Progress in Electrochemical Biosensing of SARS-CoV-2 Virus for COVID-19 Management
Abstract
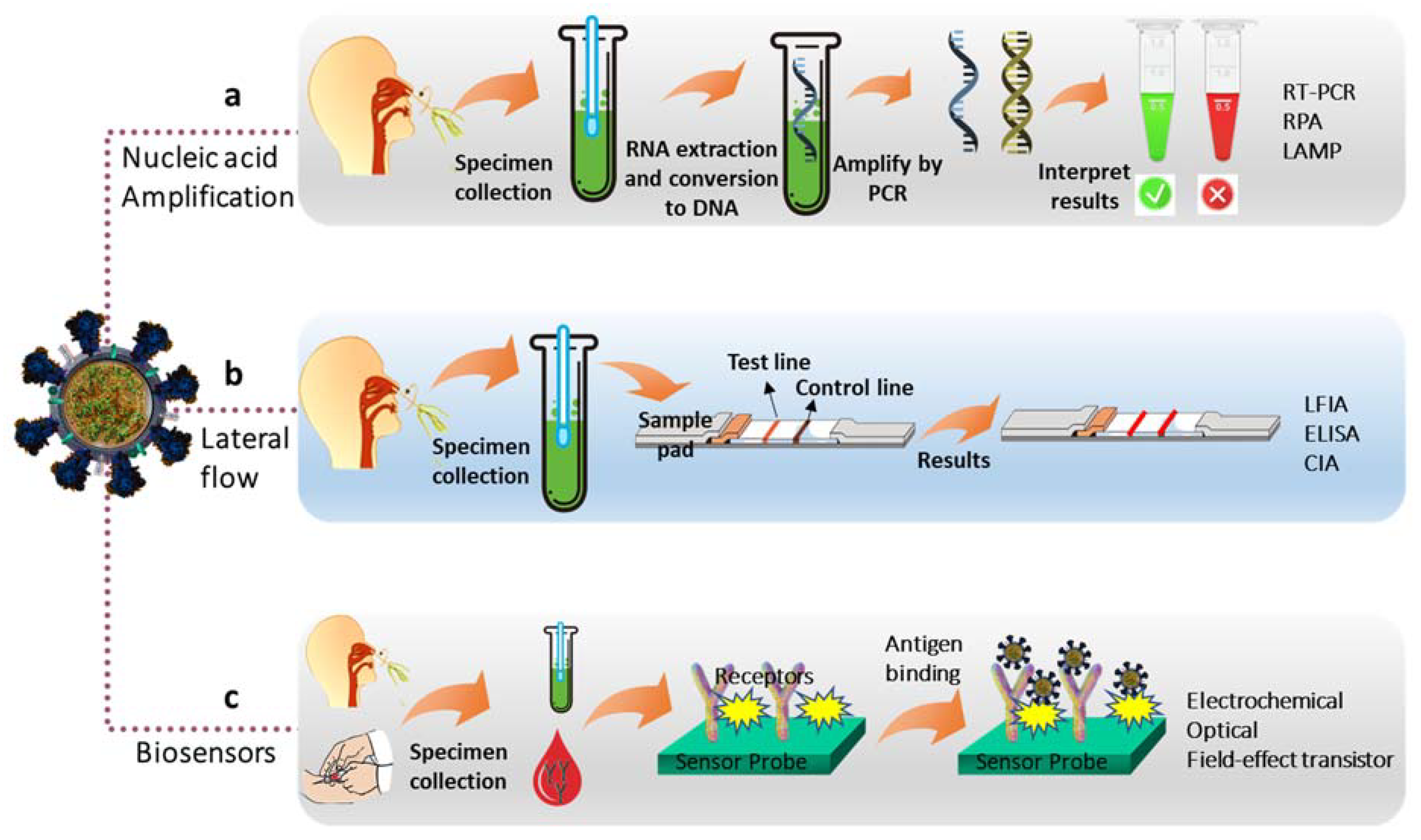
:1. Introduction
2. Structure of the SARS-CoV-2 Virus
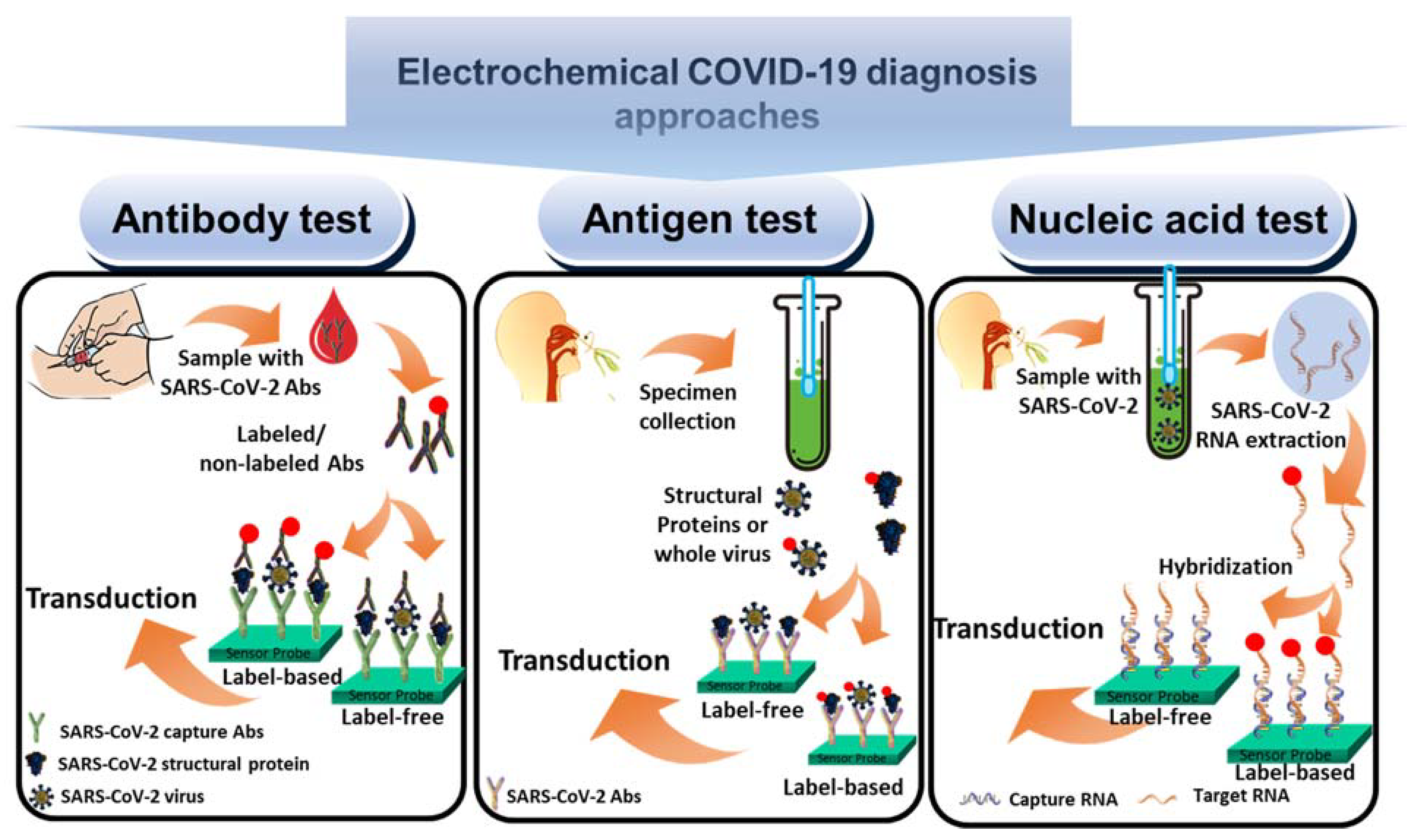
3. Designing Electrochemical SARS-CoV-2 Virus Biosensors
3.1. Antibody Biosensors
3.2. Antigen Biosensors
3.3. Nucleic Acid Biosensors
4. Electrochemical Biosensors for the Detection of SARS-CoV-2 Virus
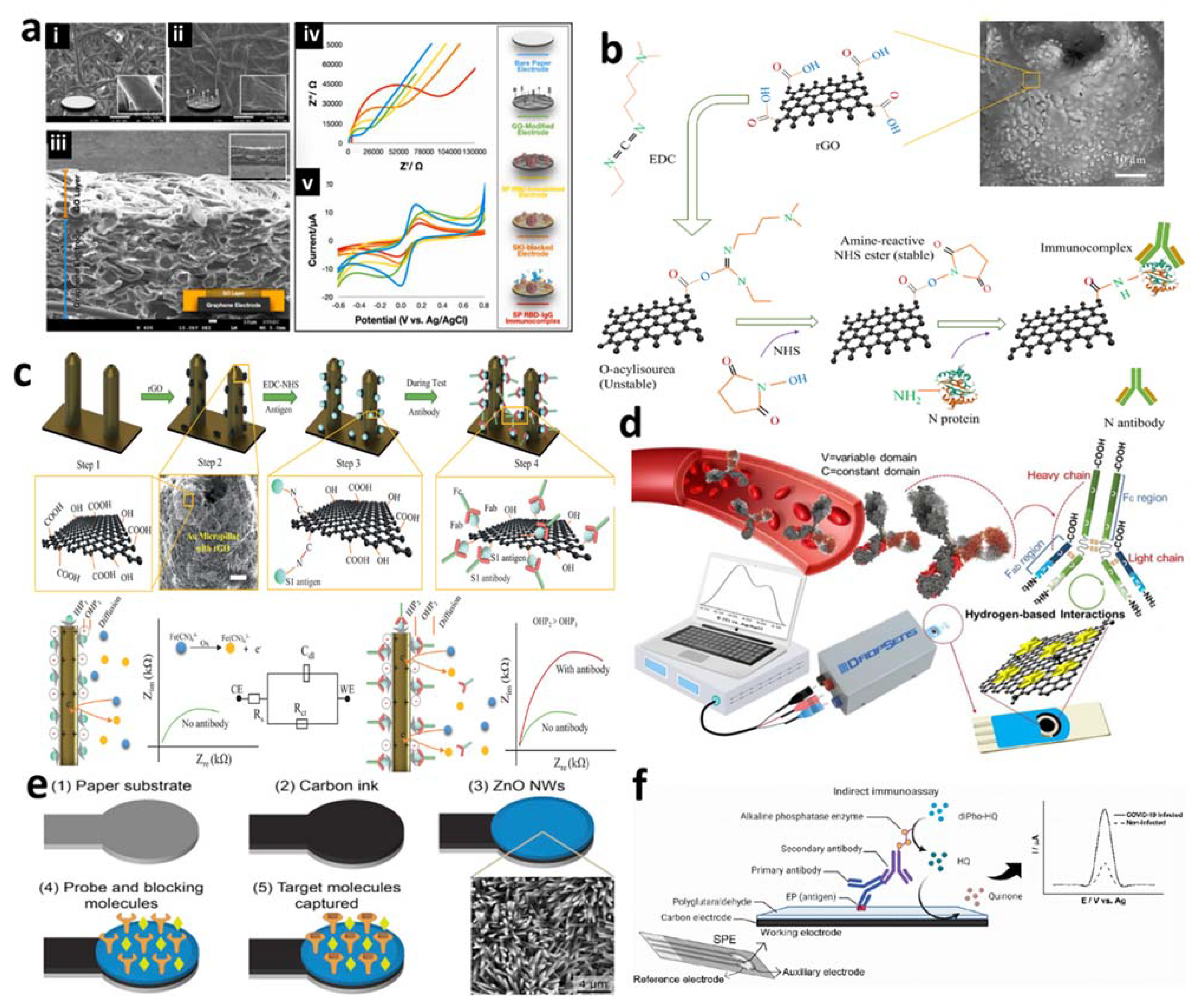
4.1. Electrochemical Antibody-Based Detection of SARS-CoV-2 Virus
4.2. Electrochemical Antigen-Based Detection of SARS-CoV-2 Virus
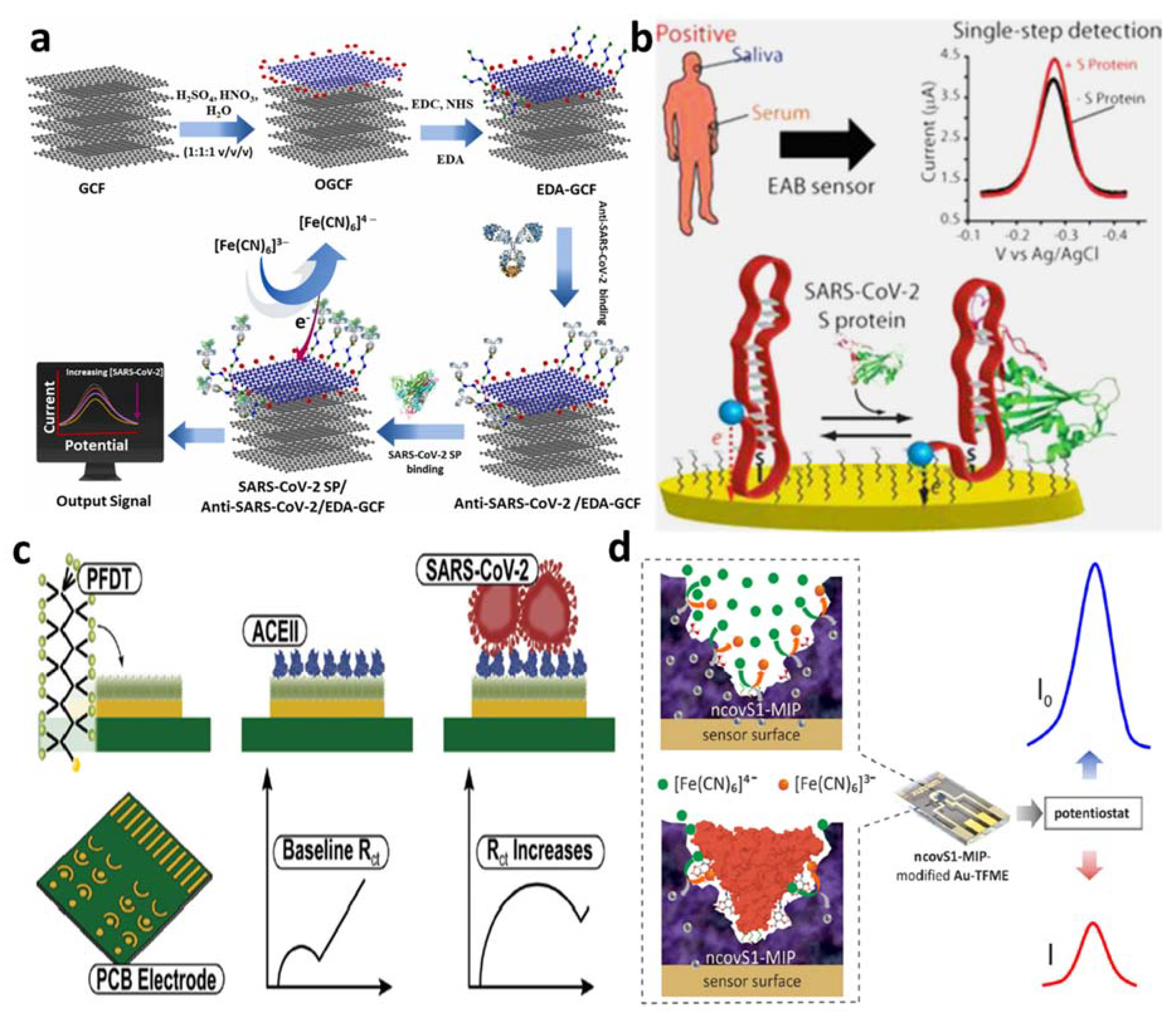
4.2.1. Detection of Spike Proteins
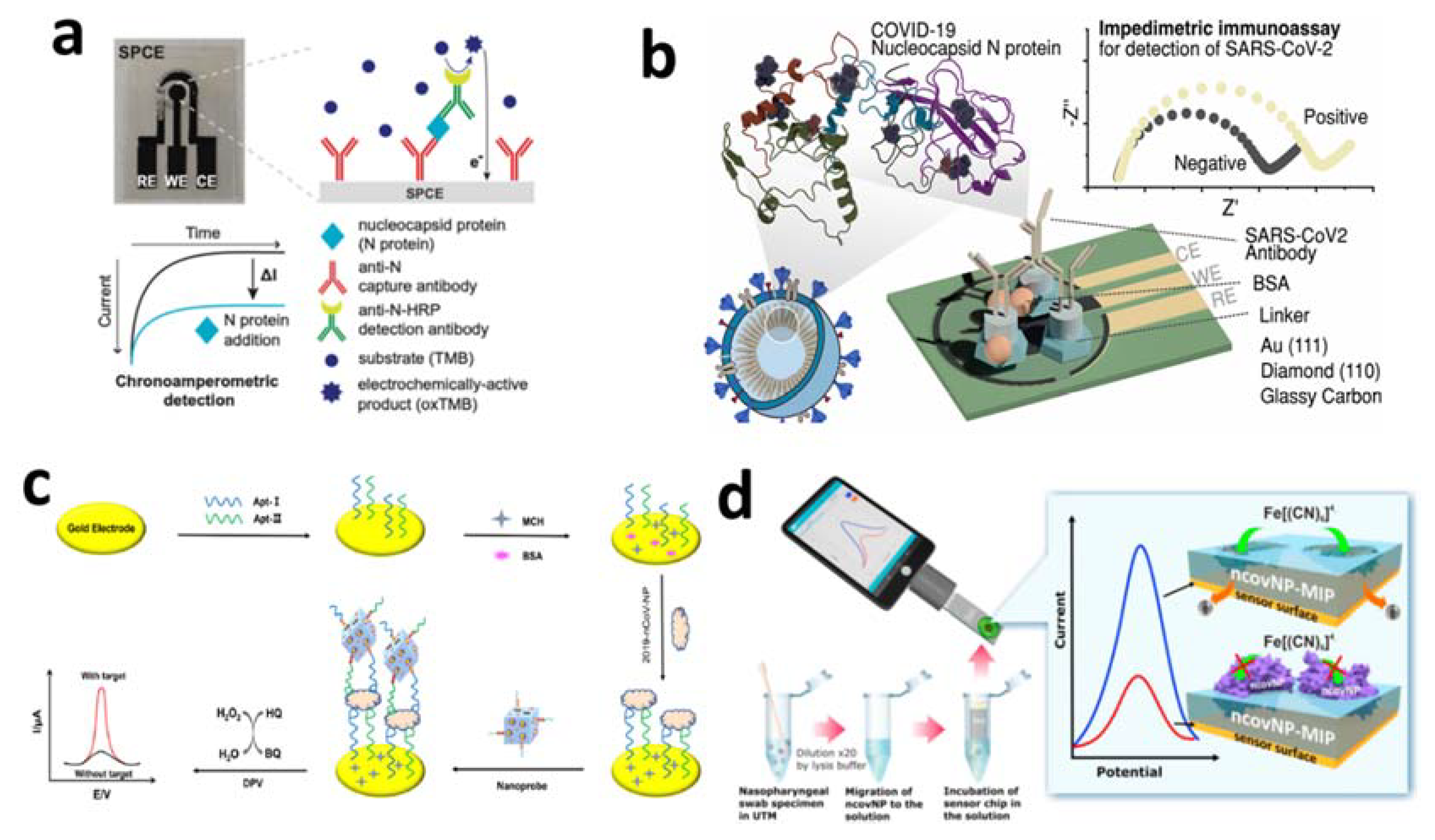
4.2.2. Detection of Nucleocapsid Protein
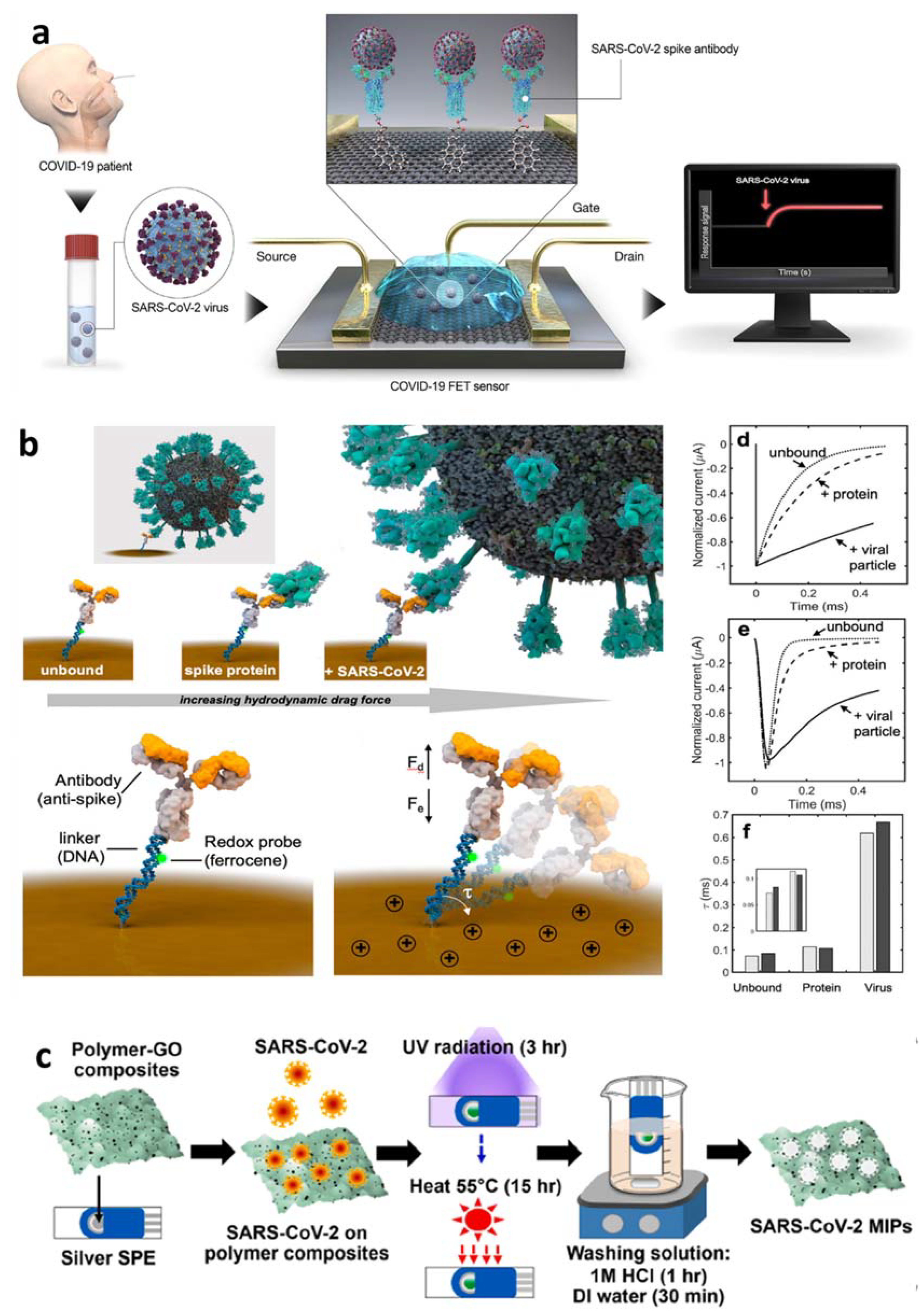
4.2.3. Detection of Whole Virus or Virus Particles
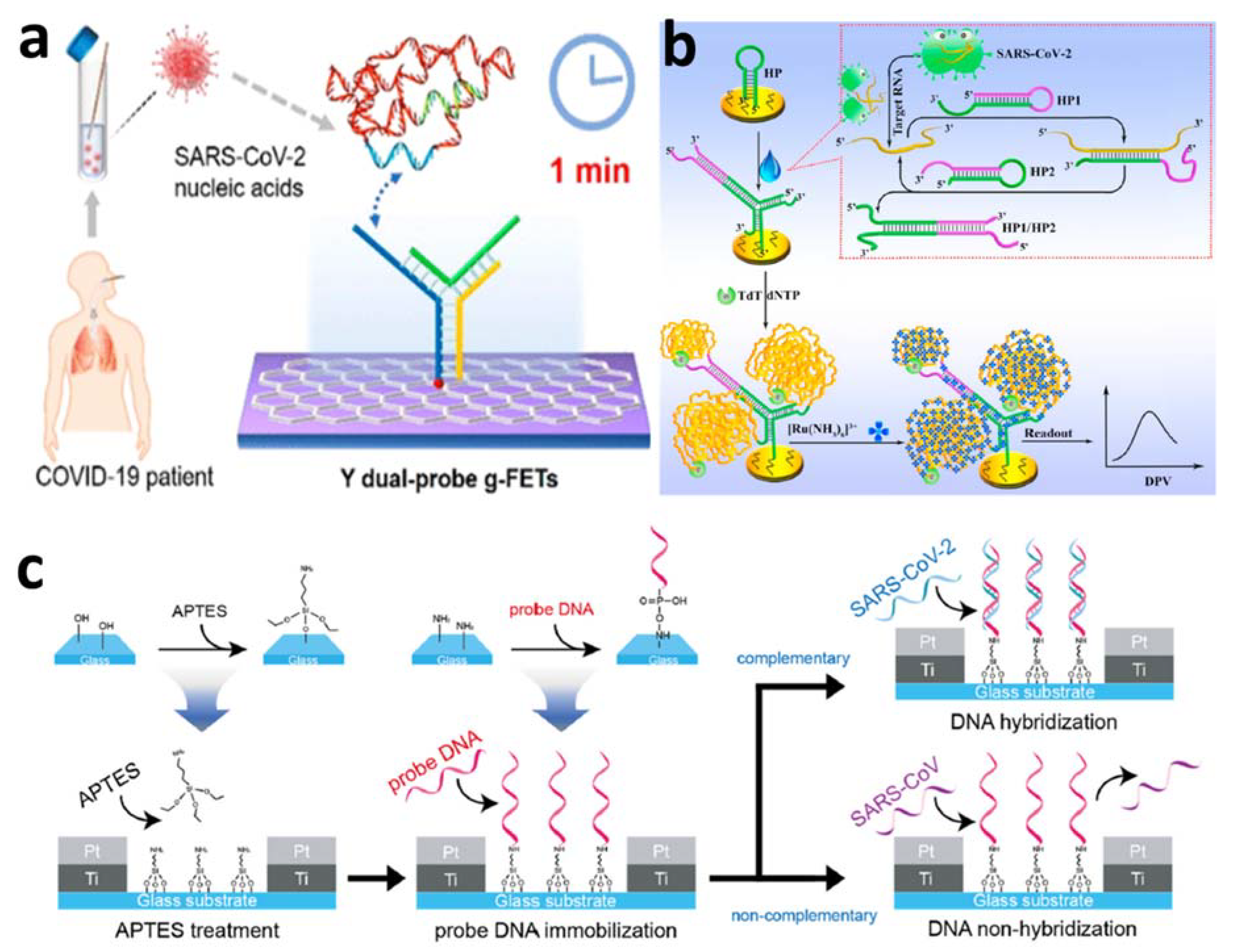
4.3. Electrochemical Nucleic Acid-Based Detection of SARS-CoV-2 Virus
5. Conclusions, Challenges, and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zong, W.; Wang, F.; Ju, S. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A review. Mol. Cancer 2020, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [PubMed]
- COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 29 May 2022).
- Available online: https://www.axios.com/2022/04/13/world-covid-cases-surpass-500-million (accessed on 29 May 2022).
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Taleghani, N.; Taghipour, F. Diagnosis of COVID-19 for controlling the pandemic: A review of the state-of-the-art. Biosens. Bioelectron. 2021, 174, 112830. [Google Scholar] [CrossRef]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis-a review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Kaushik, A.K.; Dhau, J.S.; Gohel, H.; Mishra, Y.K.; Kateb, B.; Kim, N.-Y.; Goswami, D.Y. Electrochemical SARS-CoV-2 Sensing at Point-of-Care and Artificial Intelligence for Intelligent COVID-19 Management. ACS Appl. Bio. Mater. 2020, 3, 7306–7325. [Google Scholar] [CrossRef]
- Kashir, J.; Yaquinuddin, A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Med. Hypotheses 2020, 141, 109786. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, L.; Liu, C.; Ye, S.; Chen, W.; Li, D.; Huang, W. One-tube SARS-CoV-2 detection platform based on RT-RPA and CRISPR/Cas12a. J. Transl. Med. 2021, 19, 74. [Google Scholar] [CrossRef]
- Jiao, J.; Duan, C.; Xue, L.; Liu, Y.; Sun, W.; Xiang, Y. DNA nanoscaffold-based SARS-CoV-2 detection for COVID-19 diagnosis. Biosens. Bioelectron. 2020, 167, 112479. [Google Scholar] [CrossRef]
- Serebrennikova, K.V.; Byzova, N.A.; Zherdev, A.V.; Khlebtsov, N.G.; Khlebtsov, B.N.; Biketov, S.F.; Dzantiev, B.B. Lateral Flow Immunoassay of SARS-CoV-2 Antigen with SERS-Based Registration: Development and Comparison with Traditional Immunoassays. Biosensors 2021, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.A.; Almutairi, A.Z.; Jan, A.A.; Alkhalify, A.M. Enzyme-Linked Immunosorbent Assay for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) IgM/IgA and IgG Antibodies Among Healthcare Workers. Cureus 2020, 12, e10285. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Grossi, V.; Lari, B.; Bambi, R.; Perri, A.; Manneschi, M.; Terenzi, G.; Liotti, I.; Ciotta, G.; Taddei, C.; et al. Diagnostic accuracy of an automated chemiluminescent immunoassay for anti-SARS-CoV-2 IgM and IgG antibodies: An Italian experience. J. Med. Virol. 2020, 92, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Drobysh, M.; Ramanaviciene, A.; Viter, R.; Chen, C.-F.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Biosensors for the Determination of SARS-CoV-2 Virus and Diagnosis of COVID-19 Infection. Int. J. Mol. Sci. 2022, 23, 666. [Google Scholar] [CrossRef]
- Jiang, C.; Mu, X.; Du, B.; Tong, Z. A review of electrochemical biosensor application in the detection of the SARS-CoV-2. Micro Nano Lett. 2022, 17, 49–58. [Google Scholar] [CrossRef]
- Adeel, M.; Asif, K.; Rahman, M.M.; Daniele, S.; Canzonieri, V.; Rizzolio, F. Glucose Detection Devices and Methods Based on Metal–Organic Frameworks and Related Materials. Adv. Funct. Mater. 2021, 31, 2106023. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lopa, N.S.; Lee, J.-J. Advances in electrochemical aptasensing for cardiac biomarkers. Bull. Korean Chem. Soc. 2022, 43, 51–68. [Google Scholar] [CrossRef]
- Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens. Bioelectron. 2020, 165, 112331. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, M.M.; Ahmed, F.; Ryu, T.; Sutradhar, S.C.; Lei, J.; Kim, J.; Kim, D.H.; Lee, Y.H.; Kim, W. Simple, low-cost, sensitive and label-free aptasensor for the detection of cardiac troponin I based on a gold nanoparticles modified titanium foil. Biosens. Bioelectron. 2019, 126, 381–388. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lopa, N.S.; Kim, Y.J.; Choi, D.-K.; Lee, J.-J. Label-Free DNA Hybridization Detection by Poly(Thionine)-Gold Nanocomposite on Indium Tin Oxide Electrode. J. Electrochem. Soc. 2016, 163, B153–B157. [Google Scholar] [CrossRef]
- Kumar, N.; Shetti, N.P.; Jagannath, S.; Aminabhavi, T.M. Electrochemical sensors for the detection of SARS-CoV-2 virus. Chem. Eng. J. 2022, 430, 132966. [Google Scholar] [CrossRef] [PubMed]
- Madhurantakam, S.; Muthukumar, S.; Prasad, S. Emerging Electrochemical Biosensing Trends for Rapid Diagnosis of COVID-19 Biomarkers as Point-of-Care Platforms: A Critical Review. ACS Omega 2022, 7, 12467–12473. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9, e57309. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.A. Properties of coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar] [PubMed]
- Raj, R. Analysis of non-structural proteins, NSPs of SARS-CoV-2 as targets for computational drug designing. Biochem. Biophys. Rep. 2021, 25, 100847. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-K.; Hou, M.-H.; Chang, C.-F.; Hsiao, C.-D.; Huang, T.-H. The SARS coronavirus nucleocapsid protein–Forms and functions. Antivir. Res. 2014, 103, 39–50. [Google Scholar] [CrossRef]
- McBride, R.; Zyl, M.V.; Fielding, B.C. The Coronavirus Nucleocapsid Is a Multifunctional Protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yang, C.; Xu, X.-F.; Xu, W.; Liu, S.-W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Sternberg, A.; Naujokat, C. Structural features of coronavirus SARS-CoV-2 spike protein: Targets for vaccination. Life Sci. 2020, 257, 118056. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef] [Green Version]
- Boson, B.; Legros, V.; Zhou, B.; Siret, E.; Mathieu, C.; Cosset, F.-L.; Lavillette, D.; Denolly, S. The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J. Biol. Chem. 2021, 296, 100111. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. The Structure of the Membrane Protein of SARS-CoV-2 Resembles the Sugar Transporter SemiSWEET. Pathog. Immun. 2020, 5, 342–363. [Google Scholar] [CrossRef] [PubMed]
- Kontou, P.I.; Braliou, G.G.; Dimou, N.L.; Nikolopoulos, G.; Bagos, P.G. Antibody Tests in Detecting SARS-CoV-2 Infection: A Meta-Analysis. Diagnostics 2020, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Rahman, M.M.; Lee, J.-J. Ultrasensitive and label-free detection of annexin A3 based on quartz crystal microbalance. Sens. Actuators B Chem. 2013, 177, 172–177. [Google Scholar] [CrossRef]
- Ruiz de Eguilaz, M.; Cumba, L.R.; Forster, R.J. Electrochemical detection of viruses and antibodies: A mini review. Electrochem. Commun. 2020, 16, 106762. [Google Scholar] [CrossRef]
- Ozer, T.; Geiss, B.J.; Henry, C.S. Review-Chemical and Biological Sensors for Viral Detection. J. Electrochem. Soc. 2020, 167, 037523. [Google Scholar] [CrossRef] [Green Version]
- Koyappayil, A.; Lee, M.-H. Ultrasensitive Materials for Electrochemical Biosensor Labels. Sensors 2021, 21, 89. [Google Scholar] [CrossRef]
- Ehsan Shabani, E.; Dowlatshahi, S.; Abdekhodaie, M.J. Laboratory detection methods for the human coronaviruses. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 225–246. [Google Scholar] [CrossRef]
- Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices FDA. 2020. Available online: https://www.fda.gov/medical-devices/emergency-use-authorizations-medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices (accessed on 29 May 2022).
- Rahman, M.M.; Kim, Y.J.; Lee, J.-J. Label-Free Detection of DNA Hybridization by Using Charge Perturbation on Poly(thionine)-Modified Glassy Carbon and Gold Electrodes. J. Electrochem. Soc. 2015, 162, B159–B162. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, Y.J.; Lee, J.-J. Sensitivity Control of Label-free DNA Hybridization Detection Based on Poly(thionine)-Modified Glassy Carbon and Gold Electrodes. Bull. Korean Chem. Soc. 2017, 38, 27–32. [Google Scholar] [CrossRef]
- Falasca, F.; Sciandra, I.; Di Carlo, D.; Gentile, M.; Deales, A.; Antonelli, G.; Turriziani, O. Detection of SARS-CoV N2 gene: Very low amounts of viral RNA or false positive? J. Clin. Virol. 2020, 133, 104660. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, D.; Ramadan, S.; Li, Y.; Klein, N. Facile biosensors for rapid detection of COVID-19. Biosens. Bioelectron. 2020, 170, 112673. [Google Scholar] [CrossRef]
- Pan, Y.; Li, X.; Yang, G.; Fan, J.; Tang, Y.; Zhao, J.; Long, X.; Guo, S.; Zhao, Z.; Liu, Y.; et al. Serological immunochromatographic approach in diagnosis with SARSCoV-2 infected COVID-19 patients. J. Infect. 2020, 81, e28–e32. [Google Scholar] [CrossRef] [PubMed]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Chapter 6-Adaptive Immune Responses to Infection; In Fenner and White’s Medical Virology, 5th ed.; Burrell, C.J., Howard, C.R., Murphy, F.A., Eds.; Academic Press: London, UK, 2017; pp. 65–76. [Google Scholar]
- Casali, P. IgM. In Encyclopedia of Immunology, 2nd ed.; Delves, P.J., Ed.; Elsevier: Oxford, UK, 1998; pp. 1212–1217. [Google Scholar]
- Neumann, F.; Rose, R.; Römpke, J.; Grobe, O.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Development of SARS-CoV-2 Specific IgG and Virus-Neutralizing Antibodies after Infection with Variants of Concern or Vaccination. Vaccines 2021, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.-Y.; Rahman, M.M.; Zhang, W.; Lopa, N.S.; Jin, L.; Yoon, S.; Jang, H.; Xu, G.-R.; Kim, W. An Electrochemical Immunosensor Based on a Self-Assembled Monolayer Modified Electrode for Label-Free Detection of α-Synuclein. Sensors 2020, 20, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeel, M.; Rahman, M.M.; Lee, J.-J. Label-free aptasensor for the detection of cardiac biomarker myoglobin based on gold nanoparticles decorated boron nitride nanosheets. Biosens. Bioelectron. 2019, 126, 143–150. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, D.; Lopa, N.S.; Baek, J.-B.; Nam, C.-H.; Lee, J.-J. Effect of the carboxyl functional group at the edges of graphene on the signal sensitivity of dopamine detection. J. Electroanal. Chem. 2021, 898, 115628. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lopa, N.S.; Ju, M.J.; Lee, J.-J. Highly sensitive and simultaneous detection of dopamine and uric acid at graphene nanoplatelet-modified fluorine-doped tin oxide electrode in the presence of ascorbic acid. J. Electroanal. Chem. 2017, 792, 54–60. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, N.; Chaiyo, S. Paper-Based Electrochemical Biosensor for Diagnosing COVID-19: Detection of SARS-CoV-2 Antibodies and Antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef]
- Ali, M.A.; Hu, C.; Zhang, F.; Jahan, S.; Yuan, B.; Saleh, M.S.; Gao, S.-J.; Panat, R. N protein-based ultrasensitive SARS-CoV-2 antibody detection in seconds via 3D nanoprinted, microarchitected array electrodes. J. Med. Virol. 2022, 94, 2067–2078. [Google Scholar] [CrossRef]
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.-J.; Panat, R. Sensing of COVID-19 Antibodies in Seconds via Aerosol Jet Nanoprinted Reduced-Graphene-Oxide-Coated 3D Electrodes. Adv. Mater. 2021, 33, 2006647. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.A.; Bahrani, S.; Mousavi, S.M.; Omidifar, N.; Behbahan, N.G.G.; Arjmand, M.; Ramakrishna, S.; Lankarani, K.B.; Moghadami, M.; Shokripour, M.; et al. Ultra-precise label-free nanosensor based on integrated graphene with Au nanostars toward direct detection of IgG antibodies of SARS-CoV-2 in blood. J. Electroanal. Chem. 2021, 894, 115341. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qin, Z.; Fu, H.; Li, T.; Peng, R.; Li, Z.; Rini, J.M.; Liu, X. Enhancing the performance of paper-based electrochemical impedance spectroscopy nanobiosensors: An experimental approach. Biosens. Bioelectron. 2021, 177, 112672. [Google Scholar] [CrossRef] [PubMed]
- Ameku, W.A.; Provance, D.W.; Morel, C.M.; De-Simone, S.G. Rapid Detection of Anti-SARS-CoV-2 Antibodies with a Screen-Printed Electrode Modified with a Spike Glycoprotein Epitope. Biosensors 2022, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.V.; Tran, N.H.T.; Hwang, H.S.; Chang, M. Development strategies of conducting polymer-based electrochemical biosensors for virus biomarkers: Potential for rapid COVID-19 detection. Biosens. Bioelectron. 2021, 182, 113192. [Google Scholar] [CrossRef]
- Rashed, M.Z.; Kopechek, J.A.; Priddy, M.C.; Hamorsky, K.T.; Palmer, K.E.; Mittal, N.; Valdez, J.; Flynn, J.; Williams, S.J. Rapid detection of SARS-CoV-2 antibodies using electrochemical impedance-based detector. Biosens. Bioelectron. 2021, 171, 112709. [Google Scholar] [CrossRef]
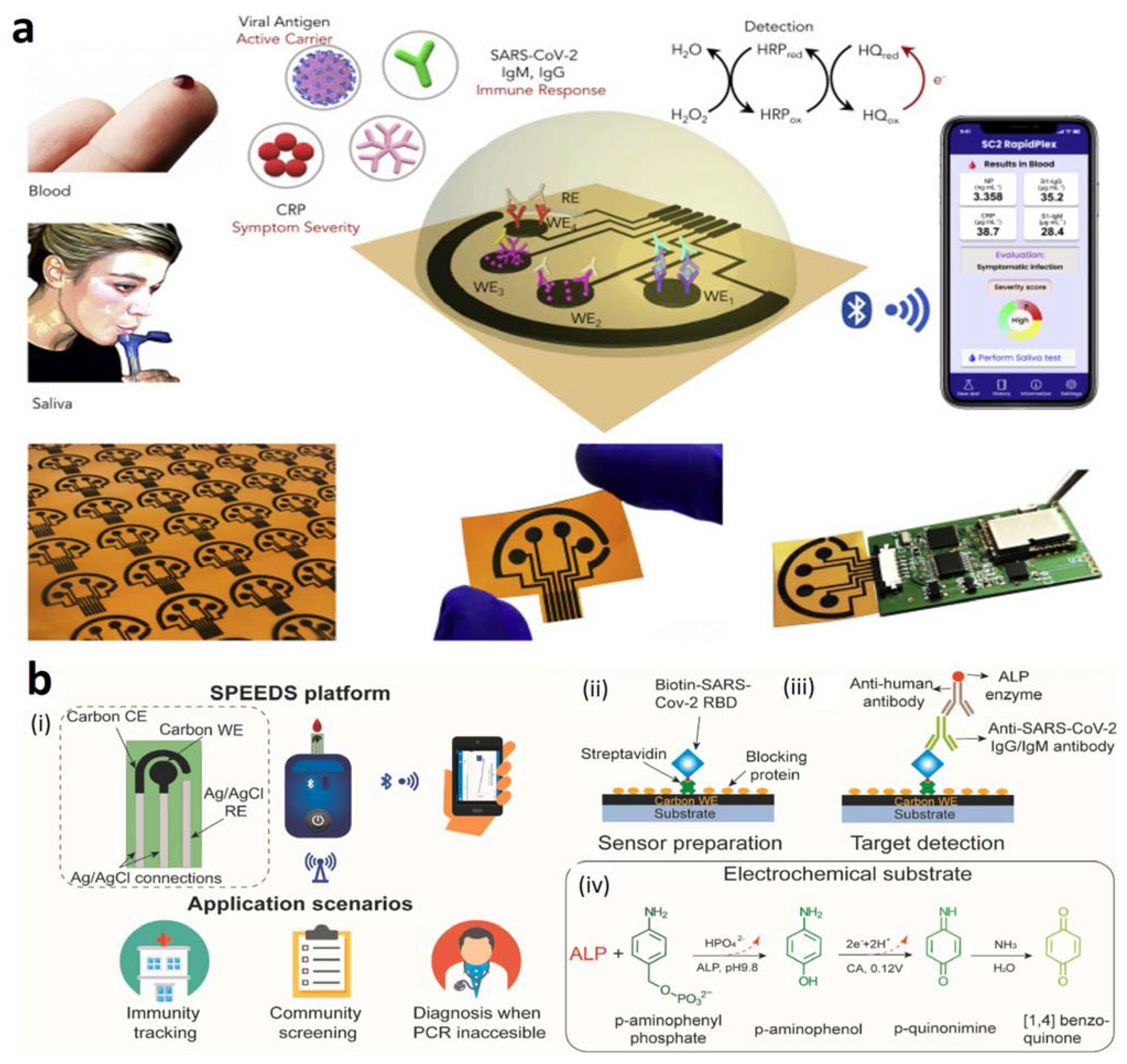
- Torrente-Rodrı’guez, R.M.; Lukas, H.; Tu, J.; Min, J.; Yang, Y.; Xu, C.; Rossiter, H.B.; Gao, W. SARS-CoV-2 RapidPlex: A Graphene-Based Multiplexed Telemedicine Platform for Rapid and Low-Cost COVID-19 Diagnosis and Monitoring. Matter 2020, 3, 1981–1998. [Google Scholar] [CrossRef]
- Peng, R.; Pan, Y.; Li, Z.; Qin, Z.; Rini, J.M.; Liu, X. SPEEDS: A portable serological testing platform for rapid electrochemical detection of SARS-CoV-2 antibodies. Biosens. Bioelectron. 2022, 197, 113762. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wei, X.; Zheng, S.W.; Lenhart, B.J.; Xu, P.; Li, J.; Pan, J.; Albrecht, H.; Liu, C. Multiplex quantitative detection of SARS-CoV-2 specific IgG and IgM antibodies based on DNA-assisted nanopore sensing. Biosens. Bioelectron. 2021, 181, 113134. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. An electrochemical immunosensor using SARS-CoV-2 spike protein-nickel hydroxide nanoparticles bio-conjugate modified SPCE for ultrasensitive detection of SARS-CoV-2 antibodies. Microchem. J. 2021, 170, 106718. [Google Scholar] [CrossRef]
- Find. Test Directory, FIND. (n.d.). Available online: https://www.finddx.org/test-directory/ (accessed on 25 May 2022).
- Abdelhamid, H.N.; Badr, G. Nanobiotechnology as a platform for the diagnosis of COVID-19: A review. Nanotechnol. Environ. Eng. 2021, 6, 19. [Google Scholar] [CrossRef]
- Liu, G.; Rusling, J.F. COVID-19 antibody tests and their limitations. ACS Sens. 2021, 6, 593–612. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Asif, K.; Canzonieri, V.; Barai, H.S.; Rahman, M.M.; Daniele, S.; Rizzolio, F. Controlled, partially exfoliated, self-supported functionalized flexible graphitic carbon foil for ultrasensitive detection of SARS-CoV-2 spike protein. Sens. Actuators B Chem. 2022, 359, 131591. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor- Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malla, P.; Liao, H.-P.; Liu, C.-H.; Wu, W.-C.; Sreearunothai, P. Voltammetric biosensor for coronavirus spike protein using magnetic bead and screen-printed electrode for point-of-care diagnostics. Microchim. Acta 2022, 189, 168. [Google Scholar] [CrossRef] [PubMed]
- Idili, A.; Parolo, C.; Alvarez-Diduk, R.; MerkoçI, A. Rapid and Efficient Detection of the SARS-CoV-2 Spike Protein Using an Electrochemical Aptamer-Based Sensor. ACS Sens. 2021, 6, 3093–3101. [Google Scholar] [CrossRef]
- Curti, F.; Fortunati, S.; Knoll, W.; Giannetto, M.; Corradini, R.; Bertucci, A.; Careri, M. A Folding-Based Electrochemical Aptasensor for the Single-Step Detection of the SARS-CoV-2 Spike Protein. ACS Appl. Mater. Interfaces 2022, 14, 19204–19211. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Vezza, V.J.; Butterworth, A.; Lasserre, P.; Blair, E.O.; MacDonald, A.; Hannah, S.; Rinaldi, C.; Hoskisson, P.A.; Ward, A.C.; Longmuir, A.; et al. An electrochemical SARS-CoV-2 biosensor inspired by glucose test strip manufacturing processes. Chem. Commun. 2021, 57, 3704–3707. [Google Scholar] [CrossRef]
- Nascimento, E.D.; Fonseca, W.T.; de Oliveira, T.R.; de Correia, C.R.S.T.B.; Faça, V.M.; de Morais, B.P.; Silvestrini, V.C.; Pott-Junior, H.; Teixeira, F.R.; Faria, R.C. COVID-19 diagnosis by SARS-CoV-2 Spike protein detection in saliva using an ultrasensitive magneto-assay based on disposable electrochemical sensor. Sens. Actuators B Chem. 2022, 353, 131128. [Google Scholar] [CrossRef]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Karube, I. Molecularly imprinted polymers for biosensor applications. Trends Anal. Chem. 1999, 18, 199–204. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Boroznjak, R.; Reut, J.; Opik, A.; Syritski, V. Molecularly imprinted polymer based electrochemical sensor for quantitative detection of SARS-CoV-2 spike protein. Sens. Actuators B Chem. 2022, 353, 131160. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Soares, A.C.; Angelim, M.K.S.C.; Proença-Modena, J.L.; Moraes-Vieira, P.M.; Mattoso, L.H.C.; Oliveira, O.N., Jr. Diagnostics of SARS-CoV-2 infection using electrical impedance spectroscopy with an immunosensor to detect the spike protein. Talanta 2022, 239, 123076. [Google Scholar] [CrossRef] [PubMed]
- Liustrovaite, V.; Drobysh, M.; Rucinskiene, A.; Baradoke, A.; Ramanaviciene, A.; Plikusiene, I.; Samukaite-Bubniene, U.; Viter, R.; Chen, C.-F.; Ramanavic, A. Towards an Electrochemical Immunosensor for the Detection of Antibodies against SARS-CoV-2 Spike Protein. J. Electrochem. Soc. 2022, 169, 037523. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. Electrochemical immunosensor with Cu2O nanocube coating for detection of SARS-CoV-2 spike protein. Microchim. Acta 2021, 188, 105. [Google Scholar] [CrossRef]
- Lasserre, P.; Balansethupathy, B.; Vezza, V.J.; Butterworth, A.; Macdonald, A.; Blair, E.O.; McAteer, L.; Hannah, S.; Ward, A.C.; Hoskisson, P.A.; et al. SARS-CoV-2 Aptasensors Based on Electrochemical Impedance Spectroscopy and Low-Cost Gold Electrode Substrates. Anal. Chem. 2022, 94, 2126–2133. [Google Scholar] [CrossRef]
- Abrego-Martinez, J.C.; Jafari, M.; Chergui, S.; Pavel, C.; Che, D.; Siaj, M. Aptamer-based electrochemical biosensor for rapid detection of SARS-CoV-2: Nanoscale electrode-aptamer-SARS-CoV-2 imaging by photo-induced force microscopy. Biosens. Bioelectron. 2022, 195, 113595. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Acedo, P. An Electrochemical Impedance Spectroscopy-Based Aptasensor for the Determination of SARS-CoV-2-RBD Using a Carbon Nanofiber–Gold Nanocomposite Modified Screen-Printed Electrode. Biosensors 2022, 12, 142. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelanye, M.; Ramanavicius, A. Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Wang, H.; Li, X.; Zhang, S.; Xu, Y.; Wei, W. Serum SARS-CoV-2 Nucleocapsid Protein: A Sensitivity and Specificity Early Diagnostic Marker for SARS-CoV-2 Infection. Front. Cell. Infect. Microbiol. 2020, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, E.N.; Indeykina, M.I.; Brzhozovskiy, A.G.; Bugrova, A.E.; Kononikhin, A.S.; Starodubtseva, N.L.; Petrotchenko, E.V.; Kovalev, G.I.; Borchers, C.H.; Sukhikh, G.T. Mass Spectrometric Identification of SARS-CoV-2 Proteins from Gargle Solution Samples of COVID-19 Patients. J. Proteome Res. 2020, 19, 4393–4397. [Google Scholar] [CrossRef] [PubMed]
- Samper, I.C.; McMahon, C.J.; Schenkel, M.S.; Clark, K.M.; Khamcharoen, W.; Anderson, L.B.R.; Terry, J.S.; Gallichotte, E.N.; Ebel, G.D.; Geiss, B.J.; et al. Electrochemical Immunoassay for the Detection of SARS-CoV-2 Nucleocapsid Protein in Nasopharyngeal Samples. Anal. Chem. 2022, 94, 4712–4719. [Google Scholar] [CrossRef]
- Białobrzeska, W.; Ficek, M.; Dec, B.; Osella, S.; Trzaskowski, B.; Jaramillo-Botero, A.; Pierpaoli, M.; Rycewicz, M.; Dashkevich, Y.; Łęga, T.; et al. Performance of electrochemical immunoassays for clinical diagnostics of SARS-CoV-2 based on selective nucleocapsid N protein detection: Boron-doped diamond, gold and glassy carbon evaluation. Biosens. Bioelectron. 2022, 209, 114222. [Google Scholar] [CrossRef]
- Karaman, C.; Yola, B.B.; Karaman, O.; Atar, N.; Polat, I.; Yola, M.L. Sensitive sandwich-type electrochemical SARS-CoV-2 nucleocapsid protein immunosensor. Microchim. Acta 2021, 188, 425. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Alhadrami, H.A.; Al-Mozaini, M.; Hassan, A.M.; Zourob, M. Voltammetric-based immunosensor for the detection of SARS-CoV-2 nucleocapsid antigen. Microchim. Acta 2021, 188, 199. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Label-free and reagent-less electrochemical detection of nucleocapsid protein of SARS-CoV-2: An ultrasensitive and disposable biosensor. New J. Chem. 2022, 46, 9172–9183. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chiang, Y.-H.; Chiang, H.-Y. A Label-Free Electrochemical Impedimetric Immunosensor with Biotinylated-Antibody for SARS-CoV-2 Nucleoprotein Detection in Saliva. Biosensors 2022, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lillehoj, P.B. Microfluidic Magneto Immunosensor for Rapid, High Sensitivity Measurements of SARS-CoV-2 Nucleocapsid Protein in Serum. ACS Sens. 2021, 6, 1270–1278. [Google Scholar] [CrossRef]
- Tian, J.; Liang, Z.; Hu, O.; He, Q.; Sun, D.; Chen, Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta 2021, 387, 138553. [Google Scholar] [CrossRef]
- Qi, H.; Hu, Z.; Yang, Z.; Zhang, J.; Wu, J.J.; Cheng, C.; Wang, C.; Zheng, L. Capacitive Aptasensor Coupled with Microfluidic Enrichment for Real-Time Detection of Trace SARS-CoV-2 Nucleocapsid Protein. Anal. Chem. 2022, 94, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Gopinath, S.C.B.; Ismail, Z.H.; Arshad, M.K.M.; Poopalan, P. Aptasensing nucleocapsid protein on nanodiamond assembled gold interdigitated electrodes for impedimetric SARS-CoV-2 infectious disease assessment. Biosens. Bioelectron. 2022, 197, 113735. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, W.; Li, Q.; Xing, W.; Luo, H.; Ji, H.; Fang, X.; Luo, Z.; Zhang, L. CRISPR/Cas12a-Derived electrochemical aptasensor for ultrasensitive detection of COVID-19 nucleocapsid protein. Biosens. Bioelectron. 2022, 200, 113922. [Google Scholar] [CrossRef] [PubMed]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, L.; Zhang, Y. Highly sensitive electrochemical determination of the SARS-CoV-2 antigen based on a gold/graphene imprinted poly-arginine sensor. Anal. Methods 2021, 13, 5772–5776. [Google Scholar] [CrossRef]
- Yousefi, H.; Mahmud, A.; Chang, D.; Das, J.; Gomis, S.; Chen, J.B.; Wang, H.; Been, T.; Yip, L.; Coomes, E.; et al. Detection of SARS-CoV-2 Viral Particles Using Direct, Reagent-Free Electrochemical Sensing. J. Am. Chem. Soc. 2021, 143, 1722–1727. [Google Scholar] [CrossRef]
- Sukjee, W.; Thitithanyanont, A.; Manopwisedjaroen, S.; Seetaha, S.; Thepparit, C.; Sangma, C. Virus MIP-composites for SARS-CoV-2 detection in the aquatic environment. Mater. Lett. 2022, 315, 131973. [Google Scholar] [CrossRef]
- Chaibun, T.; Puenpa, J.; Ngamdee, T.; Boonapatcharoen, N.; Athamanolap, P.; O’Mullane, A.P.; Vongpunsawad, S.; Poovorawan, Y.; Lee, S.Y.; Lertanantawong, B. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nat. Commun. 2021, 12, 802. [Google Scholar] [CrossRef]
- Kong, D.; Wang, X.; Gu, C.; Guo, M.; Wang, Y.; Ai, Z.; Zhang, S.; Chen, Y.; Liu, W.; Wu, Y.; et al. Direct SARS-CoV-2 Nucleic Acid Detection by Y-Shaped DNA Dual-Probe Transistor Assay. J. Am. Chem. Soc. 2021, 143, 17004–17014. [Google Scholar] [CrossRef]
- Peng, Y.; Pan, Y.; Sun, Z.; Li, J.; Yi, Y.; Yang, J.; Li, G. An electrochemical biosensor for sensitive analysis of the SARS-CoV-2 RNA. Biosens. Bioelectron. 2021, 186, 113309. [Google Scholar] [CrossRef]
- Hwang, C.; Park, N.; Kim, E.S.; Kim, M.; Kim, S.D.; Park, S.; Kim, N.Y.; Kim, J.H. Ultra-fast and recyclable DNA biosensor for point-of-care detection of SARS-CoV-2 (COVID-19). Biosens. Bioelectron. 2021, 185, 113177. [Google Scholar] [CrossRef] [PubMed]
- Miripour, Z.S.; Sarrami-Forooshani, R.; Sanati, H.; Makarem, J.; Taheri, M.S.; Shojaeian, F.; Eskafi, A.H.; Abbasvandi, F.; Namdar, N.; Ghafari, H.; et al. Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens. Bioelectron. 2020, 165, 112435. [Google Scholar] [CrossRef] [PubMed]
- Samprathi, M.; Jayashree, M. Biomarkers in COVID-19: An Up-To-Date Review. Front. Pediatr. 2021, 8, 607647. [Google Scholar] [CrossRef] [PubMed]
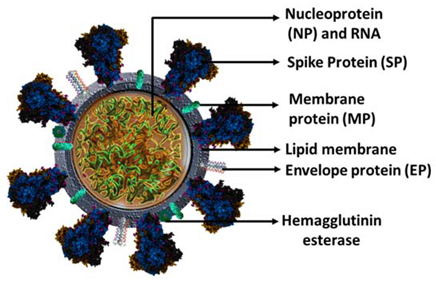 | Protein | Mass (kDa) | Function |
| SP | ~180 | Binds and fuse to the host cell receptor and induces infection, and transmission. | |
| NP | ~10 | Binding to the viral RNA genome critical for viral replication and genome packaging. | |
| MP | ~45–60 | Viral assembly and shaping viral envelope. | |
| EP | ~ 25–30 | Formation of the viral envelope. |
| Electrode | Detection Method | Target Antibodies | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|
| Paper-based ePAD | SWV | IgG/IgM | 0.96/0.14 ng/mL | 1–1000 ng/mL | [54] |
| AuNPs/rGO | EIS | IgG | 13 fm | 100 fm–1 nM | [55] |
| Au micropillar/rGO | EIS | IgG | 2.8 fm | - | [56] |
| Paper-based μPADs | EIS | IgG | 0.4 pg/mL | 10–1000 ng/mL | [58] |
| Au electrode | EIS | IgG | 1.99 nM | 30 nM–150 nM | [60] |
| Au based well plate | EIS | IgG | - | - | [61] |
| SPE | CA | IgG/IgM | 10.1/1.64 ng/mL | 10.1 ng/mL–60 μg/mL and 1.64 ng/mL–50 μg/mL | [63] |
| Ni(OH)2/SPE | DPV | IgG/IgM | 0.3 fg/mL | 1 fg/mL–1 μg/mL | [65] |
| Materials/Electrode | Detection Method | Receptors | Detection Medium | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Functionalized graphitic carbon foil | DPV | antibody | blood plasma | 27 pg/mL | 0.2–100 ng/mL | [69] |
| Graphene | FET | antibody | nasopharyngeal samples | 100 fg/mL | - | [70] |
| MB/APBA/Antibody-HRP/GLU/SPE | SWV | antibody | human saliva | 0.20 ng/mL | 3.12–200 ng/mL | [71] |
| Au | SWV | aptamer | serum and artificial saliva | - | - | [72] |
| SWCNT-SPE | DPV | aptamer | PBS | 7 nM | 20–100 nM | [73] |
| PFDT/PCB | EIS | ACE2 | human saliva | 38.6 copies/mL | - | [75] |
| MB/AuNPs/SPE | DPV | ACE2 | PBS | 0.35 ag/mL | 0.0009–360 fg/mL | [76] |
| Au-TFME | SWV | MIP | nasopharyngeal samples | 64 fM | 0–400 fM | [79] |
| CMCt/Au IDE | EIS | antibody | PBS | 0.179 fg/mL | 10−20–10−14 g/mL | [80] |
| Au | EIS | antibody | PBS | 2.78 nM | 30–150 nM | [81] |
| Cu2O NCs/SPCE | EIS | antibody | PBS | 0.04 fg/mL | 0.25 fg/mL–1 μg/mL | [82] |
| Thin-film Au electrodes | EIS | aptamer | nasopharyngeal samples | - | - | [83] |
| AuNPs/SPCE | EIS | aptamer | PBS | 1.30 pM (66 pg/mL) | 10 pM–25 nM | [84] |
| CNF/AuNP/SPE | EIS | aptamer | PBS | 7.0 pM | 0.01–64.0 nM | [85] |
| MIP-poly(pyrrole)/Pt | Amperometry | MIP | PBS | - | 0–25 μg/mL | [86] |
| Materials/Electrode | Detection Method | Receptors | Detection Medium | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Graphene | DPV | antibody | blood and saliva samples | - | - | [62] |
| COOH-SPCE | CA | antibody | PBS | 50 PFU/mL | - | [89] |
| Au, GC, and BDD | EIS | antibody | PBS | 0.227, 0.334, and 0.362 ng/mL, respectively | 4.4 ng/mL–4.4 pg/mL | [90] |
| BiWO6/Bi2S3/GC | DPV | antibody | PBS | 3.00 fg/mL | 0.01–1.00 pg/mL | [91] |
| AuNPs/SCPE | SWV | antibody | PBS | 0.4 pg/mL | 1.0 pg/mL–100 ng/mL | [92] |
| AuNPs/Pthi-Ald/ITO | EIS | antibody | PBS | 0.48 fg/mL | 0.0015 pg/mL–150 pg/mL | [93] |
| Au nanostructured/SPCE | EIS | antibody | PBS diluted saliva | 6 pg/mL | 0.01–100 ng/mL | [94] |
| SPG | CA | antibody | wholeserum | 50 pg/mL | 0–10 ng/mL | [95] |
| Au | DPV | aptamer | PBS | 8.33 pg/mL | 0–50 ng/mL | [96] |
| MEA | EIS | aptamer | PBS | fg/mL level | 10−5–10−2 ng/mL | [97] |
| Au IDE | EIS | aptamer | PBS | 0.389 fM | 1 fM–100 pM | [98] |
| Au | DPV | aptamer | PBS | 16.5 pg/mL | 0.05–100 ng/mL | [99] |
| Au-TFE | DPV | MIP | nasopharyngeal | 27 fM | 0.22–333 fM. | [100] |
| Au/graphene/SPCE | DPV | MIP | PBS | 3.0 fM | 10.0–200.0 fM | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M. Progress in Electrochemical Biosensing of SARS-CoV-2 Virus for COVID-19 Management. Chemosensors 2022, 10, 287. https://doi.org/10.3390/chemosensors10070287
Rahman MM. Progress in Electrochemical Biosensing of SARS-CoV-2 Virus for COVID-19 Management. Chemosensors. 2022; 10(7):287. https://doi.org/10.3390/chemosensors10070287
Chicago/Turabian StyleRahman, Md. Mahbubur. 2022. "Progress in Electrochemical Biosensing of SARS-CoV-2 Virus for COVID-19 Management" Chemosensors 10, no. 7: 287. https://doi.org/10.3390/chemosensors10070287
APA StyleRahman, M. M. (2022). Progress in Electrochemical Biosensing of SARS-CoV-2 Virus for COVID-19 Management. Chemosensors, 10(7), 287. https://doi.org/10.3390/chemosensors10070287






