiu, J.; Lin, Z.T.; Li, W.; Haley, C.; Mui, U.N.; Ning, J.; Tyring, S.K.; Wu, T. Identification of Novel Autoantibodies Associated With Psoriatic Arthritis. Arthritis Rheumatol. 2019, 71, 941–951. [Google Scholar] [CrossRef]
- Adobe Systems Inc, Corporate. Postscript Language Reference Manual, 2nd ed.; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 1990. [Google Scholar]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. Syst. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Zar, J.H. Significance testing of the Spearman rank correlation coefficient. J. Am. Stat. Assoc. 1972, 67, 578–580. [Google Scholar] [CrossRef]
- Patil, S.T.; Bangare, P.S.; Dubal, A.; Bangare, S.L. Reviewing Otsu’s Method For Image Thresholding. Int. J. Appl. Eng. Res. 2015, 10, 21777–21783. [Google Scholar] [CrossRef]
- Mandyartha, E.P.; Anggraeny, F.T.; Muttaqin, F.; Akbar, F.A. Global and Adaptive Thresholding Technique for White Blood Cell Image Segmentation. J. Phys. Conf. Ser. 2020, 1569, 022054. [Google Scholar] [CrossRef]
- Cheremkhin, P.A.; Kurbatova, E.A. Comparative appraisal of global and local thresholding methods for binarisation of off-axis digital holograms. Opt. Lasers Eng. 2019, 115, 119–130. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, H.; Yan, M.; Zuo, X.; Li, Q.Z. Autoantigen Microarray for High-throughput Autoantibody Profiling in Systemic Lupus Erythematosus. Genom. Proteom. Bioinform. 2015, 13, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R.; Qin, S.; Liu, B.C. The “reverse capture” autoantibody microarray: A native antigen-based platform for autoantibody profiling. Nat. Protoc 2006, 1, 452–460. [Google Scholar] [CrossRef]
- Suurmond, J.; Diamond, B. Autoantibodies in systemic autoimmune diseases: Specificity and pathogenicity. J. Clin. Investig. 2015, 125, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.; Melanthota, S.K.; Arbaaz Vaz, J.M.; Kadambalithaya, V.M.; Hussain, I.; Dutta, S.; Mazumder, N. Recent trends in smartphone-based detection for biomedical applications: A review. Anal. Bioanal. Chem. 2021, 413, 2389–2406. [Google Scholar] [CrossRef] [PubMed]
- Bange, A.; Halsall, H.B.; Heineman, W.R. Microfluidic immunosensor systems. Biosens. Bioelectron. 2005, 20, 2488–2503. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, C.; Xie, B.; Wang, S.; Yu, X.; Wen, K.; Lin, J.; Wang, J.; Wang, Z.; Shen, J. Ratiometric fluorescent sensing system for drug residue analysis: Highly sensitive immunosensor using dual-emission quantum dots hybrid and compact smartphone based-device. Anal. Chim. Acta 2020, 1102, 91–98. [Google Scholar] [CrossRef]
- Zeinhom, M.M.A.; Wang, Y.; Song, Y.; Zhu, M.-J.; Lin, Y.; Du, D. A portable smart-phone device for rapid and sensitive detection of E. coli O157: H7 in Yoghurt and Egg. Biosens. Bioelectron. 2018, 99, 479–485. [Google Scholar] [CrossRef]
- Kholafazad-Kordasht, H.; Hasanzadeh, M.; Seidi, F. Smartphone based immunosensors as next generation of healthcare tools: Technical and analytical overview towards improvement of personalized medicine. TrAC Trend. Anal. Chem. 2021, 145, 116455. [Google Scholar] [CrossRef]
- Attia, Z.R.; Zedan, M.M.; Saad, E.A.; Mutawi, T.M.; El Basuni, M.A. Association of CD14 genetic variants and circulating level with systemic lupus erythematosus risk in Egyptian children and adolescents. Biomark Med. 2021, 15, 1669–1680. [Google Scholar] [CrossRef]
- Egerer, K.; Feist, E.; Rohr, U.; Pruss, A.; Burmester, G.-R.; Dorner, T. Increased serum soluble CD14, ICAM-1 and E-selectin correlate with disease activity and prognosis in systemic lupus erythematosus. Lupus 2000, 9, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Nockher, W.A.; Wigand, R.; Schoeppe, W.; Scherberich, J.E. Elevated levels of soluble CD 14 in serum of patients with systemic lupus erythematosus. Clin. Exp. Immunol. 1994, 96, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Tripathy, R.; Das, B.K. CD14 (C-159T) polymorphism is associated with increased susceptibility to SLE, and plasma levels of soluble CD14 is a novel biomarker of disease activity: A hospital-based case-control study. Lupus 2020, 30, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Giannico, G.; Fogo, A.B. Lupus nephritis: Is the kidney biopsy currently necessary in the management of lupus nephritis? Clin. J. Am. Soc. Nephrol. 2013, 8, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Arriens, C.; Mohan, C. Systemic lupus erythematosus diagnostics in the “omics” era. Int. J. Clin. Rheumtol. 2013, 8, 671–687. [Google Scholar] [CrossRef]
- Tan, G.; Baby, B.; Zhou, Y.; Wu, T. Emerging Molecular Markers towards Potential Diagnostic Panels for Lupus. Front. Immunol. 2022, 12, 808839. [Google Scholar] [CrossRef]
- Wang, F.; Lu, Y.; Yang, J.; Chen, Y.; Jing, W.; He, L.; Liu, Y. A smartphone readable colorimetric sensing platform for rapid multiple protein detection. Analyst 2017, 142, 3177–3182. [Google Scholar] [CrossRef]
- Chellasamy, G.; Ankireddy, S.R.; Lee, K.-N.; Govindaraju, S.; Yun, K. Smartphone-integrated colorimetric sensor array-based reader system and fluorometric detection of dopamine in male and female geriatric plasma by bluish-green fluorescent carbon quantum dots. Mater. Today Bio 2021, 12, 100168. [Google Scholar] [CrossRef]
- Ra, M.; Muhammad, M.S.; Lim, C.; Han, S.; Jung, C.; Kim, W.-Y. Smartphone-Based Point-of-Care Urinalysis Under Variable Illumination. IEEE J. Transl. Eng. Health Med. 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Wen, J.; Zhu, Y.; Liu, J.; He, D. Smartphone-based surface plasmon resonance sensing platform for rapid detection of bacteria. RSC Adv. 2022, 12, 13045–13051. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Li, Y.; Tang, C.; Lin, F.; Wu, T.; Bao, J. Smartphone-Based Quantitative Analysis of Protein Array Signals for Biomarker Detection in Lupus. Chemosensors 2022, 10, 330. https://doi.org/10.3390/chemosensors10080330
Yang G, Li Y, Tang C, Lin F, Wu T, Bao J. Smartphone-Based Quantitative Analysis of Protein Array Signals for Biomarker Detection in Lupus. Chemosensors. 2022; 10(8):330. https://doi.org/10.3390/chemosensors10080330
Chicago/Turabian StyleYang, Guang, Yaxi Li, Chenling Tang, Feng Lin, Tianfu Wu, and Jiming Bao. 2022. "Smartphone-Based Quantitative Analysis of Protein Array Signals for Biomarker Detection in Lupus" Chemosensors 10, no. 8: 330. https://doi.org/10.3390/chemosensors10080330
APA StyleYang, G., Li, Y., Tang, C., Lin, F., Wu, T., & Bao, J. (2022). Smartphone-Based Quantitative Analysis of Protein Array Signals for Biomarker Detection in Lupus. Chemosensors, 10(8), 330. https://doi.org/10.3390/chemosensors10080330






