Detection of Polynitro Compounds at Low Concentrations by SERS Using Ni@Au Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
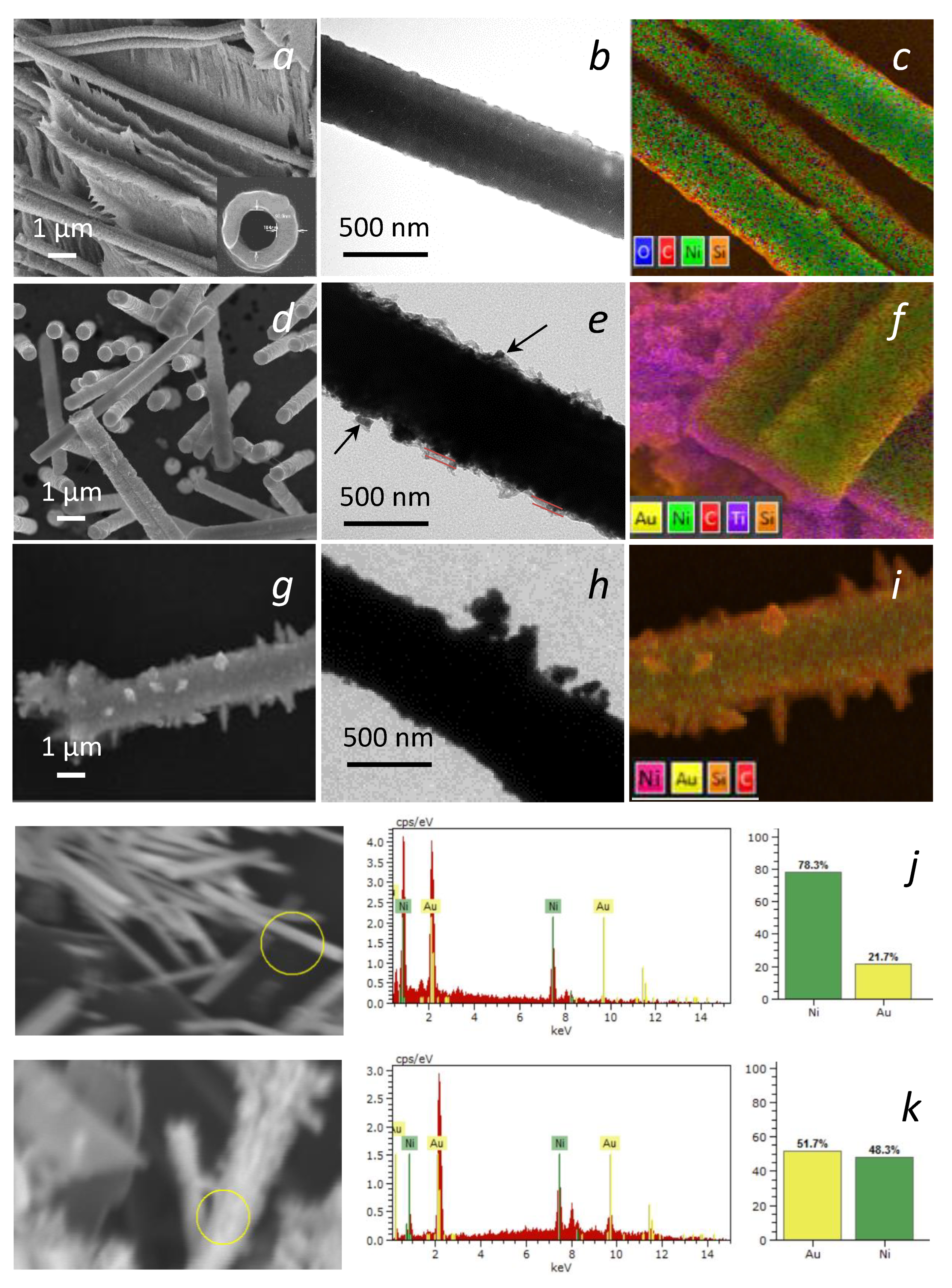
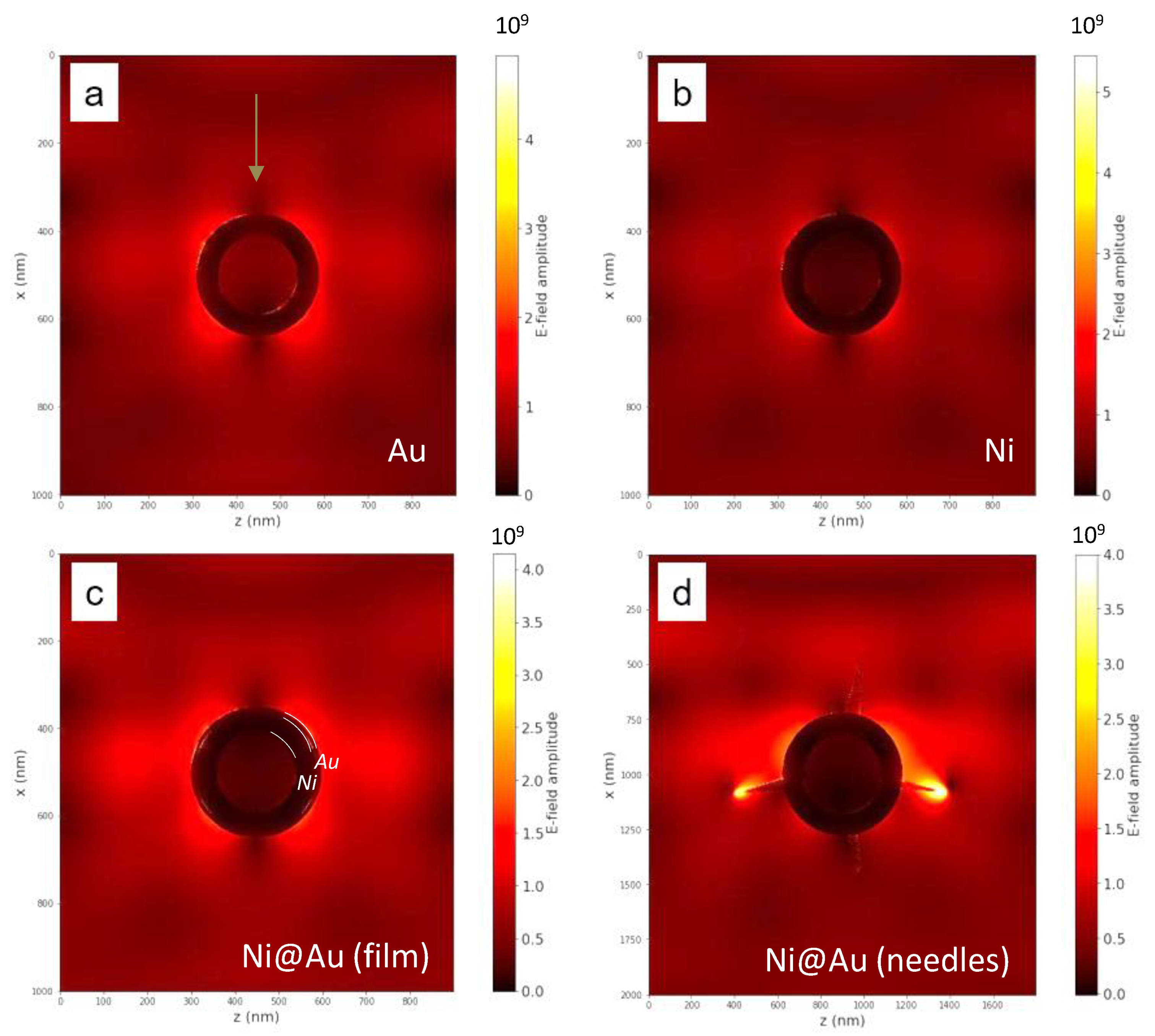
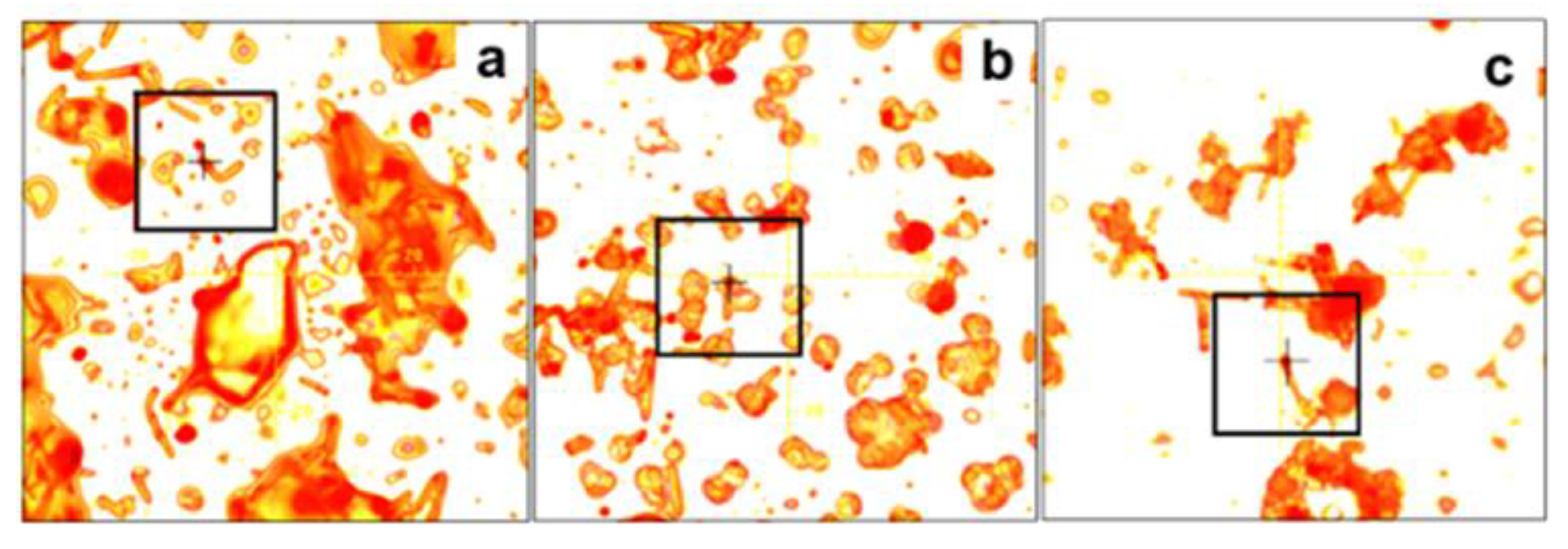
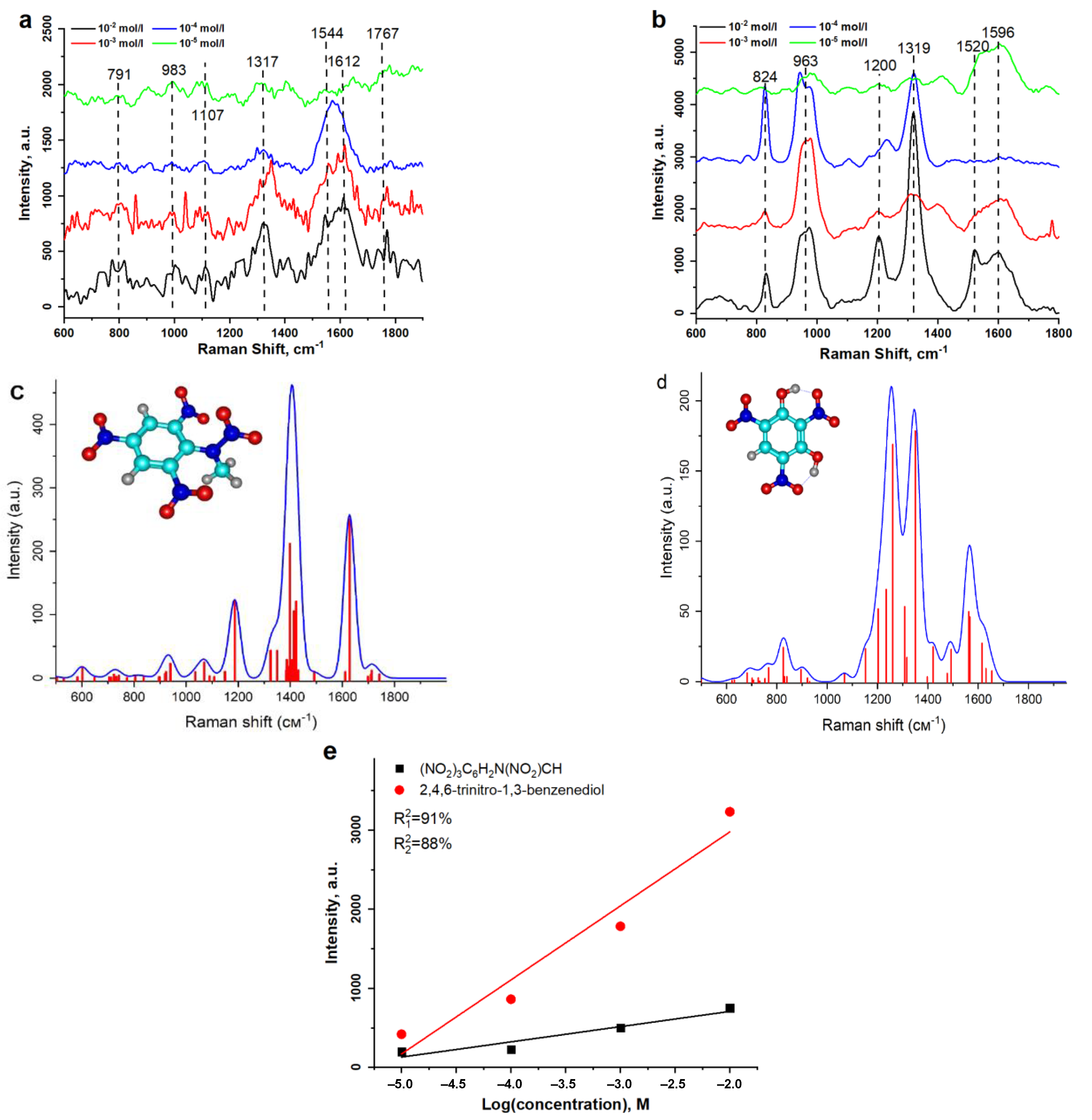
3. Results and Discussion
- Simple and efficient sample preparation and preparation of equipment for operation.
- A simple and fast detection technique with a portable device, and there should be no need to create special conditions for measurements.
- A reliable signal processing technique allowing the obtaining of a full spectrum from a partial spectrum. The option of comparison with the library of substance spectra or with calculated spectra should be provided.
- Add a suspension of functionalized nanotubes at a concentration of 1 mg/mL to the analyte solution. Then, the NTs need to be concentrated in a solution with a magnet and deposited on a single-crystal silicon substrate. The NTs are spread evenly over the surface with a magnet and dried.
- Preliminarily apply functionalized nanotubes from the suspension onto a single-crystal silicon plate, distribute them with a magnet, and dry. Apply the analyte solution.
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Barron, L.; Gilchrist, E. Ion chromatography-mass spectrometry: A review of recent technologies and applications in forensic and environmental explosives analysis. Anal. Chim. Acta 2014, 806, 27–54. [Google Scholar] [CrossRef] [PubMed]
- López-López, M.; García-Ruiz, C. Infrared and Raman spectroscopy techniques applied to identification of explosives. TrAC Trends Anal. Chem. 2014, 54, 36–44. [Google Scholar] [CrossRef]
- Ben-Jaber, S.; Peveler, W.J.; Quesada-Cabrera, R.; Sol, C.W.O.; Papakonstantinou, I.; Parkin, I.P. Sensitive and specific detection of explosives in solution and vapour by surface-enhanced Raman spectroscopy on silver nanocubes. Nanoscale 2017, 9, 16459–16466. [Google Scholar] [CrossRef] [PubMed]
- Doty, K.C.; Muro, C.K.; Bueno, J.; Halámková, L.; Lednev, I.K. What can Raman spectroscopy do for criminalistics? J. Raman Spectrosc. 2016, 47, 39–50. [Google Scholar] [CrossRef]
- Milligan, K.; Shand, N.C.; Graham, D.; Faulds, K. Detection of Multiple Nitroaromatic Explosives via Formation of a Janowsky Complex and SERS. Anal. Chem. 2020, 92, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Wackerbarth, H.; Salb, C.; Gundrum, L.; Niederkruger, M.; Christou, K.; Beushausen, V.; Viol, W. Detection of explosives based on surface-enhanced Raman spectroscopy. Appl. Opt. 2010, 49, 4362–4366. [Google Scholar] [CrossRef]
- Hakonen, A.; Wu, K.; Stenbaek Schmidt, M.; Andersson, P.O.; Boisen, A.; Rindzevicius, T. Detecting forensic substances using commercially available SERS substrates and handheld Raman spectrometers. Talanta 2018, 189, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.K.M.; Izake, E.L.; Sivanesan, A.; Fredericks, P.M. Rapid detection of TNT in aqueous media by selective label free surface enhanced Raman spectroscopy. Talanta 2015, 134, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Bachelot, R.; Kostcheev, S.; Royer, P.; Espiau de Lamaestre, R. Surface plasmon resonances in silver Bowtie nanoantennas with varied bow angles. J. Appl. Phys. 2010, 108, 124314. [Google Scholar] [CrossRef]
- Radziuk, D.; Moehwald, H. Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093. [Google Scholar] [CrossRef] [PubMed]
- Barbillon, G. Fabrication and SERS Performances of Metal/Si and Metal/ZnO Nanosensors: A Review. Coatings 2019, 9, 86. [Google Scholar] [CrossRef]
- Fateixa, S.; Nogueira, H.I.; Trindade, T. Hybrid nanostructures for SERS: Materials development and chemical detection. Phys. Chem. Chem. Phys. 2015, 17, 21046–21071. [Google Scholar] [CrossRef]
- Ward, D.R.; Grady, N.K.; Levin, C.S.; Halas, N.J.; Wu, Y.; Nordlander, P.; Natelson, D. Electromigrated nanoscale gaps for surface-enhanced Raman spectroscopy. Nano. Lett. 2007, 7, 1396–1400. [Google Scholar] [CrossRef]
- Cinel, N.A.; Cakmakyapan, S.; Butun, S.; Ertas, G.; Ozbay, E. E-Beam lithography designed substrates for surface enhanced Raman spectroscopy. Photonics Nanostruct. Fundam. Appl. 2015, 15, 109–115. [Google Scholar] [CrossRef]
- Farcau, C.; Astilean, S. Mapping the SERS Efficiency and Hot-Spots Localization on Gold Film over Nanospheres Substrates. J. Phys. Chem. C 2010, 114, 11717–11722. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, D.; Langer, J.; Henriksen-Lacey, M.; Liz-Marzán, L.M. Hybrid Au–SiO2 Core–Satellite Colloids as Switchable SERS Tags. Chem. Mater. 2015, 27, 2540–2545. [Google Scholar] [CrossRef]
- Faure, A.C.; Barbillon, G.; Ou, M.; Ledoux, G.; Tillement, O.; Roux, S.; Fabregue, D.; Descamps, A.; Bijeon, J.L.; Marquette, C.A.; et al. Core/shell nanoparticles for multiple biological detection with enhanced sensitivity and kinetics. Nanotechnology 2008, 19, 485103. [Google Scholar] [CrossRef]
- Jorio, A.; Pimenta, M.A.; Filho, A.G.S.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Characterizing carbon nanotube samples with resonance Raman scattering. New J. Phys. 2003, 5, 139. [Google Scholar] [CrossRef]
- Gohil, S.; Ghosh, S. Surface enhanced Raman scattering from multiwalled carbon nanotubes at low temperatures. Appl. Phys. Lett. 2010, 96, 143108. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Zhang, J. Few-Layer Graphene-Encapsulated Metal Nanoparticles for Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2014, 118, 8993–8998. [Google Scholar] [CrossRef]
- Dey, S.; Trau, M.; Koo, K.M. Surface-Enhanced Raman Spectroscopy for Cancer Immunotherapy Applications: Opportunities, Challenges, and Current Progress in Nanomaterial Strategies. Nanomaterials 2020, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Ahmed, E.; Somvanshi, P.S.; Sina, A.A.I.; Wuethrich, A.; Trau, M. An Electrochemical and Raman Scattering Dual Detection Biosensor for Rapid Screening and Biomolecular Profiling of Cancer Biomarkers. Chemosensors 2022, 10, 93. [Google Scholar] [CrossRef]
- Wang, F.; Du, D.; Liu, S.; Wang, L.; Jiao, T.; Xu, Z.; Wang, H. Revealing the Hemispherical Shielding Effect of SiO2@Ag Composite Nanospheres to Improve the Surface Enhanced Raman Scattering Performance. Nanomaterials 2021, 11, 2209. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gun, X.; Pang, G.; Zheng, Z.; Li, C.; Yang, C.; Wang, M.; Xu, K. Femtosecond laser patterned superhydrophobic/hydrophobic SERS sensors for rapid positioning ultratrace detection. Opt. Express 2021, 29, 16904–16913. [Google Scholar] [CrossRef]
- Kovalets, N.P.; Kozhina, E.P.; Razumovskaya, I.V.; Bedin, S.A.; Piryazev, A.A.; Grigoriev, Y.V.; Naumov, A.V. Toward single-molecule surface-enhanced Raman scattering with novel type of metasurfaces synthesized by crack-stretching of metallized track-etched membranes. J. Chem. Phys. 2022, 156, 034902. [Google Scholar] [CrossRef] [PubMed]
- Skibinska, K.; Smola, G.; Bialo, L.; Kutyla, D.; Kolczyk-Siedlecka, K.; Kwiecinska, A.; Wojnicki, M.; Zabinski, P. Influence of Annealing Time of Aluminum AA1050 on the Quality of Cu and Co Nanocones. J. Materi. Eng. Perform. 2020, 29, 8025–8035. [Google Scholar] [CrossRef]
- Bedin, S.A.; Rybalko, O.G.; Polyakov, N.B.; Zagorskii, D.L.; Razumovskaya, A.V.; Bondarenko, G.G.; Oleinikov, V.A. Metal micro- and nanowires fabricated by matrix synthesis and their application in mass spectrometry. Inorg. Mater. Appl. Res. 2010, 1, 359–364. [Google Scholar] [CrossRef]
- Shumskaya, A.; Bundyukova, V.; Kozlovskiy, A.; Zdorovets, M.; Kadyrzhanov, K.; Kalkabay, G.; Kaniukov, E. Evolution of morphology, structure, and magnetic parameters of Ni nanotubes with growth in pores of a PET template. J. Magn. Magn. Mater. 2020, 497, 165913. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zdorovets, M.; Kadyrzhanov, K.; Korolkov, I.; Rusakov, V.; Nikolaevich, L.; Fesenko, O.; Budnyk, O.; Yakimchuk, D.; Shumskaya, A.; et al. FeCo nanotubes: Possible tool for targeted delivery of drugs and proteins. Appl. Nanosci. 2018, 9, 1091–1099. [Google Scholar] [CrossRef]
- Roselina, N.R.; Azizan, A.; Hyie, K.M.; Murad, M.C.; Abdullah, A.H. Synthesis and characterization of Ni-Au bimetallic nanoparticles. Int. J. Mod. Phys. B 2015, 29, 1540006. [Google Scholar] [CrossRef]
- Song, D.; Yang, R.; Long, F.; Zhu, A. Applications of magnetic nanoparticles in surface-enhanced Raman scattering (SERS) detection of environmental pollutants. J. Environ. Sci. China 2019, 80, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.V.P.; Rangarajan, N.; Sonia, B.; Deepika, P.; Rohman, N.; Narayana, C. Metal-coated magnetic nanoparticles for surface enhanced Raman scattering studies. Bull. Mater. Sci. 2011, 34, 207–216. [Google Scholar] [CrossRef]
- Kozhina, E.; Kulesh, E.; Bedin, S.; Doludenko, I.; Piryazev, A.; Korolkov, I.; Kozlovskiy, A.; Zdorovets, M.; Rogachev, A.; Shumskaya, A. One-Dimensional Magneto-Optical Nanostructures: Template Synthesis, Structure, Properties, and Application in Spectroscopy Based on Plasmon Resonance. IEEE Magn. Lett. 2022, 13, 1–5. [Google Scholar] [CrossRef]
- Sen, L.; He, D.; Li, S.; Chen, R.; Peng, Y.; Li, S.; Han, D.; Wang, Y.; Qin, K.; Ren, S.; et al. Magnetic Halloysite Nanotube-Based SERS Biosensor Enhanced with Au@Ag Core—Shell Nanotags for Bisphenol A Determination Enhanced Reader. Biosensors 2022, 12, 387–400. [Google Scholar]
- Kazemi, M. Based on magnetic nanoparticles: Gold reusable nanomagnetic catalysts in organic synthesis. Synth. Commun. 2020, 50, 2079–2094. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Shumskaya, A.; Korolkov, I.; Rogachev, A.; Ignatovich, Z.; Kozlovskiy, A.; Zdorovets, M.; Anisovich, M.; Bashouti, M.; Shalabny, A.; Busool, R.; et al. Synthesis of Ni@Au core-shell magnetic nanotubes for bioapplication and SERS detection. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127077. [Google Scholar] [CrossRef]
- Shumskaya, A.; Panina, L.; Rogachev, A.; Ihnatovich, Z.; Kozlovskiy, A.; Zdorovets, M.; Kaniukov, E.; Korolkov, I. Catalytic Activity of Ni Nanotubes Covered with Nanostructured Gold. Processes 2021, 9, 2279. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 8, e1327. [Google Scholar] [CrossRef]
- Tarakanov, V.P. Code KARAT in simulations of power microwave sources including Cherenkov plasma devices, vircators, orotron, E-field sensor, calorimeter etc. EPJ Web Conf. 2017, 149, 04024. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguie, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Kozhina, E.P.; Andreev, S.N.; Tarakanov, V.P.; Bedin, S.A.; Doludenko, I.M.; Naumov, A.V. Study of Local Fields of Dendrite Nanostructures in Hot Spots Formed on SERS-Active Substrates Produced via Template-Assisted Synthesis. Bull. Russ. Acad. Sci. Phys. 2021, 84, 1465–1468. [Google Scholar] [CrossRef]
- Yakimchuk, D.V.; Kaniukov, E.Y.; Lepeshov, S.; Bundyukova, V.D.; Demyanov, S.E.; Arzumanyanm, G.M.; Doroshkevich, N.V.; Mamatkulov, K.Z.; Bochmann, A.; Presselt, M.; et al. Self-organized spatially separated silver 3D dendrites as efficient plasmonic nanostructures for surface-enhanced Raman spectroscopy applications. J. Appl. Phys. 2019, 126, 233105. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Shumskaya, A.; Kozlovskiy, A.L.; Kaliyekperov, M.E.; Lissovskaya, L.I.; Zdorovets, M.V. Magnetic-plasmonic Ni nanotubes covered with gold for improvement of SERS analysis. J. Alloy. Compd. 2022, 901, 163661. [Google Scholar] [CrossRef]
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.; Hu, X.; Wang, X.; Huang, Z.; Li, F.; Wu, N. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater 2016, 28, 4871–4876. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Zhang, P. Laser-MBE of nickel nanowires using AAO template: A new active substrate of surface enhanced Raman scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 91–95. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumskaya, A.; Kozhina, E.; Bedin, S.; Andreev, S.; Kulesh, E.; Rogachev, A.; Yarmolenko, M.; Korolkov, I.; Kozlovskiy, A.; Zdorovets, M.; et al. Detection of Polynitro Compounds at Low Concentrations by SERS Using Ni@Au Nanotubes. Chemosensors 2022, 10, 306. https://doi.org/10.3390/chemosensors10080306
Shumskaya A, Kozhina E, Bedin S, Andreev S, Kulesh E, Rogachev A, Yarmolenko M, Korolkov I, Kozlovskiy A, Zdorovets M, et al. Detection of Polynitro Compounds at Low Concentrations by SERS Using Ni@Au Nanotubes. Chemosensors. 2022; 10(8):306. https://doi.org/10.3390/chemosensors10080306
Chicago/Turabian StyleShumskaya, Alena, Elizaveta Kozhina, Sergey Bedin, Stepan Andreev, Ekaterina Kulesh, Alexander Rogachev, Maxim Yarmolenko, Ilya Korolkov, Artem Kozlovskiy, Maksim Zdorovets, and et al. 2022. "Detection of Polynitro Compounds at Low Concentrations by SERS Using Ni@Au Nanotubes" Chemosensors 10, no. 8: 306. https://doi.org/10.3390/chemosensors10080306
APA StyleShumskaya, A., Kozhina, E., Bedin, S., Andreev, S., Kulesh, E., Rogachev, A., Yarmolenko, M., Korolkov, I., Kozlovskiy, A., Zdorovets, M., Belyaev, V., Rodionova, V., & Panina, L. (2022). Detection of Polynitro Compounds at Low Concentrations by SERS Using Ni@Au Nanotubes. Chemosensors, 10(8), 306. https://doi.org/10.3390/chemosensors10080306










