Abstract
In recent years, wearable sensors have enabled the unique mode of real-time and noninvasive monitoring to develop rapidly in medical care, sports, and other fields. Sweat contains a wide range of biomarkers such as metabolites, electrolytes, and various hormones. Combined with wearable technology, sweat can reflect human fatigue, disease, mental stress, dehydration, and so on. This paper comprehensively describes the analysis of sweat components such as glucose, lactic acid, electrolytes, pH, cortisol, vitamins, ethanol, and drugs by wearable sensing technology, and the application of sweat wearable devices in glasses, patches, fabrics, tattoos, and paper. The development trend of sweat wearable devices is prospected. It is believed that if the sweat collection, air permeability, biocompatibility, sensing array construction, continuous monitoring, self-healing technology, power consumption, real-time data transmission, specific recognition, and other problems of the wearable sweat sensor are solved, we can provide the wearer with important information about their health level in the true sense.
1. Introduction
Perspiration is produced through sweat glands distributed throughout the human body. The interest of researchers has been centered on the composition and function of perspiration. Perspiration contains abundant biomarkers, providing insights into potential physiological and metabolic processes, making it an attractive liquid for noninvasive physiological monitoring including glucose [], lactic acid [], electrolytes [], pH value [], cortisol [], vitamins [], ethanol [] and drugs [], which are related to different physiological processes such as dehydration, fatigue, mental stress, or disease (Figure 1). Therefore, understanding the physiological level and correlation of sweat components and health evaluation is the key to establishing an intelligent health monitoring device, which means that the component analysis of sweat has attracted the attention of relevant physiological monitoring and noninvasive disease diagnosis [].
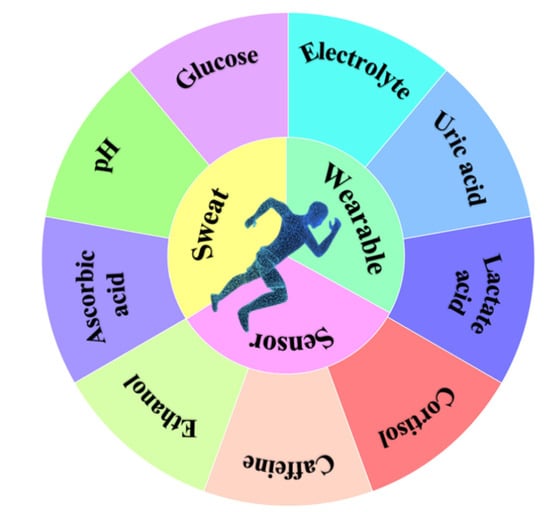
Figure 1.
A diagram of the different common components in sweat.
Blood has long been the first choice for the physical diagnosis of people because advanced clinical technology can help us deepen our understanding of health. The results of the blood test can include glucose, lactate, protein, uric acid, electrolyte ions, etc. []. Many substances are also commonly found in sweat. According to the results of the blood test, we can judge and evaluate according to the reference standard, but the frequency of our exposure to them is simply not enough to predict or to prevent health problems. From the perspective of invasive blood testing, noninvasive sweat analysis can avoid trauma to the human body and avoid infection, and at the same time, save on expensive costs, and has certain development prospects. Natural sweating behavior increases sweating due to stress, stressful psychological states, ambient temperature, and exercise behavior, and decreases due to cold as well as being affected by hormonal imbalances, an overactive thyroid and sympathetic nervous system, and certain foods and medications []. However, under certain behavioral conditions, for the purpose of sampling sweat samples, the sweating skin area can be stimulated by heat or a chemical such as pilocarpine with a low-intensity current to obtain a sufficient sweat volume for subsequent analysis [,].
Sweat has little traditional use as a clinical sample in routine clinical analysis. Moreover, the current preliminary research interest in sweat biological fluids allows us to review the current mature sweat analysis technologies, gradually evaluate the technologies that have not been evaluated thus far, and application technologies that can be developed and constructed in the future based on current cutting-edge technologies. This makes understanding the composition of sweat, the physiological levels of biomarkers, and their correlation with blood components crucial for building smart wearable health monitoring devices.
Specific disease biomarkers in sweat are closely related to the early diagnosis and treatment of disease. However, the potential applications of biomarker analysis by omics techniques are mostly aimed at studying the protein metabolism changes in common biological fluids such as blood [,] and urine [,]. There are relatively few omics studies on sweat, nevertheless, in the past decade, high-throughput omics methods such as metabolomics using sweat as biological samples have further determined the function regulation rules of the body by analyzing the metabolic changes caused by environmental stimuli or gene modification. This facilitates the study of the progression of genetic and phenotypic variation in inherited diseases [,]. The metabolomics application research of local noninvasive samples through sweat is more compliant for patients and can better reflect the local change characteristics of the disease. In-depth analysis of these specific biomolecules has practical implications in deciphering the disease markers of various diseases, understanding pathogenesis, and rapidly providing the diagnostic methods []. For example, correlational omics studies have shown that not only are antibodies such as immunoglobulin (Ig) A, IgE, and IgG found in sweat, but also cytokines including tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, and other interleukins []. These omics studies realize the transition from basic compositional research to clinical application.
In addition, common metabolites of the human body also have research value for the health and physiological state. As an important biomarker, Na+ can help predict and prevent non-contact injuries such as ion imbalance, disorientation, fatigue, and even muscle strain. K+ plays an important role in nerve and muscle cell function, cellular biochemical reactions, and carbohydrate metabolism. In addition, oxidative phosphorylation of glucose is the main energy source for the active function of sweat glands. However, the normal human sweat glucose level is significantly lower than the serum glucose concentration, and the level of glucose in the sweat of diabetic patients is significantly increased. Lactic acid also reflects the function of the energy metabolism of sweat glands and is a metabolite of anaerobic respiration during human exercise. Thus, elevated lactate concentrations in sweat indicate tissue oxygenation levels. Since hypoxia is associated with fatigue, muscle weakness, and muscle spasms, noninvasive monitoring of lactate is essential for the detection of human performance during high-endurance activities []. Therefore, it is of great significance to measure the components and concentrations in sweat to realize the early diagnosis of the disease.
In previous studies, most wearable sensors have focused more on the connection with smart devices. They mainly integrate various data in real-time through technical means and presents human physical parameters such as heart rate [], blood oxygen saturation [], exercise steps [], breathing rate [], blood pressure [], and body temperature []. However, these types of physical monitoring may not fully meet the molecular level requirements of various human health monitoring because they cannot provide the characteristics of related diseases early and establish prevention models.
In recent years, the development of sweat-based wearable devices has enabled the chemical analysis of metabolites such as glucose and lactate, electrolytes such as Na+ and K+, and hormones such as cortisol. It completes the assessment of the overall health of the human body []. The passive monitoring of various physiological parameters is transmitted to medical institutions in real-time, and the corresponding treatment plans are formulated. Notably, introducing the modulation of humidity sensing in a wearable sweat sensor can improve its flexibility and application lifetime []. Therefore, actively promoting the commercialization of sweat-based wearable medical equipment is of practical significance for the tracking of health indicators and predicting disease.
In this paper, first, the sweating behavior, sweat composition, and physiological research analysis are expounded from the physiological point of view. Second, the analysis of specific sweat markers, different types of wearable sweat sensors, and different preparation methods and material properties are discussed in depth. Then, the ideal wearable sweat sensor devices and challenges are discussed in detail. Finally, the sweat wearable sensors are prospected.
2. Physiological Perspective of Sweat Research
2.1. Analysis of Sweating Behavior
As a normal physiological phenomenon, human sweating is a behavior of body temperature regulation, evaporation, and heat dissipation, and a response to emotions. The amount and speed of human sweat are affected by environmental temperature, humidity, and other factors such as the higher the environmental temperature, the faster the sweating speed, and the greater the amount of sweat. The greater the environmental humidity, the more difficult the sweat is to evaporate, and the stimulation of the heat dissipation center is enhanced, and the amount of sweat is relatively increased due to the difficulty in heat transfer [].
Sweat mainly comes from the secretion of sweat glands. There are many sweat glands in each position of the human skin, so there are sweating positions in all parts of the skin of the human body such as the forehead, neck, and waist, back of the hand, limbs, and inside the thigh. The sweat glands of the human body are divided into two types. The first type includes the large sweat glands, which are distributed in the armpit and other locations, and are relatively easy to sweat. The second is the small sweat gland, which is distributed under the epidermis of the skin across the whole-body. The sweating at these positions is during exercise or under high temperature []. Sweat is mainly divided into active sweating and passive sweating. The former is the continuous movement of human muscles, which consumes fat and energy, and takes away waste and heat generated in the process of sweating to maintain body temperature. The latter is caused by sweating due to weather, the environment, and psychological factors. Although sweating is a physiological regulation, a large amount of sweat loss can take away the body’s water and electrolytes, leading to dehydration, thus affecting health [].
2.2. The Physiological Role of Sweat
The first is the body temperature regulation function of perspiration: the small sweat gland, also known as the exocrine gland, is related to the body temperature regulation function, and the main role of perspiration is to avoid excessive body temperature caused by the rise in ambient temperature, resulting in dysfunction of the body. Likewise, when the outside temperature decreases, the perspiration system stops working. The heat production and heat dissipation functions of the human body maintain a dynamic balance under normal circumstances, and 2.43 kJ of vaporization heat is required for each gram of water to evaporate. The evaporation of water inside the body at room temperature is called insensitive evaporation, and sensible evaporation is the phenomenon of sweating caused by the temperature of the outside world in daily life. In contrast, if the heat dissipation balance is broken, it is easy to generate heat and heat storage. Therefore, sweating is a protective response of the human body to adapt to the external high temperature environment.
The second is the protective function of perspiration. As an important accessory organ of the skin, sweat glands secrete sweat, which is also an important component to maintain the skin barrier. Perspiration is a slightly acidic and low-permeability solution, which can be used as the first barrier to protect the skin such as wetting the skin keratinocytes, maintaining the hydration state to prevent drying, and acidification of the skin surface to maintain the acidity of the skin. At the same time, antimicrobial peptides and immunoglobulins also play an immune defense function at the skin interface. In addition, sweat acts as an aqueous phase to emulsify with sebum, creating a sebum film that limits evaporative water loss and becomes an important part of the skin barrier.
Finally, the metabolic excretion of sweat can regulate the acid–base balance and metabolism of the body, discharge protein metabolites or garbage in the body, and maintain the relative stability of the internal environment []. It acts to assist or replace part of the kidney function. The metabolic process of the body can be divided into two categories: one is that carbon dioxide is excreted in a gaseous state, and the other is that part of the metabolites dissolved in water are excreted through perspiration. Therefore, through excretion, water and salt metabolism and acid–base balance in the body can be regulated, and protein metabolites are discharged to maintain the relative stability of the body’s internal environment. Table 1 shows the main related biomarkers and their dynamic concentrations in human sweat.

Table 1.
The common components and relative contents in sweat and related diseases.
2.3. Specimen Collection
The collection and monitoring methods of human tissue components are generally divided into invasive collection and noninvasive collection. Invasive collection, as the name implies, is to use medical-grade instruments to penetrate the skin area to extract quantitative blood samples, drop them on the test paper, and directly measure the chemical composition information of the target substances required in the samples by colorimetry and electrochemical methods. In the field of biochemical analysis, invasive analysis has high credibility. However, the analysis method also has many shortcomings: traumatic exposure to humans increases the likelihood of infection or requires laboratory analysis; biological information at a certain time point can only be obtained for each blood sampling and blood samples cannot be continuously monitored; and chemical information on the determination of glucose is affected by diet and medication []. In contrast, the noninvasive collection of sweat provides an ideal alternative scheme, which can collect biological fluids from the epidermis of patients without stabbing the skin tissue and has certain compliance. Through the conditions of sweating time, rate and volume, the concentration of target biomarkers can be monitored with time [].
Sweat samples obtained from different collection sites of the human body may have a certain impact on the relevant analysis results. For example, when using a sweat collection analysis system, normalizing the flexor surface of either forearm is the preferred site for sweat collection, and the flexor surface of either forearm is the preferred site for sweat collection. However, if the forearm cannot be used, the upper arm, thigh, or calf can also be used [,]. The selection of the collection site should be positively correlated with the distribution of hair, sweat glands, and whether it is flat and easy to collect. In general, sweat from the hands and feet is rarely used as a test specimen, even though they have many sweat glands. The reason for consideration is that there is more contact with the outside world, and the existence of more pollutants will lead to the contamination of the specimen. In addition, when the sweat samples are collected, the strict transport and storage method is to use a dry ice incubator and then place them in a freezer at −80 °C for freezing. The storage conditions of the experiment will be adjusted according to different experimental purposes and detection methods.
The collection of sweat can be divided into systemic collection and local collection. The former is to wash the whole body after exercise or to collect sweat samples from clothes soaked in sweat, while the latter mainly uses filter paper, gauze, sponge pads, arm bags, etc. to collect the sweat secreted from specific parts such as chest and arms. In addition, the induction methods of sweat currently mainly include the method of pilocarpine iontophoresis, thermal stimulation and exercise, and sweat patch. Among them, the most widely used and mature sweat induction method at this stage is the pilocarpine iontophoresis method, which mainly uses the Macroduct sweat stimulation and collection system to collect samples []. The sweat inducer adopts bilateral stimulation collection and analysis, with two electrodes rich in pilocarpine ion gel in contact with the skin of the forearm, and a stimulation current of 1.5–4 mA is applied for a stimulation time of 5 min. During this process, care needs to be taken to use a fail-safe circuit for battery power and regular testing. Sweat was collected from each subject using a Wescor Macroduct collector at the positive site after sweat stimulation for about 30 min after the electrodes were removed. Generally speaking, the average sweat rate of sweat should be greater than or equal to 1 g·m−2·min−1 or the minimum volume is greater than or equal to 15 μL. Furthermore, even if the sweat sample is not sufficiently collected, further analysis should not be performed. Similarly, samples collected multiple times cannot be used collectively.
Second, thermal stimulation and exercise are also commonly used sweat induction methods. In this process, the collection methods of sweat included direct suction with a pipette, glass roller collection, etc., and indirect methods included the use of cotton paper, filter paper, gauze, etc. to absorb and then extract and separate sweat. In the early days, the perspiration collection devices mainly existed in the form of cotton, gauze, or towel closed belts, which consisted of one to three layers of filter paper, or a closed belt composed of several pieces of filter paper []. However, the disadvantage of this kind of patch is that it is cumbersome to operate, easy to fall off, and has a poor experience. At the same time, it may change the skin homeostasis and cause irritation, and it enables only a small amount of sweat to be analyzed. With its development, its application range is now also wider, and different sweat patches have different compositions. In general, it consists primarily of an outer transparent surgical waterproof dressing, a sweat-absorbing pad such as a sterile gauze block [] or a detection probe [], and an adhesive layer that is integrally attached to the radial region of the arm. The dressing film is a semi-permeable membrane that allows substances such as oxygen, carbon dioxide, and water molecules to pass through, and non-volatile substances in the air cannot pass through. It should be noted that the skin in the collection area also needs to be cleaned before the collection, and the collection time can vary from 1 days to 7 days. After the collection is completed, centrifugation is required to separate the sweat, or a non-sticky cavity is formed in the center of the waterproof dressing, and a syringe is used to extract the pooled sweat [].
2.4. Sweat Metabolomics
Sweat is rich in protein and peptide sources, and also contains a variety of cytokines, which have the potential to uncover disease biomarkers. At present, relevant research results have confirmed that there are differences in the composition of sweat in various diseases. Sweat metabolomic analysis is expected to further optimize disease screening, diagnosis, and monitoring methods through the application of new technologies and the formulation of personalized diagnosis and treatment methods. The complexity of target analytes in sweat determines the complexity of sweat analysis equipment, inevitably requiring metabolo-proteomic analysis utilizing high-throughput techniques [].
The Wescor Macroduct System (MCS) above-mentioned is a typical device that combines sweat sampling and analysis. It requires less sweat sample volume and can combine sweat sampling with osmotic pressure and conductivity measurements to maximize the minimal errors. In addition, the Nanoduct sweat test system, which requires less sweat volume and provides reliable results, is very popular in the field of newborn screening []. This is followed by general-purpose analytical equipment liquid chromatography (LC) and capillary electrophoresis (CE) for high-resolution separations, which often need to be coupled with mass spectrometers (MS) for the analysis of drugs or proteins in sweat and complex metabolites were analyzed. It is also possible to combine LC with time-of-flight mass spectrometers, ion traps, and triple quadrupoles, which are common in metabolomic studies using sweat as a clinical sample. The method enables qualitative differences between the control and disease groups in the form of a Venn diagram by comparing the results obtained in the sweat of healthy people with those from diagnosed individuals, in which many different proteins and peptides can be found. Abundance visualization was used to investigate the origin of unique disease-relevant biomolecules.
Additionally, gas chromatography (GC) can be used to analyze the volatile organic compounds (VOCs) present in sweat. Gas chromatography coupled with mass spectrometry (GC-MS) [] can also be used to analyze the assessment of cancer biomarkers in sweat and to distinguish the differences in sweat volatiles between different populations. Meanwhile, nuclear magnetic resonance spectroscopy (NMR) [] also provides a fast and highly reproducible platform for sweat metabolome analysis, but the main disadvantage of this technique is its lower sensitivity compared to MS.
Sweat is mainly composed of abundant electrolytes, metabolites, proteins, and some hormones including a variety of cytokines. Since sweat contains a small number of impurities, it is not easy to be polluted. Continuous monitoring of the observation indicators can be realized on a noninvasive basis. There is growing interest in using sweat analysis as an anchor for disease biomarker discovery, which means that it has the potential to explore disease biomarkers. At present, many relevant research results have confirmed that there are differences in sweat components in various disease samples. Therefore, it is expected that through the analysis of sweat metabolomics, the formulation of personalized diagnosis and treatment methods will be realized, and the methods of disease screening, diagnosis, and monitoring will be optimized []. The current trend in the application of sweat specimen analysis technology focuses on the metabolomic analysis of sweat in order to discover relevant biomarkers that can characterize disease. There have been studies on cystic fibrosis (CF) [], Koyanagi–Harada disease (VKH) [], atopic dermatitis (AD) [], and other diseases.
The most mature diagnosis at this stage is the research on cystic fibrosis. CF is caused by a mutation in the gene encoding CFTR (cystic fibrosis transmembrane conductance regulator), resulting in disturbed chloride levels in the patient’s sweat. It causes lung disease, pancreatic and liver insufficiency, loss of electrolyte ions, and male infertility. Sodium or sodium/chloride ratios in sweat can provide useful information and thus sweat chloride ions can be used as biomarkers for cystic fibrosis diagnosis. The conventional diagnostic methods for CF currently mainly use pilocarpine ions into the skin to stimulate the secretion of sweat for collection and analysis []. Regarding the measurement of chloride concentration in sweat, identification is mainly performed by the coulometric titration of chloride or by colorimetry. In general, sweat chloride concentrations greater than 60 mmol/L are considered positive [].
In addition, VKH is a syndrome that affects the eyes, ears, skin, hair, and meninges. The study found significant differences in the proteins and metabolites in the sweat of VKH patients, revealing the important role of amino acid metabolic pathways in the pathogenesis of Koyanagi–Harada disease []. Moreover, AD is one of the most common inflammatory skin diseases, and some AD patients have sweat secretion disorders []. Studies have found differences in the sweat protein composition, antimicrobial peptides, and glucose concentrations in AD patients. The glucose concentration in the sweat of patients with acute AD increased more significantly than that of healthy people and patients with chronic AD. This suggests that glucose may be a biomarker associated with disease severity [].
Currently, high-throughput proteomics and metabolomics provide valuable information on key biomolecules in sweat, which is helpful for the study of disease progression and accelerates the interpretation of the pathophysiological process and pathogenesis of various diseases [].
3. Composition Analysis of Sweat Wearable Sensor
The health parameters detected by sweat are almost as rich as those detected by blood, which hides a lot of human health information. Human sweat contains a variety of substances such as glucose, lactic acid, electrolyte ions, cortisol, vitamins, ethanol, drugs, water, hormones, proteins, polypeptides, and other secretions. Through the monitoring of the sweat composition, people can analyze the electrolyte imbalance degree, lactic acid index, dehydration status, potential diseases, calorie burning value, and then analyze the vital signs of the human body []. Table 2 lists typical examples of these types of sensors, showing that the type of analyte can be detected using different sensing platforms. Table 3 compares the types of sensors, substrate materials, component materials, detection range, detection limit, sensitivity, and related application details, followed by the above references.

Table 2.
The types of sweat wearable sensors and the related analytes in the review.

Table 3.
The details of the wearable sweat sensors in this review.
3.1. Glucose
Glucose is one of the most important biological compounds in human blood and participates in many human reactions. The blood glucose level of healthy people should be maintained at 3.9–6.1 mM. A glucose concentration outside this range will lead to renal dysfunction and other diseases []. For example, diabetes is a chronic disease caused by insufficient insulin, which can seriously threaten human life and quality of life. With many serious complications, the blood glucose level needs to be continuously controlled. Glucose detection is of great significance in the fields of diabetes diagnosis and blood glucose monitoring.
Sweat can replace blood samples for the noninvasive assessment of glucose levels in vivo. Zhao et al. [] developed a gold fiber-based three-electrode electrochemical biosensor platform for the detection of glucose content in wearable fabrics. The sensitivity of the textile glucose biosensor was 11.7 μA·mM−1·cm−2, and the linear range was 0–500 μM. Furthermore, the textile glucose biosensor showed a stable amperometric response in 8 days of storage. Due to the selective oxidation of glucose oxidase and the low potential assisted by Prussian blue, the sensor showed a strong response to glucose detection, even if other interfering substances caused some slight electrochemical response fluctuations. The combination of intrinsic ductility and the external spiral structure of the highly conductive gold fiber makes the sensor maintain an excellent sensing performance at a strain of 0–200%. The feasibility of the sensor is manifested in the high tensile state and high selectivity. The glucose level in perspiration before and after food intake is monitored by observing the amperometric current level. Furthermore, this type of sensor can play a role in artificial sweat under high tension and has the potential for real-time monitoring and diagnosis in wearable areas. Previous research work on sensory sensors has focused on monitoring physical parameters such as pressure and strain associated with human activity. The high-performance fiber-shaped wearable sensors studied by the authors have important implications for the real-time and extra-clinical health monitoring of next-generation smart textiles and enables the enzyme-based noninvasive monitoring of health information such as sweat glucose levels.
Xiao et al. [] developed a wearable device based on a microfluidic chip for colorimetric analysis and the detection of glucose in sweat. The linear range of detection was 0.1–0.5 mM, and the detection limit was 0.03 mM. There was also a significant difference in the content of glucose in sweat between fasting and satiety subjects. The colorimetric wearable sensor could reveal subtle differences about the glucose concentrations in sweat. However, in the colorimetric analysis of perspiration glucose, it is necessary to take into account the potential chemical harm caused by the contact of colorimetric reagents and reaction products with the skin during sampling and perception [,]. The microfluidic-based wearable device studied by the authors was designed to keep the sampling process sealed to avoid evaporation and contamination of the sweat samples. Microfluidic chips can be integrated with check valves to prevent the backflow of chemicals in the microchambers. The sensitivity of the oxidative colorimetric reaction of biological enzymes is sufficient for the colorimetric detection of glucose in low concentration sweat. The problems of sweat sampling, in general, are the sweat volume, evaporation, and contamination details. The avoidance of colorimetric reagents and reaction products in contact with the skin should also be considered to avoid any risk of potential chemical damage to the skin during sampling and sensing.
Poletti et al. [] believe that the realization of a real wearable biosensor requires a driving force to ensure the continuous transport of sweat rather than any complex external equipment. The bioenzyme-based biosensors were prepared by functionalizing glucose oxidase and lactate oxidase in the graphene oxide and chitosan composites. The sensing signals remained linear and stable within 2 h, which are suitable for analysis in real samples. The detection limits were 32 nM and 68 nM for glucose and lactate, respectively, which can avoid the interference of other substances in complex matrices. In addition, driving forces in wearable biosensors regarding sweat are required to ensure the continuity of sweat delivery. Therefore, the highlight of the authors’ research is the use of continuous capillary flow to renew the sample on an electrochemical platform for continuous and stable sensing. In practical applications, the device is embedded in nitrocellulose strips integrated in the capillary flow to enable the collection and transport of sweat over a sensor platform. The sensing signal of the sensor is continuous, stable, and linear within two hours, which is suitable for the monitoring of glucose and lactate in human sweat at the same time.
Wang et al. [] successfully prepared a sensitive PET-based glucose sensor by casting Prussian blue and glucose oxidase on a gold electrode. It can successfully resist the interference of lactic acid, urea, paracetamol, dopamine, and ascorbic acid. The glucose sensor had a good sensitivity of 22.05 μA·mM−1·cm−2, linear detection range of 0.02–1.11 mM, and a low detection limit of 2.7 μM. Considering the flexibility of the substrate, the sensor can be used as a wearable device for the real-time detection of glucose in sweat. The overall performance of the sensor designed by the authors showed excellent sensitivity, linear range, detection limit, selectivity, reproducibility, and long-term stability to interfering substances.
Zhao et al. [] successfully prepared a portable enzyme-free electrochemical sensing device by synthesizing AuNPs@CuONWs/Cu2O/CF hierarchical nanostructures based on polydimethylsiloxane as a sensing electrode, integrated circuit elements, and smart mobile devices. The sensor had excellent electrocatalytic detection performance for glucose, the sensitivity was 1.619 μA·μM−1·cm−2, the linear detection range was 2.8–2000 μM, and the detection limit was 0.9 μM. In addition, the portable sensor could also detect the change trend of the glucose content in sweat, which is expected to meet the needs of family medical care, field monitoring, and rapid detection in the actual environment. Numerous studies have shown that combining in situ synthesis, morphology regulation, and optimized inert metal modification is an effective method to develop high-performance electrocatalytically active enzyme-free glucose sensors, which can simultaneously achieve synergistic effects in the determination of glucose. The authors designed a sensor based on hierarchical nanostructures to effectively utilize the above method to achieve high-sensitivity glucose measurements based on enzyme-free catalysis, with superior anti-interference and stability.
Yoon et al. [] successfully prepared a wearable sensor based on glucose detection in perspiration. The porous laser-induced graphene (LIG) achieved the purpose of surface modification by acetic acid impregnation, successfully increasing the proportion of carbon–carbon bonds, effectively increasing the surface conductivity, reducing the resistance and hydrophobicity, improving the intrinsic electrical properties, and ensuring the stable and uniform diffusion of platinum nanoparticles in the LIG layer. The sensitivity of the sensor was 4.622 μA/mM, the detection limit was less than 300 nM, and the dynamic linear range was up to 2.1 mM, which could successfully verify the change in the glucose level before and after meals, showing unique commercial potential and practical feasibility. The authors’ study was characterized by the use of the acetic acid treatment of laser-induced LIG surface modification to improve the defect of its own low electrical conductivity, and to achieve a fine and uniform distribution of catalytic metal nanoparticles and higher sensitivity. This method is very useful for large-scale production and effectively avoids nanoparticles that might cause aggregation during electrodeposition, effectively reducing sheet resistance. We believe that the increased content ratio of carbon–carbon bonds contributes greatly to the electroplating state, which improves the conductivity and the low detection limit due to high charge.
Yu et al. [] prepared a flexible sweat sensor based on a gold nanoarray by the electrochemical deposition method, which could detect the glucose and lactate content in actual sweat samples. Among them, the gold nanoprobe could successfully amplify the signal and increase the specific surface area. Polyethylene glycol diglycidyl ether (PEGDE) could successfully immobilize biological enzymes and retain the activity on the chip surface. The detection limits of the prepared sweat biosensor for glucose and lactic acid were 7 μmol·L−1 and 54 μmol·L−1, respectively, with good reproducibility, long-term stability, and selectivity. This can be used as a wearable skin sensing device to verify the value of the determination of glucose and lactate in human sweat samples. The authors constructed a flexible sweat sensor programmed with gold nano-pine needles for the real-time monitoring of glucose and lactate in sweat at the micromolar and millimolar levels. The growth of pine needles by electrochemical deposition could effectively improve the electrochemical signal. Furthermore, its presence in the deposition increased the surface-to-volume ratio, allowing for more enzyme immobilization. Thus, the detection sensitivity was improved, and the detection limit reached the micromolar level and the millimolar level, respectively.
At present, wearable sensors for glucose monitoring in sweat are mainly divided into two trends based on enzymatic and non-enzymatic applications. Although enzyme-based sensors have the advantage of high sensitivity and specificity, they are susceptible to limitations such as temperature, range, and humidity. The sensing mechanism of non-enzymatic sensors is that glucose is directly oxidized on the electrode material. Therefore, electrode sensing materials are one of the key components, which largely determine the performance and potential practical applications of such sensors. For sensors, it is also a challenge to develop materials with high selectivity, low detection limit, wide detection range, and fast response time. Nano-sensing materials have high specific surface area to improve the sensitivity of the sensor, and good biocompatibility can improve the long-term stability of the sensor by enzymatic activity. In addition, features such as high porosity provide the basis for enzyme immobilization and enhance glucose catalysis.
Moreover, the excellent flexibility of the sensor is also important, which can be exploited by the inherent stretchability of multidimensional nanomaterials, which is crucial for flexible wearable devices. The next research plan can continue to introduce nanomaterials with unique structures and properties to achieve the development of low-volume, low-concentration portable wearable devices. Nanostructured metal oxides can provide a larger surface area for glucose oxidation, and the high electrical conductivity of carbon nanotubes and graphene can also improve the electron transport and sensing sensitivity. Compared with traditional electrochemical detection methods, the emerging spintronic detection technology has the advantages of more efficient, faster, and less power consumption. These supports can provide potential methods to fabricate biosensors with excellent performance. Although the research on sweat-wearable glucose sensors is developing rapidly, there is still room for development in medical, fitness, and military applications.
3.2. Lactic Acid
The lactic acid in sweat is the energy metabolism in the secretory gland, and the increase in exercise intensity will lead to the increase in lactic acid content in sweat. Appropriate sweating can be used for individual function analysis without traumatic blood collection. The imbalance of lactic acid production and clearance may lead to lactic acidosis, and more seriously, anaerobic metabolism may lead to hemorrhagic shock. Therefore, lactic acid can be used as a sensitive marker of tissue cells, providing early warning for ischemia, and reflecting insufficient oxidative metabolism and tissue damage [].
Lactic acid can be used as a biomarker for tissue oxidation to evaluate the physical performance of a human body. Zhang et al. [] prepared lactic acid molecularly imprinted polymers (MIPs) through the electropolymerization of 3-aminophenylboronic acid (3-APBA) and lactic acid imprinted electropolymerization on the carbon working electrode to detect lactic acid in sweat. On the flexible substrate of polyethylene terephthalate, a silver nanowire (AgNW)-based epidermal electrochemical biosensor (MIPs-AgNWS) was established by screen printing technology, which was used for the noninvasive monitoring of lactic acid in the sweat produced by human movement. The biosensor showed a stable electrochemical response after 200 bending and twisting cycles. The measurement by pulse voltammetry showed that the monitoring range of lactic acid was 10−6 M−0.1 M, and the detection limit was 0.22 μM. The sensitivity of the biosensor remained at 99.8% ± 1.7% after 7 months of storage at room temperature and dark conditions, which was beneficial to the health care and physiological monitoring of the athletes and soldiers. The enzymatic lactate wearable sensor utilizes an enzymatic reaction to catalyze lactate to obtain an electrochemical response, but the activity of the enzyme is easily affected by temperature, pH, and ionic strength. Enzymes may be degraded due to improper storage and use in the long-term, affecting the sensitivity and long-term use of the sensor. In contrast, molecularly imprinted polymers have the advantages of low cost, good reproducibility, and high stability, but few works on wearable MIPs have been reported. The authors’ research idea was to prepare flexible electrochemical biosensors based on AgNW MIPs, providing a sustainable noninvasive method for monitoring sweat lactate metabolites, avoiding the use of biological enzymes. The device usage results showed a high electrochemical stability as well as high sensitivity and specificity detection.
Under constant pressure, blood vessels are partially or completely occluded, and tissues in the body cause malnutrition and cause skin or deep tissue necrosis. A large amount of lactic acid will be produced when the oxyphiles in the blood cannot meet the metabolic needs and turn to anaerobic metabolism, so stress ischemia has a significant impact on health []. Tur-García et al. [] developed an enzyme-based highly flexible biosensor designed for real-time and noninvasive monitoring of lactic acid in human sweat to detect the occurrence of pressure ischemia in the early stage. The selective working range of lactic acid displayed by the sensor was 0–70 mM, covering the physiological concentration related to pressure ischemia. Through the vigorous exercise test of volunteers, the results showed that the sensor system could detect the fluctuation of lactic acid level produced by sweat glands under low oxygen and load conditions. The authors constructed flexible electrodes based on sputter-coated polycarbonate films, constituting a highly flexible laminate, for lactate sensing monitoring of passively expressed eccrine sweat without the use of redox molecules. This was capable of measuring the lactate stress ischemia in the physiological range and is suitable for the detection of low volume sweat. In the interference test, no significant interference was observed in synthetic sweat, which can be measured directly in diluted human sweat. Furthermore, we suggest that there are still some challenges to be addressed, for example, the effects of changes in the dielectric strength, pH, and the electrical conductivity of biological fluids.
Lin et al. [] successfully prepared a low-volume (<5 μL) sweat lactate electrochemical sensor based on illegal charge transfer in a flexible substrate electrode system by integrating graphene oxide nanosheets with immobilized lactate oxidase. The detection limit of the affinity sensor was 1 mM, and the upper limit of the detection range was as high as 138.6 mM within 45 min. The working range of the sensor was matched with the physical expression range of lactic acid concentration in the human sweat samples, and the correlation was as high as 0.955. Therefore, it is suitable for the continuous detection of high concentration lactic acid in low-volume sweat samples, but the influence of the pH value, current intensity, and other factors on the sensing response needs to be considered. The highlight of this author’s research was the construction of a low-volume monitoring sweat lactate sensor, which utilized Faradaic electron-ion charge transfer to achieve sensitive impedance detection. This was used for real-time, continuous, and noninvasive detection of lactate expressed in passively secreted sweat, enabling the monitoring of sweat lactate levels under stress ischemia in humans.
Wang et al. [] successfully prepared an enzyme-free lactic acid biosensor by synthesizing the transition metal layered hydroxide NiCo LDH, a derivative of ZIF-67, as a catalyst. The material showed uniform pores, a large specific surface area, and a hierarchical layered structure in structure, and the sensitivity of the sensor reached 83.98 μA·mM−1·cm−2 in the range of 2–26 mM lactic acid concentration. Thus, the ZIF-67 derivative biosensor can realize noninvasive monitoring of lactic acid in human sweat. This is of great significance in anaerobic exercise and aerobic exercise. The authors proposed a ZIF-67-derived NiCo LDH with a three-dimensional layered structure as a non-enzymatic electrocatalyst for lactate oxidation. The layered structure had a large area and uniform porosity for lactate oxidation. The addition of NiCo LDH provides additional electrocatalytically active sites, thus providing a shortcut for charge transfer. We believe that this study provides a potential application for wearable bioelectronics for noninvasive human sweat monitoring utilizing a low-cost, high-efficiency enzyme-free electrochemical catalyst with a reliable and stable performance.
Shitanda et al. [] successfully prepared a screen-printed lactic acid sensor by the grafting polymerization of MgO template carbon, and the covalent fixation of lactate oxidase and 1,2-naphthoquinone. The sensor had a detection range of up to 50 mM and a detection limit of 0.3 mM, which is suitable for the process from anaerobic to aerobic metabolism. Continuous monitoring of lactate biomarkers in physical training and related sports has great practical significance. The author’s research idea was to use MgO as a template for mesoporous carbon materials, graft polymerized lactate oxidase and functionalized polymer materials, and then screen-print after covalent bonding. This flexible electrode fabrication method can achieve mass production. The high specific surface area of the material increases the effective surface area of the electrodes, which in turn leads to biosensors with high response currents, enabling continuously monitored responses for sweat lactate sensing. Additionally, the sensor is lightweight and fits comfortably on the skin for real-time lactate threshold detection.
At present, many sweat-based lactic acid-based wearable monitoring sensors have been developed. The detection methods of different types of the designed sensing platforms are also different, and common methods include colorimetry, voltammetry, enzymatic methods, and chromatography. Among them, colorimetry and voltammetry are relatively simple in operation and low in cost, but colorimetry has a high detection accuracy error, low specificity, and sensitivity. In addition, the disadvantages of the enzymatic method are high cost, cumbersome operation, time-consuming sample preparation, etc., but the enzymatic method and the chromatographic method have specific detection performance, high sensitivity, and reliable and accurate detection results. Therefore, the introduction of a biosensing method for the determination of lactate in sweat can be considered because the method is very simple, sensitive, and selective, uses complete automation, and provides fast results. In addition, a lot of current research is to improve the sensing performance of sensors by designing nanomaterials with special structures or introducing lactase. Here, we introduce the concept of enzyme nanoparticles, which are aggregates of nanoscale enzyme molecules with excellent electronic, mechanical, optical, and thermal properties. The following research can focus on the use of enzyme nanoparticles to construct biosensors based on sweat-based lactate detection methods, perfecting the development of simpler, more accurate, reliable, and low-cost sensing platforms.
3.3. Electrolyte
Electrolyte abnormalities can lead to acidosis, renal failure, and other high mortality and morbidity diseases, and the sweating rate and electrolyte concentration vary from person to person. Real-time electrolyte and acid–base parameters are particularly important for the human body []. Sweat contains many inorganic ions such as Na+, K+, Cl−, etc. These components more or less reflect the metabolic process of human organs. The purpose of early diagnosis can be achieved by analyzing the concentration of specific components. For example, by monitoring the content of Na+, we can see the loss of electrolytes in the process of ultra-endurance exercise, and prompt patients to urgently supplement their electrolytes to prevent the reduction in physical exercise function due to excessive sweating. The irregular concentration of Na+ in sweat has clinical significance in the diagnosis of cystic fibrosis []. Na+ and K+ in sweat provide the basis for maintaining cell infiltration and balance.
Rose et al. [] allowed for the integration of sweat sensing components such as low-cost and highly self-sufficient ion selective sensing electrodes on a standard copper/polyimide flexible substrate by electroplating and prepared a kind of adhesive radio frequency identification sensor bandage, which had significant performance advantages in monitoring the performance of biomarkers in sweat. The dynamic range from 20 mM–70 mM to 235 mV–255 mV was realized in the transmission from the solution to the intelligent equipment by using an RFID minimum chip component. Furthermore, the potential sensing accuracy of Na+ and surface temperature in sweat was 96% and the sensitivity was 0.3 mV/mM. The wear resistance of the patch could be maintained for 7 days. The wearable RFID patch sensor adopted by the authors conformed to the skin better, minimized the problem of sweat trapping, and avoided the lag response of the sensor. It also had obvious advantages for continuously monitoring the dynamic changes of different biomarkers in sweat in chronological order. In addition, according to the author’s research progress, it is suggested that the hydration monitoring of Cl−, K+, Mg2+, NH4+, and Zn2+ plasma in sweat can be expanded to realize the monitoring of electrolytes in human sweat to a greater extent.
Choi et al. [] successfully prepared a salt bridge integrated sweat chloride monitoring electrochemical sensor. When the chloride ion concentration was lower than 10 mM and higher than 150 mM, the measurement deviations were less than 2 mM and 5 mM, respectively. A small concentration drift below 4 mM was achieved in 12 h. The test sample volume was only 110 μL, and the concentration range covered healthy people and sick people. Considering the influence of exercise intensity on chloride ion concentration in the human body, the sensor had the influence of wearable monitoring. One challenge in wearable potentiometric sensors is that the reference and test solutions can cause measurement errors over time. The innovation of the authors’ study lies in the integration of the presence of salt bridges in the sensing platform, which can minimize measurement errors due to non-equilibrium concentration changes. Even if the concentration is small, the drift of the sensor is small, but it cannot continuously monitor the chloride ions in sweat. The detection range of the sensor spans healthy people and cystic fibrosis patients, with potential for health monitoring applications.
Alizadeh et al. [] prepared a wireless wearable sweat sensing device that was suitable for the continuous monitoring of electrolytes in medium-intensity motion and could be used as a measurement standard for the hydration state. The sensitivities of Na+ and K+ were 55.7 mV and 53.9 mV per logarithm, respectively. The device can be used to monitor electrolytes during high-intensity perspiration by predictive analysis. The authors’ research direction was to design a fluid system to adapt to the operating conditions of sweat extraction, minimize sensor lag, and quickly remove sweat from the sensing site. Second, the microfluidic system was integrated with electronic component signal processing algorithms to determine the stability of monitoring with low noise. To further improve its reliability in practical operation and increase the shelf life and stability of the potentiometric sensor, we propose performing extensive exercise tests to evaluate the correlation of sweat volume to the electrolyte concentration and hydration state at different levels.
Pirovano et al. [] used poly(3,4-ethylenedioxythiophene) (PEDOT) and poly(3-octylthiophene-2,5-diyl) (POT) as conductive polymers and used solid-state ion sensitive electrodes (ISEs) to prepare wearable sensors based on the monitoring of sodium and potassium ions in sweat. The sensor showed that the sensitivity of PEDOT to the sodium and potassium ions was 52.4 ± 6.3 mV/dec and 45.7 ± 7.4 mV/dec, respectively. The sensitivity of POT to the sodium and potassium ions was 56.4 ± 2.2 mV/dec and 54.3 ± 1.5 mV/dec, respectively. In addition, the cycling experiment of athletes showed that the dynamic ranges of sodium and potassium ion concentrations in 90 min were 1.89 mM–2.97 mM and 3.31 mM–7.25 mM, respectively, which could be used to measure the electrolytes in the sweat of high-quality athletes. However, it is inevitable to consider more verification of the response mode obtained by electrolyte changes. In this study by the authors, the SwEatch platform was extended for the analysis of multiple electrolytes, and the sensing platform had dual channels for direct electrolyte continuous monitoring and resistance to interference. In addition, the dual-mirror fluidic unit could draw sweat from the skin through passive capillary action and bring it into contact with two independent electrodes, and the potential signal was measured by an integrated electronic board and transmitted to a laptop via digitized Bluetooth. However, it was also suggested that validation of the response patterns obtained for the electrolyte changes, showing similar trends, is needed to determine utility.
Mazzaracchio et al. [] prepared a screen-printed electrochemical sensor based on carbon black nanomaterials. The detection range of the sodium ions was 10−4 M−1 M, the sensitivity was 58 ± 3 mV/dec, and the detection limit was 63 μM. This can be used to detect the content of sodium ions in actual sweat samples. In addition, they also developed the application of a carbon black modified screen-printing electrode in the field of wearable devices. This can be considered to further improve the stable transmission of sensing signals in the configuration of screen-printing and develop the application of screen-printing wearable sensors. This is the first time that the authors reported the use of carbon black modified screen-printed electrodes for the potentiometric detection of sodium ions in sweat. Among them, it is considered that due to the high surface area, good dispersibility, and abundant defect sites of carbon black, it provides advances in the electrochemical properties in reducing the applied potential, resisting contamination, and improving the sensitivity to several analytes such as ascorbic acid and hydrogen peroxide. The sensing platform had good stability and shelf life and was resistant to interference from oxygen and light. Compared to the study by Pirovano et al., the detection range was wider, and the sensitivity was further improved, but the dynamic changes of sodium ions could not be continuously monitored. This study opens up further applications of screen-printed electrodes decorated with carbon black in potentiometric measurements, which can be further applied in the field of wearable devices.
Currently, in contrast to biosensing, a wide range of signaling methods for wearable systems for electrolyte monitoring in sweat include electrochemistry, optical contrast, and colorimetry. Among them, although the colorimetric system is simple to make, the disadvantages re that the sampling frequency is low and the resolution of the sensor is low. Therefore, it is not necessary and not the primary choice for the sensing device. The advantage of electrochemical sensing such as signal processing is that it is simpler than optical systems. In addition, microfluidic devices have been increasingly used for the determination of electrolytes in sweat in recent years. In the future development of this equipment, we make several suggestions here. The first is to eliminate the interference of impurity ions and realize the selective monitoring of the target sample to improve the detection sensitivity. Second, analytes can be chelated to achieve specific target electrolyte ions. In addition, the detected signal needs to be converted into a readable signal to realize the signal acquisition of the mobile device. Combining the ultrasensitive detection mechanism of microfluidic devices and designing inexpensive chips will facilitate the development of sweat electrolyte analysis in microfluidic wearable devices.
3.4. pH
The pH value of the normal human body is slightly acidic, about 3.0–8.0. However, the increase in pH will cause some health problems such as dermatitis and fungal infections. Therefore, excessive changes in pH play an important role in the health mechanism, and the pH value of sweat is related to the hydration state of the body [].
The acid–base balance is crucial in biological processes. Monitoring pH is helpful to understand our physiological and pathological conditions, and its content is directly related to Na+ concentration, indicating human dehydration []. Promphe et al. [] prepared a noninvasive fabric colorimetric sensor for the simultaneous detection of sweat pH and lactic acid by depositing chitosan, sodium carboxymethyl cellulose, indicator dyes, or lactic acid on cotton fabrics. The changes can be distinguished by the naked eye or a spectrophotometer, with the increase in pH, the color changes from red to blue, and the lactic acid changes from purple with the increase in concentration. Through experimental comparison, the platform could be used to determine the pH range of 1–14 and the lactic acid level of 0–25 mM. In addition, it could distinguish the movement of the main body and potential muscle fatigue to achieve continuous real-time monitoring. The authors’ research strategy was to achieve continuous monitoring of the pH and lactate in sweat by an indicator dye and the lactate enzymatic method, which have the advantages of easy observation and signal acquisition. In addition, the addition of CTAB prevents the color fading of this sensor indication. With the help of textile features, the sensing platform can be the next step to integrate wearable devices with bed sheets, pajamas, shirts, tights, wristbands, headbands, and other items for the real-time monitoring of human health and sports performance in a noninvasive way. Compared with the sensor strategies of other authors introduced in this section, this noninvasive fabric colorimetric sensor achieved the widest detection range of pH. However, there is a disadvantage in that it cannot be continuously monitored. Once the indicator color is measured and changed, it cannot return to the original state. The next step is to consider the reuse of the sensing element through redox, starting from the indicator dye.
Mazzaracchio et al. [] prepared flexible wireless wearable sensor equipment for the epidermis based on a polyimide film by using electrochemical deposition iridium oxide screen printing technology to provide a pH sensitive layer and used cotton-based materials as the micro-fluid transmission channel. The pH data of the sweat samples could be wirelessly transmitted to obtain a longer reading range (2 m) than the NFC. It had no effect on the response of common interfering ions in sweat such as the Na+, K+, and Cl− sensors, and is suitable for the large-scale automatic monitoring of athletes and household users. The author’s research feature lies in the use of printing technology to construct an electronic base layer and the electrochemical deposition of biocompatible sensing materials. Among them, the low volume of bright sweat can directly contact the electrode through the cotton material, avoiding the effect of the external pump of the microfluidic. Structurally, an integral module is formed, and there is no need to connect to each other in other ways such as wires, resulting in a low-cost requirement. In addition, the integrated longer read range and better power autonomy of the RFID platform can be used in a real-time measurement mode in a completely battery-free situation.
Yoon et al. [] successfully prepared a pH cable sensor with high sensitivity and high flexibility by coating a self-healing polymer SHP on a carbon fiber wire electrode, which can be woven into the headband to achieve a wearable effect. The sensor verified the spontaneous self-healing efficiency of the sensor through the cutting and self-healing cycle. The pH measurement range of the sensor was 4.73–8.02 and the sensitivity was 58.7 mV/pH. The self-healing efficiency reached 97.8% within 30 s, and it had the mechanical tensile properties of 3.5 MPa and tensile toughness of 9.9 MJ·m−3. The actual test is similar to that of a pH meter, which can measure the low-volume sweat sample, and can be used for the real-time noninvasive monitoring of the pH value of sweat during cycling. The author’s research feature was the development of a cable-type flexible and repairable sensing platform that met the characteristics of portable limited use and mechanical flexibility and robustness. This guarantees the stability and reliability of the equipment, avoids the equipment being damaged by overuse, scratches, and cuts during its service life, and can measure the pH value of acidic or alkaline sweat continuously, accurately, and quickly. Considering the next work, the wireless electronic circuit board can be integrated to improve the device to further realize noninvasive diagnosis and health care monitoring applications in continuous real-time monitoring during indoor exercise.
Dang et al. [] used a graphite/polyurethane composite as the sensing electrode and prepared a wireless scalable potentiometric sweat pH monitoring device. The data were transmitted to intelligent mobile devices through a stretchable radio frequency identification antenna. The sensitivity of the sensor to pH was 11.13 ± 5.8 mV/pH, and the maximum response time was 8 s. The sensor had a response characteristic to pH in the range of 5–9. The study of sodium ions, potassium ions, and glucose interference showed that their influence were negligible. This sensor can also be extended to monitor the exudate fluid at the wound and construct a multi-biological fluid network. Considering the current biosensors based on graphite–polyurethane composites, the sensing mechanism is usually based on the electrochemical oxidation reaction between the analyte and the composite. Therefore, the authors featured a unique combination of novel graphite–polyurethane composites to form an electric double layer, and proposed a stretchable sensing system with the same detection range as the sensing strategy designed by Yoon et al. In the interference test, the major ions and glucose in sweat were tested and the results showed negligible interference. This research can also be extended to wearable system smart bandages to form multi-biofluid solutions.
Mazzara et al. [] prepared a pH electrochemical sensor based on polyaniline using indium tin oxide coated polyethylene terephthalate as the flexible substrate. A polyaniline film was deposited at constant potential, and graphene oxide was modified to construct a bilayer electrode. The ITO-PET/rGO/PANI sensor had a sensitivity of 62.3 mV/pH and a repeatability of 3.8%, which can be used to test the biofluids in the range of pH 2–8. The pH measurement results of the real sweat samples were consistent, which confirms the possibility of the quantitative determination of pH in real sweat samples. The characteristic of the author’s construction strategy was to avoid the complicated steps of chemical synthesis or the in situ polymerization of rGo and PANI, but to adopt a simple layer-by-layer deposition to obtain a double-layer electrode, which saved most of the steps in the work. In terms of material design, utilizing the excellent electrocatalytic properties of rGO ensures enhanced sensitivity, the thin films of electrochemically deposited PANI are easy to scale, and the physicochemical properties allow for control. In terms of performance, the sensing platform has a high reproducibility at pH 2 to 8, which is very close to the Nernst value. In selective interference tests, it is possible to efficiently select from interfering ions and metabolites.
Yun et al. [] prepared a stretchable electrode on a polydimethylsiloxane stretchable substrate by the layer-by-layer deposition of carbon nanotube (CNT) film prepared by the vacuum filtration method on self-assembled nanosheets (AuNS). Then, cobalt tungstate/carbon nanotube and polyaniline/carbon nanotube composites were prepared by hydrothermal synthesis and coated on the CNT-AuNS electrode, respectively. Because the AuNS material itself can form a permeation network, an electrochemical sensor to monitor the glucose and pH in sweat was successfully fabricated. The sensor had good adhesion to moist skin. The detection limit was 1.3 μM. The sensitivity of glucose and pH were 10.89 μA·mM−1·cm−2 and 71.44 mV·pH−1, respectively. It was not interfered with by the other ions and chemical components in sweat. It could also maintain long-term stability in air for 10 days. After repeated stretching and releasing for 1000 times, the mechanical stability reached 30%. It can be predicted that such devices can be stably attached to the skin surface with medical care equipment for health management. In the authors’ research work, an infiltrated network was formed on a stretchable substrate and modified by layer-by-layer deposition. It avoided the use of complex and multi-step processes such as CVD and photolithography, and the overall engineering operation was simple, low in cost, and controllable in film thickness. In terms of material design, nanomaterials with porous structures with large specific surface areas were selected to construct sensing devices to enhance the signal, and interfering substances such as ascorbic acid, dopamine, and uric acid were oxidized at a fixed voltage, which can further enhance the selectivity of glucose monitoring. Furthermore, PANI with low cytotoxicity and low skin irritation is widely used as a wearable pH sensor. We propose that this work can connect electrochemical sensing elements for high-performance health care wearable devices for the continuous monitoring of health management.
The sensing performance of wearable sensors based on sweat pH monitoring depends on the effects of the monitoring response time, stability, selectivity, and hysteresis and drift. Therefore, the design and study of new sensing biocompatible materials is crucial for the stable operation of flexible electrochemical sensors. Currently, most sensors are constructed based on metal oxides, but their sensing performance in solution is slow. Therefore, it is possible to consider mixing metal oxides with conductive materials, which will act as a path for ion transfer. The electrochemical layers formed in the conductive elements jointly participate in the electrochemical reaction. In addition, flexible RFID patches with wireless electronic communication will facilitate the online monitoring of sweat, while flexible triboelectric nanogenerators can be used to power electronic components and can be integrated with wearable sensors, which will facilitate online monitoring.
3.5. Cortisol
Cortisol, synthesized by the adrenal gland, is a stress hormone that plays an important role in physiological processes such as energy metabolism and electrolyte balance, affecting cognitive processes such as memory, sleep, and emotion. It usually exists in blood, urine, sweat, and saliva. The cortisol level in human sweat is about 8–50 ng/mL, and the increase in cortisol content will lead to symptoms such as elevated blood pressure, muscle weakness, osteoporosis, hyperglycemia, and obesity. In contrast, low cortisol content will cause non-specific symptoms such as weight loss, hypotension, and abdominal pain [].
Kinnamon et al. [] integrated and dispersed molybdenum dioxide (MoS2) into a polyamide (PA) membrane nanoporous flexible electrode system, which allowed the polymer to make direct contact with the skin area in a composite human morphology conformation. Vertically arranged metal electrodes were used to limit the semiconductor MoS2 nanosheets, and a stacked design was used to realize small volume (1–5 μL) sensing. MoS2 nanosheets were functionalized with the cortisol antibody. A kind of affinity mechanism biosensor for a cortisol physiological range (8.16–141.7 ng/mL) in human sweat was developed. The specificity of the biosensor for cortisol was realized by measuring the impedance change in cortisol binding along the interface of MoS2 nanosheets by electrochemical impedance spectroscopy. The dynamic monitoring range was 1–500 ng/mL, the detection limit was 1 ng/mL, and the regression coefficient was 0.998. During the study period of more than three consecutive hours, the equipment could distinguish the concentration ranges of 0.5, 5, 50, and 500 ng/mL cortisol. The authors constructed a sweat-based Faraday and label-free cortisol sensing platform for the first time. One of its innovative features is the realization of a low-volume perspiration sensing setup that avoids the passive method of iontophoretic stimulation to induce perspiration. The diffusion-driven coverage sensing area of the hydrophilic PA membrane realizes transmembrane sensing, improves the influence of microgravity and operation time on sample kinetics, and indirectly maintains the stability of the electrode–electrolyte interface. In the material design, MoS2 nanosheets are dispersed between vertically aligned electrodes to advance the 3D sensing mechanism. This sensing device has great potential for the health monitoring ability of long-term sitting people, namely, people with the smallest amount of sweat. For example, in travel and other applications, portable noninvasive sensing can provide valuable diagnostic feedback for high-risk users.
Torrente-Rodríguez et al. [] integrated and prepared a laser-induced graphene-based perspiration pressure sensing wireless mHealth device. Combined with the competitive immune sensing method, the dynamic changes in the stress hormone cortisol in perspiration triggered by day and night changes and acute external stimuli were studied. The results established the empirical correlation between the cortisol in serum and perspiration. The detection limit of the sensing device was 0.08 ng/mL, and the detection range was 0.43 ng/mL–50.2 ng/mL. This could be used as a whole-day noninvasive dynamic pressure monitoring of cortisol in perspiration, providing continuous and real-time assessment of psychological state for the subjects. The authors presented stress-related physiological studies for the first-time such as the cortisol circadian cycle and the constructed dynamic stress response curve. This revealed a great potential for the monitoring of circadian changes in cortisol in sweat. Another of his research features was the unique combination of laser-induced graphene synthesis and immunosensing, enabling the sustained highly sensitive, selective, and efficient sensing of cortisol, validating the stress response and adaptive analysis. The advantages of the graphene sensor array are its low cost and can be mass produced. However, although a physiological stress stimulation test was performed in the study, similarly, psychosocial stress stimuli trigger similar neuroendocrine and behavioral responses, so extensive experimental exploration is required to refine the sensing mechanisms that can advance the development of mental health management.
It is very important to monitor the biological molecules related to chronic health in a dynamic and noninvasive way, because for the people who are usually sedentary or completely unexercised, a lack of physical activity can cause some chronic health problems such as diabetes, heart disease, and its sweating is also very weak. Therefore, a sensor for quantifying biomarkers from low-volume human sweat provides an effective approach. Madhu et al. [] used the hydrothermal method to deposit zinc oxide nanorods (ZnONRs) with a flexible and biocompatible ZnONR array on flexible carbon yarn (CCY) to immobilize specific anti-corticosteroid antibodies. An electrochemical immunosensor platform based on yarn non-adhesion and superhydrophobicity was proposed for the high selectivity and sensitivity detection of cortisol in sweat. The ZnONR integrated carbon yarn showed excellent mechanical stability and super wettability, indicating that the detection range was 1 fg/m–1 g/mL. The detection limits of the CV method and DPV method were 0.45 fg/mL and 0.098 fg/mL, respectively. The sensitivity was 2.12 A/(g·mL−1) and the regression coefficient was 0.998. The in-depth study of materials can provide a stable environment for the immobilization of biological receptors. In other studies, the exploration of the biocompatibility of fabric-based sensor materials is mostly unreported. For the first time, the author designed a sensor for the continuous monitoring of cortisol in sweat using ZnONR integrated flexible fabrics, and carried out cell viability studies, biocompatibility studies, and cytotoxicity studies of the immunosensor. The sensing performance had a wide detection range and low detection limit. The sensing platform with biocompatibility and cytotoxicity would be beneficial to the development of collaborative modern wearable devices.
Barman et al. [] constructed a flexible wearable electrochemical impedance sensing patch based on highly conductive Ti3C2Tx MXene loaded laser-burned graphene (LBG) sheets. The device has microfluidic channels and cavities, which can move sweat to the cavity under natural pressure through channels and can be used for the real-time noninvasive monitoring of cortisol, a biomarker in human sweat. The detection limit of the sensor was 3.88 pM and the detection range was 0.01 nM–100 nM. The impedance signal used to monitor biomarkers can be transmitted through wireless data through integrated intelligent mobile devices. The authors explored Ti3C2Tx MXene nanosheets for wearable and flexible sweat biomarker monitoring. In the constructed 3D electrode network impedance immunosensor, the polydimethylsiloxane penetrated the voids of the LBG 3D structure, reducing the special surface of the LBG. The two were combined with each other while curing into a film. Compared with the design strategy of Kinnamon et al., the sensing performance in the detection range and detection limit was improved.
Cheng et al. [] prepared a flexible electrochemical immunosensor patch. The sensor could be rapidly detected by the differential pulse voltammetry module, and the data could be wirelessly transmitted to the intelligent mobile device of the NFC module. It did not need batteries to be installed and could react with the cortisol level in human sweat in situ and in real-time. Through the sweat test of volunteers, the results showed that cortisol could be continuously detected in situ under relaxation and stress conditions, which has important physiological significance in evaluating individual psychological stress. In this study, the authors constructed a wireless, battery-free flexible patch for the in situ analysis of cortisol in sweat. In terms of design, the impact of the size and battery power consumption on flexible devices is greatly reduced. Combined with NFC-enabled smartphones and electrochemical detection technology, an epidermal biochemical detection system was flexibly constructed.
Liu et al. [] prepared a flexible immune sensing platform for the highly selective and sensitive determination of cortisol in sweat. The detection limit of the sensor was 0.3 fg/mL, and the detection range was 1 fg/mL–1 mg/mL. The detection results were basically consistent with the commercial chemiluminescence immunoassay method, which has a high sensitivity to cortisol. In terms of material design, the authors combined the mechanical stability of multi-walled carbon nanotubes, the electrical conductivity of gold nanoparticles, and the flexibility of polydimethylsiloxane. The next step is to build a flexible stretchable immunosensing platform with excellent mechanical properties and electrochemical performance. In addition, compared with the sensing strategies designed by Kinnamon et al., Barman et al., and Torrente-Rodríguez et al., the sensing performance was improved, which manifested in the advantages of a lower detection limit and wider detection range. This sensing platform can be used as a noninvasive health monitoring and clinical diagnosis tool for sweat cortisol.
Reliable and accurate quantitative analysis equipment is a key inflection point in the medical diagnosis of disease. At present, many electrochemical immunosensors for the real-time and continuous monitoring of cortisol levels in sweat are sufficient to detect cortisol in the physiological range and have the advantages of a low detection limit and fast response time. At the same time, there is a clear trend toward portability, reliability, and ease of use. Part of the equipment is also integrated with microelectronics to form a portable mobile nursing device. Despite their immunosensor properties, current research has only shown that most are disposable sensors.
Therefore, we present our outlook for the next steps in electrochemical immunosensors for cortisol. First, the antibody in the electrochemical immunosensor should have high selectivity and specificity for the target analyte, so the sensitivity and specificity of the sensor must be increased. Among them, imprinted polymers and aptamers can significantly improve the performance of cortisol immunoassays. Second, sensor reusability must be considered, and techniques must be developed to restore the sensor to its original state, since the bonds that react between cortisol and the immobilized biomolecules are often irreversible. Finally, the reproducibility of the sensor is still unresolved. Most of the current research is based on the fabrication of a set of sensors containing multiple detection channels. Therefore, more efforts are needed to study to achieve a faster commercialized cortisol electrochemical immunosensor.
3.6. Vitamins
Vitamins [] play an important role in the normal metabolism of the human body and are essential material and nutrient elements for the collective to maintain normal functions. Vitamin C can prevent and treat hematological diseases, restore the immune system, accelerate wound healing, skin care, and enhance the antioxidant capacity of the body. It is clinically used for the adjuvant treatment of various tumors and cancers, anti-aging, and the treatment of immune system diseases. However, a large amount of vitamin C intake will cause kidney disease, thrombosis, and stones [].
A wearable bio-electronic platform by Sempionatto et al. [] was proposed to track the intake concentration and dynamic trend of vitamin C in the epidermal sweat noninvasively. The content of vitamin C consumed by the enzymatic reaction was proportional to the concentration of ascorbic acid, so the change in the redox current could be measured by monitoring after taking vitamin C tablets or drinking fruit juice without the interference of other sweat components such as uric acid and acetylaminophenol. Through the tensile and bending deformation tests of sensor patches, it showed strong elasticity. It was concluded that such a sensor could be used to evaluate the tracking of dietary nutrition, so that the wearer can improve their dietary behavior and correct nutritional intake. Furthermore, the authors investigated the selectivity specificity of the first wearable enzyme-based sensor for vitamin C by continuous monitoring in sweat through ascorbate oxidase modification to catalyze the oxidation of the sensor. However, the authors’ work requires the incorporation of a local iontophoretic sweat stimulation system to induce perspiration. Therefore, further research work can be suggested to expand the research of low-volume sweat monitoring or replace ion stimulation with long-acting sweat drugs and integrate perspiration sensors to address potential changes in sweat volume, which will also extend the life of biosensors.
Zhao et al. [] developed a wearable sensor based on the oxidation of vitamin C and l-ascorbic acid oxidase to selectively measure the concentration of vitamin C in biological fluids including sweat, urine, and blood, with a wide detection range of 0–5000 μM, high selectivity, and long-term stability. The sensitivity of the sensor was about 12% and the detection limit was about 4 μM. The sensor was flexible and could maintain contact with the skin through a thin water absorbent pad to ensure that the biological liquid was absorbed into the sensor. The results also showed that the concentration of vitamin C in sweat increased when the intake of vitamin C increased from 0 to 1000 mg. Under the control of drinking water intake, the concentration of vitamin C in urine generally increases with its intake. The correlation coefficients between sweat and urine and blood were 0.81 and 0.72, respectively. Therefore, the sensor can facilitate regular nutritional analysis, clinical nutritional assessment, and dietary monitoring. The L-ascorbate oxidase-based wearable sensor constructed by the authors can and selectively measure the concentration of vitamin C in biological fluids. Monitoring is accomplished by absorbing a relatively low volume of sweat, approximately 1.5 µL, by maintaining flexible contact with the skin through a thin absorbent pad. In contrast to the research strategy of Sempionatto et al., perspiration can be induced without the need for ion to electric stimulation.
Currently, electrochemical sensors for the detection of water-soluble vitamins such as VB1, VB2, VB6, VB9, VB12, and VC have attracted great attention based on the outstanding advantages of simplicity, cost-effectiveness, high sensitivity, and easy miniaturization. All vitamins are electrochemically active, so they can be detected by electrochemical sensing. However, there have been few studies on electrochemical sensors for detecting vitamins in sweat, and new electrode materials can be designed to improve the sensitivity and selectivity. Therefore, we can envisage the real-time and remote detection of vitamins based on wearable mobile device-based electrochemical sensors combined with medical IoT for detecting different vitamins in sweat.
3.7. Ethanol
Alcohol abuse and addiction can cause acute alcoholism and multiple organ damage such as heart, liver, and lung. Therefore, it is urgent to develop efficient diagnostic methods to detect high-risk drinking behavior and alcohol-induced tissue damage []. Biomarkers of ethyl glucuronate (EtG), a direct metabolite of ethanol, can be used as an objective indicator for evaluating the intervention of alcohol intake behavior and can be detected in body fluids such as sweat and urine. Although EtG only accounts for about 0.1% of the total ethanol, it is detected within one day or more after drinking one or two cups and within 2–4 days after drinking more ethanol [].
Selvam et al. [] designed the label-free electrochemical impedance sensing technology to continuously monitor the metabolite EtG of ethanol in human sweat to determine the consumption of ethanol. After drinking, EtG can stay in the human body for three days, so it can monitor withdrawal and find out whether to drink again. The detection range of the EtG sensor based on the gold electrode was 1–10,000 μg/L, the detection time was up to 9 h, the detection limit was 1 μg/L, and the sensitivity was 0.001 μg/L, which is more suitable for monitoring and limiting alcohol intake. The detection range of the EtG sensor based on the ZnO electrode was 0.001–100 μg/L, the detection time was up to 4 h, and the detection limit was 0.001 μg/L, which is more suitable for the withdrawal process. Therefore, the monitoring and sensing field of ethanol has considerable potential value for relapse from abstinence and heavy drinking. For the first time, the authors realized the monitoring of alcohol in sweat by quantifying ethanol and the ethanol metabolite EtG through a wearable device. The constructed sensor electrodes with different modifications simultaneously continuously monitor coplanar sensors, which are configured in a lateral current or multiplexing manner, among which ZnO-based electrodes are more sensitive.
The relationship between alcohol consumption and diabetes is approximately U-shaped. Alcohol intake changes the internal environment of glucose, has a contradictory impact on blood glucose level, and has a higher risk of developing into diabetes []. Bhide et al. [] integrated the surface-functionalized zinc oxide film of ethanol oxidase and glucose oxidase into a nanoporous flexible electrode system to fabricate a non-invasive, label-free, and simultaneous detection and monitoring of the physiological concentrations of glucose and ethanol in perspiration. They used 1–3 μL of low-volume perspiration of the human body without external stimuli for sensing. The corresponding impedance changes of the biomarkers on the sensor interface were measured by electrochemical impedance spectroscopy in the dynamic range of 0.01–200 mg/dL. The detection limit of alcohol in sweat was 0.01 mg/dL, and the sensitivity was 0.2 ± 0.02 μA/mM. By changing the pH level of synthetic sweat and in the presence of other interfering substances, the selectivity of the sensor for the immunoassay was calibrated, and glucose and ethanol in sweat were detected. Studies have shown that the detection of ethanol molecules is the change in the capacitive reactance component of the measured impedance, which is due to the high charge accumulation at the ZnO sweat buffer interface and the charge transfer between the enzyme biomolecular complex and the ZnO surface. Moreover, the nano-scale target biological molecules in the basal pores can reduce the noise of other molecules in the sweat matrix and obtain a higher signal-to-noise ratio and better impedance signals. The authors were the first to demonstrate the combined monitoring of alcohol and glucose via biosensors in human sweat-based fluids. Compared with the strategy of Gamella et al., the constructed sensing device realized the sensing response of low volume sweat, without the need for iontophoresis or a sweat patch to induce perspiration, which is more practical. In addition, synthetic sweat samples tested with different interferents showed no significant difference in the performance of the constructed sensor compared to the BACtrack® S80 Pro breath analyzer. Compared with the strategy of Selvam et al., which also used a ZnO thin film functionalized sensing system, the author’s sensing device had a wider linear range and higher sensitivity. Therefore, the glucose and alcohol levels in diabetic patients can be detected in combination.
Gamella et al. [] prepared a noninvasive and passive dual-enzyme amperometric biosensor that could rapidly monitor the concentration of alcohol in blood by measuring the ethanol content in sweat. In a single test, it could continuously and provide relevant information in a timely manner, allowing for the determination of ethanol content within 5 min after alcohol intake. The sensor device constructed by the author realized the continuous determination of the sensitive change of ethanol by adding biological enzymes. In addition, the ethanol in the sweat of different volunteers was measured by gas chromatography. There was no significant difference between the reference results and the sensor device results, and was comparable to the current commercial alcohol meters. In addition, this type of sensor has a protective effect on the prediction and early warning of drunk driving behavior. However, the device uses pilocarpine ions to stimulate the skin to induce perspiration, and the addition of biological enzymes also makes the experimental process cumbersome. Therefore, it is also a development direction to build a low-volume sweat sensing device.
Sánchez et al. [] constructed an alcohol colorimetric sensor by impregnating non-woven materials with colorless photochromic heteropoly phosphotungstic acid (PTA). This sensor avoids the use of high analytical cost and environmentally sensitive enzymes, and reduces PTA to blue by UV irradiation to quantitatively detect the concentration of ethanol. One of the significant advantages of the colorimetric sensor constructed by the authors is the use of a photochromic agent such as PTA and an ultraviolet lamp. The photochromic factor POM contained in PTA can redox color change. When the device is exposed to air, PTA can be oxidized again to a colorless state, potentially offering a unique potential for reusability. The designed device can still detect blue at lower ethanol concentrations, and the intensity of the color is proportional to the ethanol concentration. At the same time, it overcomes the impact of high cost and environmental instability. Compared with the strategies of Gamella et al. and Bhide et al., monitoring in a colorimetric sensor avoids the use of more cumbersome biological enzymes, which is also one of its features. This provides a powerful platform for the noninvasive detection of ethanol in sweat and can be used repeatedly to determine whether the driver has drunk driving behavior during driving.
Biosensing of alcohol is of great interest in the clinical, forensic, and food and beverage industries. Alcohol addiction is also a major cause of violence and drinking and driving can also pose a serious threat to the lives of drivers. Blood alcohol content (BAC) is an accurate indicator of alcohol intake in the human circulatory system. It has been widely used as a key parameter in tests to measure alcohol intake, but it cannot be measured in situ. It requires cumbersome procedures and professional related personnel to analyze samples such as the monitoring of alcohol through the release of volatile organic compounds (VOCs) through the skin. At present, noninvasive, rapid, and regular monitoring and the detection of post-consumed ethanol has been a hot topic of research in recent years. The research on ethanol through sweat monitoring is gradually being promoted. Next, we can consider using these wearable devices to quantify and dynamically report the user’s relevant information.
3.8. Drugs
Caffeine, belonging to xanthine alkaloids, is a relatively safe psychoactive drug and widely exists in coffee, tea, and other products. Because of its addiction, caffeine is closely related to life and health []. At present, the commonly used caffeine drugs are Ganmaoling capsules, compound aminophenol amine tablets, aminopyrine caffeine tablets, etc.
Tai et al. [] developed a wearable skin sensing platform for the noninvasive, real-time, and continuous in situ drug monitoring, which was realized by the electrochemical differential pulse voltammetry sensing module. Studies have shown that the linear response sensitivity of the sensor to caffeine concentration was 110 nA·μM−1, the detection range was 0–40 μM, and the detection limit was 3 × 10−6 M. The response of the sensor to interference substances such as urea, glucose, lactic acid, ascorbic acid was less than 9.2%. The ability of the sensor to capture the physiological trend of the highest concentration of caffeine was expected within 30–120 min. Drug monitoring for human therapeutic properties or drug-related medical application interventions play a pivotal role in precision medicine. The wearable sweat wristband constructed by the authors successfully realized the continuous monitoring of methylxanthine drugs in sweat. The authors also performed an experimental comparison of the detection of the collected sweat samples by ion-induced and exercise methods, which were identical to those reported for the wristbands. In addition, other types of methylxanthine drugs could also be detected by this platform’s DPV detection technology, paving the way for continuous noninvasive drug monitoring.
Carbamazepine, a drug used in nerve therapy, is widely used in the treatment of mental attacks, seizures, trigeminal neuralgia, and other diseases. Carbamazepine can also spread resistant genes, resist antibiotics, and cause infections in sweat, blood, and other biological fluids. Monitoring the carbamazepine levels in health care is therefore essential [].
Sushmitha et al. [] developed a low-cost and flexible amperometric sensing platform based on two-dimensional metallic NiSe2 nanoclusters for the high selectivity and high sensitivity detection of carbamazepine in human sweat. Due to the high conductivity of NiSe2 metal, the high electroactive surface area of nanoclusters and the interacting Ni2+/Ni3+ oxidation state, the NiSe2 metal showed an excellent sensitivity of 65.65 μA/nM to carbamazepine. The linear range of the carbamazepine detection concentration was 50 nM–10 μM, and the detection limit was 18.2 nM. It was found that more than 10 times the interfering substances such as lactic acid, glucose, uric acid, and ascorbic acid did not affect the accurate determination of carbamazepine, showing good selectivity. Currently, most studies based on carbamazepine sensors are conducted in blood, urine, food, and water. For the first time, the wearable sensing platform constructed by the author has considerable practicality in medical diagnosis using sweat as a biological fluid. The low-potential sensing platform avoids the use of biological enzymes or redox reagents and avoids complicated operation steps in sensing. In the synthesis process of NiSe2, the nickel element will form unnecessary impurities due to thermal decomposition, and the insolubility of selenium atoms will cause slow diffusion and require intermittent processing steps. The stoichiometric elemental composition also affects the existence of 2D phase NiSe2. Therefore, the authors adopted a hydrothermal synthesis technique to synthesize layered NiSe2 nanoclusters, typically avoiding the existence of defects.
In short, there are few designs of wearable sensors for sweat microbial fluids at this stage, but there is still no lack of device applications for drug monitoring such as dipyridamole, acetaminophen, and levodopa. During the research process, it is also necessary to pay attention to the presence of many low molecular weight, high lipophilic exogenous drugs, and many high abundance endogenous substances in the samples of the sweat complex. Therefore, it is necessary to decouple endogenous electroactive substances to avoid repeated superposition of voltametric currents, and to construct an electrochemical sensing interface with high sensitivity and high signal-to-noise ratio for sensitive monitoring of targeted drug molecules at ten points []. Among them, voltametric electrochemical methods can utilize the electroactivity of target detection drugs to quantify molecules. It can eliminate relevant recognition species that depend on physiological disturbances and can suppress Faradaic background currents. Therefore, we can continue to use wearable devices to monitor drugs that may be present in sweat and expand a series of applications such as drug compliance, drug abuse, and the formulation of personal drug treatment plans.
4. Types of Sweat Wearable Sensors
A set of standard wearable sensing equipment or monitoring device can mainly be divided into sample data acquisition, data processing, and signal transmission. For sweat sensing equipment, this includes the sweat collection, determination of the sweat analyte, circuit interface, sample data processing, and signal transmission []. For example, a sweat sensing system wristwatch based on sweat conductivity sensors can absorb sweat from the arm and read conductivity values, and transmit them to smart phones via Bluetooth, providing breakthrough progress in real-time sweat monitoring. In view of the complexity of sweat secretion and the importance of the selective screening of target biomarkers, it is necessary to integrate comprehensive systems to ensure the accuracy of measurement. Figure 2 demonstrates various flexible skin-interface electrochemical sensors utilizing sweat as a rich source of biomarkers. These wearable sensors can analyze analytes by continuously monitoring multiple dynamic concentration levels. Widely used glasses platforms [], polymer adhesive patches [], soft, wearable fabric sensors [], light temporary tattoos [] and available paper [,] can directly contact the skin, more suitable for real human contact sensing. In addition, the microfluidic channel in the device can also guide and absorb sweat into the designed substrate for in situ analysis. Table 4 summarizes the electrochemical detection modes of the various sensor examples reviewed in this paper and the relevant characteristics of the common electrochemical detection methods for sensors.
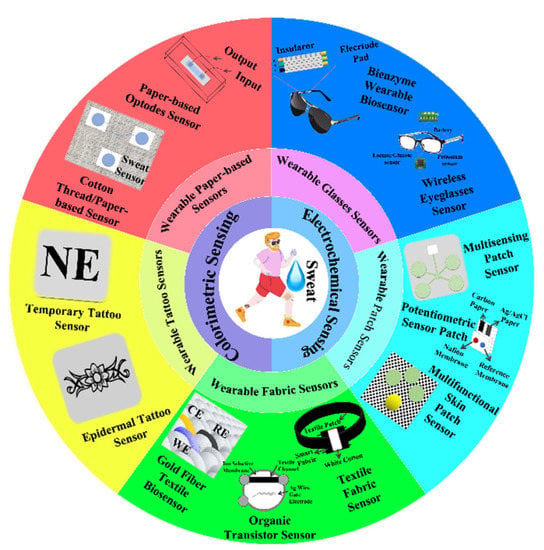
Figure 2.
The representative examples of sweat based wearable electrochemical sensors. Starting from the top including five types of wearable glasses sensors, wearable patch sensors, wearable fabric sensors, wearable tattoo sensors, and wearable paper-based sensors.

Table 4.
A comparison of the electrochemical detection method of the wearable sweat sensor included in this paper with other common methods.
4.1. Wearable Glasses Sensor
In recent years, the demand for wearable sensors has grown rapidly with people’s attention to epidermal chemical sensors. As a fashion accessory, glasses are widely used in various occasions. Tens of thousands of people wear glasses, which can provide clear visual effects and certain protection. The inherent shape and quality of the glasses are consistent with the face. The bracket is close to the head skin and maintains friction with the skin. The pressure of the nose pad also promotes the contact between the sensor and the skin, and the microbiosensor can be placed to form an attractive platform. A chemical sensing platform based on glasses provides comfort to wearers [].
Zhang et al. [] prepared an electrochemical epidermal biosensor, which can be flexibly attached to the glasses to continuously monitor the lactic acid level in sweat during exercise. Glucose, uric acid, and other common interfering substances in lactic acid showed little current interference in the buffer matrix. The epidermal biosensor itself had the advantages of non-invasive, continuous, and was easy to use. In the detection range of 0–25 mM, the current signal had a high linear relationship with the concentration of lactic acid. The sensitivity was 0.74 A·mM−1 and the detection limit was 0.04 mM, reflecting the excellent characteristics of biosensors for lactic acid detection. The development of the equipment showed that mainly through the prefrontal position measurement, the deformation interference could be reduced. It can be concluded that the integrated glasses and wireless transmission signal acquisition system are one of the future development trends. Here, it needs to be mentioned that the glasses provide visual aid while also protecting the eyes from injury. The frame of the eyeglass frame is close to the skin and keeps friction, forming an attractive platform. Based on this, the authors demonstrated a platform of electrochemical biosensors hidden in glasses that could continuously measure the lactate levels without interruption, presenting a stable signal free of mechanical interference. The sensor exhibited good high sensitivity and selectivity when detecting the electroactive components that can exclude human sweat. The research in this work opens a new avenue for the dynamic and continuous assessment of metabolite changes.
Sempionatto et al. [] integrated amperometric lactate biosensors and K+ selective electrode systems, printed on polyethylene terephthalate paper, placed on both sides of the nasal pad of the glasses. A fully integrated chemical sensing platform for glasses based on wireless was prepared by placing a radio interface device in the glasses’ bracket. The device is connected to the host through Bluetooth wireless data transmission by converting wireless signals and the real-time monitoring of lactic acid and potassium levels in sweat without obvious interference. The detection range and sensitivity of the two were 0–10 mM and 0.1–100 mM and 0.39 mM and 10−3.9 M, respectively. The chemical sensing platform integrated the necessary functions and practical shape factors, provided comfort and was easy to wear while ensuring good contact with the skin. This device can replace the sensing adhesive to monitor metabolites and electrolytes. Therefore, the system can be easily expanded to monitor various biological analytes. The temperature sensing module and pH microsensor can be integrated into the multi-channel analysis glasses system to compensate for the skin temperature or pH effect and further improve the platform device. The uniqueness of the authors lies in the first demonstration of an attractive sweat sensor bioglass platform for the simultaneous real-time monitoring of sweat electrolytes and metabolites. The new platform addresses the challenges of incorporating wearables, chemosensing capabilities, and attention to key design as well as the technical hurdles to be aware of when incorporating eyewear accessories. It provides the wearer with comfort and ease of use, plus the nose pad promotes good sensor-skin contact, and the overall shape and weight fit well with the user’s face and pressure. Therefore, the sensor can be used in fields such as health care. We also suggest that the monitoring of other important metabolites, electrolytes, or hormones can also be extended to create a combined development in the field of fashion and wearables.
4.2. Wearable Patch Sensor
In terms of sports and health, biomarkers need to be continuously and noninvasively monitored to assess human performance. A flexible electronic substrate and multi-layer microfluidic channels were integrated and optimized to prepare programmable and automatic wearable sensor patches. Thus, the detection and in situ analysis of metabolites, electrolytes, and other biomarkers in sweat can be realized, and the changes in the biological physiological indices, and the real-time feedback of chemical information can be analyzed [].
A flexible multi-sensoring patch was designed for the simultaneous and selective measurement of sweat metabolites and electrolytes by Anastasov et al. []. Poly(3,4-ethylenedioxythiophene) (PEDOT) was deposited on a polyvinyl chloride (PVC) membrane by the potentiostatic deposition method to prepare a potentiometric Na+ sensor with a sensitivity of 56 ± 1 mV/unit. Irox film prepared through electrochemical oxidation was used as a pH sensitive layer with a sensitivity of 71.90 ± 0.8 mV/unit. The relative standard deviations of the two types of sensors were 0.6% and 4%, respectively. A 0.65 V constant potential lactic acid sensor was prepared by depositing the doped lactic acid oxidase on the surface of a semi-permeable polymer membrane and the outer layer of polyurethane. The temperature and humidity sensors were used for internal calibration. The wireless transmission electron technology was integrated to collect and store real-time data. The sensing signal reached a stable state within 10 s after the initial measurement. The authors’ work focused on simultaneous and selective measurement and internal temperature control calibration by integrating sensors with different functions. As well as additional features that allowed for continuous sweating for the analysis and wireless data transfer, the platform is robust, wearable, and easy to use. However, more working research is also needed for validation such as a grasp of the test population, and a better understanding of the health of the collected data and their clinical significance. In addition, other research directions can deploy more biomarker monitoring.
Calamak et al. [] successfully prepared a multifunctional wearable sensing patch based on super absorbent sodium polyacrylate (SP) particles immobilized on a fiber patch. The pH sensitive element of the sensor could provide a colorimetric response in the pH range of 4–9. The circular hydrogel particles with high water absorption could quantify the sweat volume and sweat rate and showed a rapid response to NaCl, KCl, and urea. The overall characteristics are its low cost and simple method, which can be used to measure the local sweat volume at nursing points. The authors’ research highlight was the construction of a patch containing superabsorbent SP hydrogel microparticles for fast, low-cost, and standardized real-time sweat analysis. The presence of SP particles was beneficial in enhancing the surface area for water absorption, and the circular shape could quantify the sweat volume and sweat rate. We believe that the significance of this work goes beyond monitoring electrolytes because predicting the perspiration rate and perspiration volume is more important for quantifying the perspiration volume loss and perspiration rate at the skin surface. We also believe that further work studies can achieve early timely reminders for lost electrolytes and early replenishment recovery.
Hoekstra et al. [] designed a disposable solid-state wearable patch to monitor the activity of all electrolyte ions in sweat by casting a cationic polymer substrate onto carbon ink-coated paper. The sensitivity of Na+ in the aqueous solution was 55.3 ± 1.0 mV/dec. Because the ion exchange membrane had weak selectivity to different types of electrolyte ions and showed different Nernst response, the Nafion membrane could reduce biological adsorption and improve the stability of the system. The linear working range of the sensor was 0.1–1000 mM, and the detection limit was 48.3 ± 5.0 μM. The sensor was wirelessly transmitted to the mobile phone application through Bluetooth to track the conductivity distribution and chemical data of sweat during the movement of athletes. The authors aimed to introduce, optimize, and validate a wearable potentiometric sensor to monitor sodium ion activity in sweat. The results showed that the system can be used as an alternative to conductometric measurements, and the sensor is overall simple and flexible with excellent reproducibility. We suggest that the next step in this work can be achieved through the design of materials to monitor the ionic activity of various ions in sweat.
4.3. Wearable Fabric Sensor
Due to the soft, wrinkle-prone, and irregular dynamic characteristics of the skin, it is usually a challenge to establish contact between patches and human skin. Some elastic patches are usually waterproof, which will lead to uncomfortable and unbreathable wear experience. In contrast, a textile’s inherent soft air permeability gives the textile clothing a good wearing experience. Fabricating a scalable, biocompatibility sensing platform provides a higher availability for wearable sensors [].
Coppedè et al. [] prepared an organic textile electrochemical transistor by continuously functionalizing a series of textile fibers with selective membranes, while maintaining the hydrophilicity and flexibility of the device. Using poly(3,4-dioxythiophene):polystyrene sulfonate (PEDOT:PSS) as the active layer of the conductive polymer channel and ion-selective membranes based on different ion carriers, a wearable biosensor based on ion-selective membranes was designed to measure the different electrolytes in sweat by potentiometric titration. The sensor could distinguish Na+, K+, and Ca2+ in the concentration range of 10−5−1 mM, which provides an application prospect in sweat analysis. The authors’ research focused on the integration of functionalized ion-selective membranes in textile biosensors, addressing the insufficient selectivity of conventional organic electrochemical transistors. Simplifying the absorption of sweat samples as a whole and utilizing the wearable properties of the device enables the construction of wearable devices based on everyday clothing. What we propose is that the authors further tune the modification work to improve the ability to respond to a variety of specific ions for further device development before implementing organic electrochemical transistors on textiles.
Caldara et al. [] successfully prepared a smart textile wearable sensor platform by modifying cotton fabric with organic silicate, integrating low power electronic devices, and using stamen as a pH indicator, which can continuously monitor the pH value of sweat. This textile equipment can provide medical disease diagnosis and drug monitoring in the fields of health care and fitness. The highlight of the authors’ research work is that mesoporous films were prepared by sol–gel and low-temperature block copolymerization extraction under acidic conditions. It can be effectively used as a pH sensing sensitive material. By combining it with textiles, the application of a smart textile wearable device was demonstrated. We suggest that the potential for further work to focus on organic modification on the spectral properties and analytical performance of thin films will further enhance the development of biosensors.
Wang et al. [] prepared stretchable, strain-insensitive, and highly conductive gold fibers for a three-electrode system by the dry spinning process and wove them into fabrics in the standard plane. The textile lactic acid biosensor had high specificity and sensitivity. The sensitivities in artificial sweat and phosphate buffer solution (PBS) were 14.6 μA/mM∙cm2 and 19.13 μA/mM∙cm2, respectively. The linear range was 0–30 mM, and the detection limit was 0.137 mM. Under the high tensile strength of 100% strain, the sensor maintained high performance and the most ductility. The advantage of a textile-based wearable design is that it can be easily integrated with everyday clothing without interfering with daily activities. Behaviors such as bending, twisting, stretching, etc. need to be maintained under the normal motion of skin deformation. Based on this, the authors’ work focused on the development of stretchable fiber textiles for wearable devices. The textile lactate biosensor had high stretchability and excellent sensitivity and can be used for the real-time monitoring of lactate in sweat.
4.4. Wearable Tattoo Sensor
Noninvasive monitoring based on skin epidermis should take into account the elastic characteristics of electrochemical sensors. Therefore, flexible, anti-mechanical stress, and compatible non-planar skin tattoo sensors are created. The tattoo sensor can be greatly expanded in fitness, military, and other fields by integrating a variety of electrochemical technologies [].
Kim et al. [] developed a skin-based tattoo printable platform on temporary transfer tattoo inkjet paper with a modified screen-printed bismuth/perfluorosulfonic acid film, which was applied to wearable electrochemical sensors for skin wear and the noninvasive monitoring of trace metals in human sweat. The sensor could withstand mechanical fatigue stress repeatedly. The device showed a clear and stable Zn2+ response, and the detection limit was about 0.05 μg/mL. This mobile temporary skin sensor can easily expand to the measurement of other skin-related heavy metals by a noninvasive detection method. The highlight of the authors’ research was that they were the first to construct a flexible temporary tattoo electrode based on adhesion to the skin for the noninvasive monitoring of the electrolytes in sweat. The sensor achieved skin-compliant elastic properties, mechanical stress, and compatibility with skin non-planarity, further extending to the monitoring of trace electrolytes. We suggest that future work can focus on the miniaturization and integration of trace metal monitoring equipment, and improve the electronic equipment for data acquisition, processing, and wireless transmission.
Bandodkar et al. [] designed a temporary transfer tattoo potential sensor based on skin wear by screen printing carbon ink onto a flexible polyethylene terephthalate substrate. Furthermore, the Bluetooth wireless transceiver was integrated inside, which avoided the cumbersome circuit of recording data and could monitor the Na+ content in human sweat in real-time. After in vitro experiments and skin measurement, the sensor showed a fast Nernst potential close to the nerve reaction. The linear working range was 0.1–100 mM, and the sensitivity range was 64.1 mV/log10[Na+]. The sensor had good recovery ability after various fatigue mechanical deformations such as bending, stretching, and punching. In the in vitro characterization of motion experiments, the real-time monitored Na+ concentration was transmitted to the computer through the sensor wirelessly. The device had a long shelf life and showed excellent analytical performance. The author’s highlight was the integration of pass-through stock combining a thick film, laser printing, solid-state potentiometric sensing, and wireless technologies. The potentiometric sensor that constructs the skin-based tattoo has good elasticity and resists various mechanical deformations experienced by the human epidermis. However, we still recommend improving the sensor signal drift, integrating temperature sensors to address temperature changes, and the further miniaturization of transceivers to meet the real-life applications to a greater extent.
4.5. Wearable Paper-Based Sensor
Paper has excellent properties such as light weight, porosity, hydrophilicity, flexibility, and biocompatibility. As a result, it has been used to manufacture microfluidic devices and sensor platforms for medical diagnosis [,].
Xiao et al. [] integrated wearable cotton/paper-based microfluidic devices and smartphones that met the requirements of wearable systems for flexibility and biocompatibility. By analyzing the colorimetric changes caused by glucose in sweat, it can be used for real-time, noninvasive, and quantitative monitoring of glucose concentration in human sweat. The dynamic monitoring range was 50–250 μM and the detection limit was 35 μM. The device introduced low cost and easy to operate sensing elements, which makes wearable devices more valuable for monitoring analysis and field diagnosis. The development of microfluidic devices integrated on cotton thread and paper materials met the requirements for flexibility and biocompatibility in wearable systems, but it has not been used for in situ sweat analysis. Therefore, the authors constructed a sensor for colorimetric sensing guided by a hydrophilic cotton thread as a sweat collection channel connected to a cotton absorbent patch. We suggest that the wear resistance of the material can be used for compatibility with the traditional textile industry to achieve further development of the sensing device.
Petar et al. [] prepared a simple paper-based K+ ion selective photoelectric sensor in sweat by using valinemycin as the potassium selective carrier combined with a lipophilic pH indicator. When the concentration range was 10−3 M–10−1 mM, the related reaction was linear, which covered the concentration of potassium in sweat. Moreover, the sensor could continuously monitor the small signal of the output signal in 100 min. The drawback is that the method used by the author requires the addition of specific ion carriers to determine the ions needed in sweat and their concentrations. Paper-based materials can be used as ion-selective electrode materials for ionophores, but their potential for reversible sensing has not been investigated. The author’s research aimed at the gradual promotion of reversible ion monitoring in the emerging field of wearable sensing platforms. A highly integrated low-cost platform was developed using the combination of the versatility of paper. The sensor had reversible and repeatable responses, and its general application in paper-based microfluidic systems can be integrated into wearable systems for continuous sweat analysis.
5. Ideal Sweat Wearable Sensor Devices and Challenges
The rapid development of emerging information technology, printing technology, and material science has brought new opportunities for wearable sensors. However, many core challenges need to be solved before practical application. It is imperative to verify the progress of new sweat sensing devices in the field of sweat disease monitoring. The following suggestions are put forward:
ScreenPrinting: For wearable electrochemical sensors, the structure mainly includes a flexible substrate and functional layer, in which the functional layer can be divided into the electrochemical active layer, insulating packaging layer, microfluidic channel, and some biomarker enrichment layers. Most of these circuit structures use serpentine geometries, which can meet the need for flexibility and ductility. However, how to assemble the circuit on ta flexible substrate through an electronic printing technology with simple steps, low cost, and simple design is a problem that needs attention. By including 3D printing and inkjet printing, there are advantages in their low cost and scalable production. However, printing on flexible substrates still presents some challenges that need to be further optimized. Screen printing technology is generally used for functional layer assembly on the substrate. In screen-printing technology, the mesh size and pattern on the screen-printing plate are required to be related to the thickness of the printing substrate, which affects the mechanical properties of the sensor. High viscosity printing ink is needed to make it easy to penetrate into the substrate. Therefore, screen printing technology has more integration potential in different types of sensors [].
Temperature Calibration: The wearable sensor will be affected by temperature in practical work, which will affect the conductivity, sensing performance, and data transmission, more or less. It may not meet the characteristics of temperature control and flexibility, and the development of application fields may be limited. Therefore, it is very important to embed the corresponding temperature sensor in the equipment for temperature self-calibration. It can be considered to connect the power supply and the control element to realize the conversion of thermal energy to electric energy. The thermistor is connected to an analog circuit such as a bridge circuit to obtain an output voltage proportional to the temperature difference [].
Energy Consumption: Power or energy is one of the most important issues for sweat wearable electronics. If the energy problem is not resolved, they will not work properly to provide relevant power to the sensors. Therefore, research on R&D issues of wearable energy will be beneficial and necessary, and developing novel low-power or self-powered devices is also a feasible route. At present, the capacity of flexible thin-film batteries that provide electrical energy is generally very small, and other types of energy generation such as generating electricity through human friction, and collecting radio frequency power from the surrounding environment are very inefficient. It is not enough to drive the peripheral signal processing and transmission circuit of the sweat sensor chip to work. In practical work, to reduce the load and energy consumption of the sensor, the sensor has a low working voltage and suitable resistance value [].
System Integration: In the practical application of wearable sensors, the requirements for integrated circuits are high. It is necessary to meet the requirements of large area sensing in matrix sensing and the integration of organic field effect transistors, diodes, RF inductors, and supercapacitors as a unit embedded system for application. Current smart wearable devices only have primary signal processing capabilities, and external devices are required for complex analysis processes involving big data and complex algorithms. With the development and use of miniaturized smart modules with greater computing power within the sensor, it is expected to eliminate the reliance on rigid external devices and maximize the use of data to expand the scope of wearable applications [].
Flexible Chip Construction: The peripheral signal processing and transmission circuit part is responsible for amplifying, filtering, noise reduction, and other signal processing of the signal measured by the sweat sensor, and then converting it into the target analyte concentration value for transmission and display. However, due to the single structure and low integration of the existing flexible electronic devices, many complex functions cannot be completed automatically. Therefore, it is often necessary to integrate rigid chips. Based on this, the development of mechanical properties such as flexibility and stretchability of the constructed rigid–flexible sweat wearable device is limited. The difference in Young’s modulus is large, which will cause unstable transmission performance, resulting in a decrease in the overall fatigue resistance of the device and unstable working conditions. Therefore, the construction strategy for a rigid–flexible sweat wearable devices needs to be optimized and the development of flexible integrated chips to realize multifunctional and fully flexible wearable sweat sensors [].
Unit Functional Design: The micro-computing system is embedded in each structural unit. When the array of sensitive units is assembled, the complete sensor network can self-process signal processing and data transmission to meet more practical applications. Moreover, it should be noted that the integration of multi-unit functions is not simply to build the sensor by stacking, but to minimize the interference between different components and retain the flexibility of the sweat sensor [].
Multifunctionalization: A variety of sweat analysis can provide abundant information, but most sweat sensors only meet the specific detection of a single signal. In the application of wearable electronic devices, it is important to be able to fully compete with biological skin, and to be able to acquire various forms of data in one device to enhance the multifunctional detection of sweat. Therefore, a wearable sweat sensor with multiple signal monitoring functions and the detection of external strain signals such as pressure, distortion, and bending is a major trend in the future [].
Physiological Measurement: The physiological characteristics of sweat vary from person to person, but even the sweat information measured in the same area of the same person is very different, and it will be affected by objective factors such as diet and drugs. In daily activities, the human body produces various types of physiological signals including common electrophysiological signals, molecularly labeled biochemical signals, and biological tissue dynamics signals. Different types of signals contain specific physiological sign information, and through real-time monitoring of these signals, an objective evaluation of the physical condition of the human body can be achieved. Therefore, the analysis of sweat is absolute. It is necessary to design and develop a normalization scheme so that the equipment can obtain the reference information and measurement information at the same time for correlation and comparison, and then carry out subsequent research [].
Transmission Range: One of the main functions of wearable sensor equipment is to transmit the measured data to the relevant applications of smart phones through wireless technology, which solves the problem of distance transmission. However, distance is, relatively speaking, in large-scale activities such as competitions and games, which requires a wider signal range for real-time data collection. Flexible sensors need to maintain signal stability, but the sensing signal is susceptible to drift. In the process of continuous detection, errors will accumulate over time, affecting the service life of the sensor. In addition, signal drift also plagues enzyme sensors, which can cause the enzyme to lose activity or detach from the attached support, resulting in signal attenuation and decreased sensitivity. Therefore, to ensure the consistency and repeatability of the measurement performance of each sweat sensing device, it is necessary to study and set up a common calibration curve, which can minimize the operational complexity of setting and calibration for users during use [].
Signal Crosstalk: As an important component of the measurement system, the bioelectric monitoring electrode directly contacts the human body to collect bioelectric signals and is the core component of the bioelectric sensor. However, when detecting bioelectrical signals, due to the large size of the electrodes, it is easy to be interfered with by signals from other parts. Conversely, when the electrode size is reduced, the contact impedance of the electrode increases, which affects the quality of the signal acquisition. Therefore, in future bioelectric detection, it is very necessary to use new materials or new structures to ensure that the electrode size is reduced and has a small contact resistance [].
User Experience: Consider the device size factor embedded in wearable devices. It is necessary to evaluate the size of all of the integrated components in the device to meet miniaturization, stabilization, and not to limit the sensing performance due to size. When the equipment is in contact with human skin, it is necessary to consider the wear resistance, respiration, adhesion, and whether it is harmful to the skin. Note that to ensure comfort, the anchorage between the device and the skin needs to be guaranteed. During large movements, the device avoids problems such as poor fit and reduced measurement accuracy [].
Based on this, researchers need to pay more attention to common substances in sweat such as glucose, lactic acid, various electrolyte ions, and so on. The existence of these components occupies a large part of life significance. In addition, there are some macromolecules of exosomes and proteins in sweat. For example, Wu et al. [] reported that exosome proteins were secreted in human sweat by collecting sweat samples from healthy people produced through aerobic exercise. Proteins are also phosphorylated [] as well as glycosylation [], acetylation [], and other translations, leading to Alzheimer’s disease, cancer, heart disease, accelerated aging, and other hazards. However, there are still only a few reports on sensors for proteins in sweat. Therefore, researchers need to examine the practical significance of protein sensors in sweat. Recently, our groups reported an intercalated structural sensing composite based on g-C3N4@Fe3O4, which interacted with phosphoproteins via the synergistic mechanism of the composites for the detection of trace phosphoproteins in sweat. The sensing platform had good selectivity, reproducibility, and stability, with a detection range of 0.01–0.01 mg/mL and a detection limit as low as 9.7 μM. The highlight of the author’s research was that the noninvasive monitoring of trace phosphoproteins in sweat was achieved for the first time. The establishment of this work also lays the foundation for the next design of sweatomics-based sensors to monitor human diseases [].
6. Summary and Outlook
With the development of various technologies, intelligent wearable devices that are comprehensively applied in materials, chemistry, biology, physics, and other disciplines have developed rapidly. In addition to the above-mentioned application fields, wearable devices that can closely adhere to human skin are also rapidly developing in wristbands, bracelets, and other fields.
However, some problems are inevitable. The first problem is with regard to the collection and utilization of sweat. It is necessary to avoid the problem of sweat evaporation, untimely collection, or too long a residence time, which causes the sensing performance to lag. The composition of sweat on the same person may change due to changes in dietary habits, environmental changes, and other factors, or it can be said that the composition of sweat varies from person to person, which will affect the normalization of the monitoring data. Large-scale sweat wearable sensors due to bulky, clumsy defects will hinder the wearer’s daily life, so the lack of biocompatibility also has to be considered. Finally, it should be considered that the power consumption of equipment monitoring, recording, and transmitting information is large.
In the future development of electrochemical technology and integrated device components, we believe that the continuing challenges to the future can be met by considering the ideas described below.
Sweat Collection: The collection methods of sweat samples in previous studies have been quite different. Common methods include the sweat patch method, exercise method, and thermal stimulation method, etc., and there is a lack of certain quality control standards. In addition, the most widely used and most mature sweat induction method is the pilocarpine iontophoresis method. It mainly collects sweat samples using the Macroduct Sweat Stimulation Collection System, which includes a sweat inducer and a sweat collector. The collection methods of sweat include direct methods such as direct pipette suction, glass roller collection, and scraper scraping as well as indirect methods such as using cotton paper, filter paper, gauze block, and other materials to absorb and extract components or separate sweat. However, the latter can contaminate the sample. Therefore, in wearable sweat microfluidic devices, it is necessary to improve sweat sampling and subsequent detection functions, and capillary action can work in deep skin pores to collect and transmit sweat. The focus of subsequent work can be combined with the microfluidic element while improving the interaction between the geometric shape of the sensitive material and the surface tension of the sweat. The microvalve structure and a hydrophilic channel similar to cotton thread can be set up for continuous and controllable transmission. The sensitive area provides samples for testing, and the required sample volume is small.
Air Permeability: Air permeability can be said to be the basic parameter of wearable devices. Although the polymer film substrates commonly used in wearable textile devices satisfy inherent ductility, flexibility, and comfort to wear, they have slightly poorer air permeability and complicated and expensive preparation processes during the assembly of materials and the packaging of sensors. The current flexible electrodes do not take air permeability into consideration, and it is easy to cause discomfort to the skin during long-term monitoring. Moreover, due to the shedding of skin keratin, the long-term accumulation of sweat, oil, etc. will also cause the signal quality to gradually decline. Therefore, it is also a trend for future development to prepare electrodes that are easy to fit, harmless to organisms, have good gas permeability, and can be naturally degraded. The interwoven fiber material with a layered structure can be prepared by a textile process and integrated into the equipment to solve such problems. The fiber can form interlaced voids and pores during the weaving process, and the surface can be further coated with a sensitive layer. In addition, punching holes on the fiber surface is also an effective strategy for restoring air permeability. It can not only prepare diversified patterns, but also apply to clothing and is very practical in wearable sweat smart textiles.
Biocompatibility: However, in addition to excellent sensing performance, good biocompatibility and environmental friendliness also need to be considered for wearable sensors. Most of the traditional flexible substrates use polymer substrates such as polyurethane, polyimide, polyethylene terephthalate, etc., which can cause abnormal skin sensitivity when worn for a long time. Therefore, the development of bio-sensitive materials with natural biocompatibility and biodegradability for use as a wearable period is a huge demand. Therefore, it can be considered to add fibrin to the sensitive material to construct a nano-film, which can not only ensure flexibility, naturalness, and sensitivity, but also realize the monitoring of biomarkers. On the other hand, due to the differences in the skin of different groups of people, the contact between the flexible substrate and the skin needs to be further optimized and improved. For example, although most of the existing flexible sensors have achieved good monitoring results, most of them are in areas with less hair. It is still difficult to ensure the high-quality monitoring of bioelectrical signals in areas with more hair. Therefore, how to ensure that the sensor and the skin form a more stable contact impedance and a larger contact area can stably acquire high-quality bioelectric signals in various complex situations such as the hair area. This is also a problem that needs to be studied and solved in the future. The multi-layered structure and large specific surface area of MXenes can provide a sufficient specific surface area for target detection, biomolecule loading, and electrocatalytic reactions. The intrinsically abundant surface groups can bind to or adsorb other biological recognition elements to enhance the signal response. The most important thing is that it has good biocompatibility. After being modified by biomolecules, the activity of the surface biomolecules can be maintained for a long time, which ensures the stability of the sensor. High sensitivity and low detection limit can be obtained to facilitate the real-time detection of human physiological indicators, and further applications can be expanded in the prevention, diagnosis, and treatment of diseases.
Sensing Array Construction: The traditional sensor design revolves around designing specific markers to develop sensitive materials that specifically bind to it, but it is difficult to form a strong affinity reaction in a complex environment even with related antibodies and enzymes. However, the number of applications of multifunctional sweat flexible sensing devices is relatively small, especially for sensor integration to measure physical and chemical signals. Among them, the array of sensitive materials in the sensor was the focus in order to achieve high selectivity and analytical recognition. On one hand, it is very important to obtain various forms of data to fully mimic and surpass biological skin in one device. On the other hand, more accurate results can be obtained based on different forms of information. Therefore, for wearable sweat sensors, building a sensor array that can analyze and recognize many biomarkers is the main work at present. The sensor array can be continuously functionalized to achieve the simultaneous testing of biomarkers. Obtaining different oxidation–reduction potentials under voltage improved the accuracy of the sensor output. Then, the flexible printed circuit was integrated to conduct the next step of conduction, adjustment, processing, and wireless transmission of the signal of the sensor array. Among them, the molecularly imprinted polymer can select different types of templates to adjust different selection modes during the imprinting process. MIP can be quickly prepared and has excellent thermal, chemical, and mechanical stability.
Continuous Monitoring: At the same time, as we have always emphasized, sweat contains abundant metabolites, electrolytes, hormones, and many small molecules, and continuous multiple data health monitoring has a high potential for the early detection of abnormal conditions of disease. Although many components are only present in extremely low concentrations, the dynamics of sweat affect human metabolism and physiology. Therefore, achieving accurate biochemical continuous monitoring with guaranteed sensing performance in wearable sensing platforms poses a major challenge. For example, most colorimetric-based wearable sweat sensors are only sufficient for one-time monitoring purposes. Typically, for continuous monitoring of a marker signal, a promising architecture is considered to construct an attachable flexible multimodal sensing system. The multimodal biometric acquisition layer is mainly integrated through independent or multiple acquisition methods, and multiple biometric technologies are integrated. Next, it is combined with the hardware layer and the decision layer to realize the continuity of different components starting from human skin sweat. However, what needs to be considered is the integration of complementary sensors or carrying out certain preprocessing, calibration, and response operations as well as combining the action selection layer, the actuator driver layer, and the hardware layer as the action operation module and conducting the next step of precise sensing on the skin interface. In addition, the development of sensing systems also requires decoupling of the target signal from external noise sources. It is believed that under the support of flexible and flexible platform conditions, the early diagnosis of diseases in wearable medical applications will also be more accurate and safer.
Self-Healing Technology: Notably, the self-healing properties of polymer materials extend the durability and long-term use of flexible polymer devices in the health care field. Self-healing sensors that emerge spontaneously in the wearable field not only overcome environmental limitations on detection devices, but also expand the applications of sensors in human health and biomedicine. Hydrogels are currently considered as ideal matrix materials in flexible electronic devices due to their excellent flexibility, self-adhesion, self-healing, high electrical conductivity, and biocompatibility. In addition, compared with flexible sensors based on high-modulus elastomers, hydrogel-type sensors have better stretchability, and can be tightly attached to the skin surface. Typically, a self-healing hydrogel-based sweat-based wearable sensor can be considered for continuous damage detection and localization, with the goal of restoring full functions almost instantaneously at room temperature, ensuring that the healing process is completely autonomous. Among them, electrical conductivity, mechanical strength, flexibility, and antibacterial properties are the most important prerequisites for flexible self-healing sensors. However, if the conductive network polymer hydrogel is constructed with organic conductive polymers, the color of the filler will affect the transparency of the flexible sensor, thus limiting its application in the biomedical field. On the other hand, if filled with inorganic conductive particles, the phase-separated filler and matrix usually reduce the tensile, toughness, and fatigue resistance, and even affect the self-healing ability. Therefore, it can be considered to construct a conductive network with free ions to prepare ion-conducting hydrogels. Ionic conduction is abundant in biological systems, and hydrogels also provide an ideal environment for ion conduction due to the fixed charges on the chains. However, the hydrogel with water as the solvent freezes at low temperature, which will lead to the loss of sensing performance, and the volatility of water will gradually affect the sensing sensitivity. Therefore, the research on anti-freezing and moisturizing performance is also important to promote the development of self-healing technology. We can consider using a binary solvent system using low temperature-resistant organic solvents such as glycerol and ethylene glycol to replace the presence of water solvents. In addition, the mechanosensing capability of the sensor needs to be tested. It mainly uses related technologies to monitor and locate the damage of one-dimensional and two-dimensional surfaces to ensure that they can be used accurately in daily life.
Power Consumption: In order to ensure the basic needs of real-time monitoring throughout the day, capacitive devices such as supercapacitance are generally necessary to achieve more persistent power stability, but repeated charging and working can also result in the high temperature of sensors that affect the sensing performance. Therefore, there is also a tendency to achieve a wearable device without batteries. Long battery life is also an important factor in the application and promotion of sweat wearable sensors. In addition to battery devices, flexible self-generating elements and flexible energy storage elements are also effective ways to provide electrical energy for sensor electronic devices. Different materials directly generate electricity by friction on the surface due to contact. The triboelectric nanogenerator is produced based on the coupling relationship between static and frictional effects, which can effectively acquire mechanical energy and for further conversion to electrical energy. Therefore, designing a suitable structure to increase the power density for use in the field of wearable electronics also has certain potential. Installing TENG on clothes can obtain electrical energy from daily body movement. It has high sensitivity, adaptability, and flexibility, and effectively avoids a series of power consumption problems caused by the use of batteries. In addition, new flexible wearable biofuel cells are increasingly entering the field of vision of researchers. Because biofuel cells rely on small molecules in biological fluids such as lactic acid and glucose in human sweat for energy conversion, they have high biocompatibility and have good application prospects in the wearable field.
Real-Time Data Transmission: Moreover, it can also be used as a medical health assessment system. Finally, 5G technology promotes digital medical services closer to life, and medical personnel can use communication base stations to provide patients with care in remote medical care, public health, and health management. Through the 5G communication link in wearable sweat sensor equipment, data can be calculated to realize big data analysis and an overall case study. This is very suitable for people’s daily fitness exercises and healthy lifestyles to avoid interference caused by distance, environment, and other factors in the signal transmission process, and to provide users with real-time data visualization. According to different application scenarios, corresponding flexible medical sensing devices can be developed to monitor the required health and physiological indicators. Through the 5G network, the data are transmitted to the cloud big data center in real-time. After preliminary screening by artificial intelligence medical algorithms, early changes in human health conditions can be detected in time, and various chronic diseases can be accurately monitored and managed. It can truly achieve preventive health care, reduce the probability of individual illness, and improve the health level of individuals. In addition, it is inevitable to protect the privacy of telemedicine data to avoid the theft of data by third-party software without the user’s knowledge. Therefore, the synergy between 5G technology, medical monitoring, and wearable devices is more in line with the needs of people in the future.
Specific Recognition: The specific recognition element of the sensor is an important part of the sweat sensor. It is involved in the specific identification and capture of target analytes in sweat and converts them into readable signals for output, which is closely related to the selection performance of the sensor. Traditional recognition elements are mainly enzymes, antibodies, etc. isolated from living organisms. However, they cannot meet the needs of rapid diagnosis, improved stability, and cost-effectiveness. Therefore, the gradual development of new identification elements to improve the identification performance of the sensor is the most sustainable development trend in the future. It strives to improve the comprehensive analysis capability of the sweat sensor in terms of the sensitivity, selectivity, detection limit, and signal-to-noise ratio, and further expands the application range of the sweat sensor. In future development, new recognition elements such as aptamers, MIPs, affinity bodies, deoxyribozymes and phages that can specifically recognize the target can be gradually screened out through artificial synthesis and in vitro screening. In addition, the use of genetic engineering technology to synthesize specific proteins and the application of ribosomal RNA probes is also a promising research direction.
In summary, with the development of material manufacturing, nanotechnology, communication technology, and biotechnology, the application of wearable sweat sensing devices will not be limited to the fields of glasses, patches, fabrics, tattoos, and paper. However, new integration methods, substrate materials, and platform designs are also needed to fill it. The ultimate goal is to achieve reliable, accurate, and complete real-time noninvasive medical diagnosis by different paths.
Author Contributions
Writing—original draft, Y.Q. and L.Q.; Data curation, Z.C.; Writing-review and editing, B.L. and L.G.; Visualization, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Qinghai Province (grant number 2020-ZJ-764) and the Xining Science and Technology Bureau (grant number 2021-Y-08).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ya, G.; Yu, H.; Jun, G.; Lei, S.; An, Z.; Shu, F.; Yu, X.; Xing, N. An interrelated CataFlower enzyme system for sensitively monitoring sweat glucose. Talanta 2021, 235, 122799. [Google Scholar]
- Massimo, O.; Stefano, G.; Tommaso, L.; Silvia, G.; Pietro, S.; Fiodor, S.; Emilia, B. Potentiometric sensor for noninvasive lactate determination in human sweat. Anal. Chim. Acta 2017, 989, 80–87. [Google Scholar]
- Youhanna, S.; Devuyst, O. Devuyst, Editors’ Digest–Basic Science a Wearable Sweat Analyzer for Continuous Electrolyte Monitoring. Perit. Dial. Int. 2016, 36, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Arie, T.; Akita, S.; Takei, K. Wearable, Flexible, and Multifunctional Healthcare Device with an ISFET Chemical Sensor for Simultaneous Sweat pH and Skin Temperature Monitoring. ACS Sens. 2017, 2, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ghaffari, R.; Baker, L.B.; Rogers, J.A. Skin-interfaced systems for sweat collection and analytics. Sci. Adv. 2018, 4, 3921–3931. [Google Scholar] [CrossRef] [Green Version]
- Rajamanikandan, R.; Ilanchelian, M. Simple and visual approach for highly selective biosensing of vitamin B1 based on glutathione coated silver nanoparticles as a colorimetric probe. Sens. Actuators B Chem. 2017, 244, 380–386. [Google Scholar] [CrossRef]
- Egmond, K.V.; Wright, C.; Livingston, M.; Kuntsche, E. Wearable Transdermal Alcohol Monitors: A Systematic Review of Detection Validity, and Relationship between Transdermal and Breath Alcohol Concentration and Influencing Factors. Alcohol. Clin. Exp. Res. 2020, 44, 1918–1932. [Google Scholar] [CrossRef]
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Wang, J. Wearable Electrochemical Sensors for the Monitoring and Screening of Drugs. ACS Sens. 2020, 5, 2679–2700. [Google Scholar] [CrossRef]
- Cho, E.; Mohammadifar, M.; Choi, S. A Single-Use, Self-Powered, Paper-Based Sensor Patch for Detection of Exercise-Induced Hypoglycemia. Micromachines 2017, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Venkatesh, H.A. The utility of blood components in the care of sick neonates: An evidence-based review. Glob. J. Transfus. Med. 2020, 5, 27–33. [Google Scholar]
- Mena-Bravo, A.; Luque de Castro, M.D. Sweat: A sample with limited present applications and promising future in metabolomics. J. Pharm. Biomed. Anal. 2014, 90, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Gallardo, E.; Vieira, D.N.; Queiroz, J.A.; López-Rivadulla, M. Bioanalytical procedures and recent developments in the determination of opiates/opioids in human biological samples. Anal. Bioanal. Chem. 2011, 400, 1665–1690. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.L.; Graça, G.; Oliva, A. Characterization of sweat induced with pilocarpine, physical exercise, and collected passively by metabolomic analysis. Ski. Res. Technol. 2017, 24, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jan, V.; Matthew, P.D.; Phuoc, T.T.; Evan, W.; Alexander, W.W.; Edmond, M.K.; Sofie, V.; Jo, V.D.; Valerie, F.; et al. Tissue- and Blood-derived Genomic Biomarkers for Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review. Eur. Urol. Oncol. 2021, 4, 914–923. [Google Scholar]
- Natalie, D.; Douglas, L.; Richard, H.; Erik, W.; Julia, E. Comprehensive genomic profiling (CGP) via peripheral blood liquid biopsies identifies therapeutically relevant genomic alterations in tubo-ovarian and peritoneal cancer. Gynecol. Oncol. 2021, 162, S14. [Google Scholar]
- Stéphane, L.; Philippe, C.; Eva, C.; Sixtina, G.D.D.M.; Olivier, T.; Morgan, R.; Philippe, S.; Géraldine, C.; Karim, S.; François, L.; et al. Diagnostic, Staging, and Grading of Urothelial Carcinomas from Urine: Performance of BCA-1, a Mini-Array Comparative Genomic Hybridisation–Based Test. Eur. Urol. 2011, 59, 250–257. [Google Scholar]
- Ai, Z.; Hui, S.; Xiu, W.; Xi, W. Urine metabolomics Clinica. Chim. Acta 2012, 414, 65–69. [Google Scholar]
- Jian, X.; David, B.; Michael, W.; David, W. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar]
- Domenico, M.; Giuseppe, G.; Federica, V.; Francesco, P. Proteomics for the identification of biomarkers in testicular cancer–review. Front. Endocrinol. 2019, 10, 462–469. [Google Scholar]
- Alharbi, R.A. Proteomics approach and techniques in identification of reliable biomarkers for diseases. Saudi J. Biol. Sci. 2020, 27, 968–974. [Google Scholar] [CrossRef]
- Melissa, D.H.; Sarah, L.S.; Young-Eun, C.; Chen, L.; Caroline, S.; Laken, R.; Jessica, G. Using sweat to measure cytokines in older adults compared to younger adults: A pilot study. J. Immunol. Methods 2017, 454, 1–5. [Google Scholar]
- Jian, Y.; Peng, Z.; Teng, C.; Qiu, L.; Li, G.; Bing, L.; Jun, D.; Zhao, W.; Jian, L. Construction of flexible and wearable 3D TiO2 NTs@Ti mesh for physiological detection based on sweat. JCIS Open. 2021, 2, 10007. [Google Scholar]
- Hashim, U.N.; Salahuddin, L.; Rina, R.; Rabaah, U.; Hariz, M. The Design and Implementation of Mobile Heart Monitoring Applications using Wearable Heart Rate Sensor. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 168–173. [Google Scholar] [CrossRef]
- Mohsen, S.; Zekry, A.; Youssef, K.; Abouelatta, M. On Architecture of Self-Sustainable Wearable Sensor Node for IoT Healthcare Applications. Wirel. Pers. Commun. 2021, 119, 657–671. [Google Scholar] [CrossRef]
- Nouriani, A.; McGovern, R.A.; Raiamani, R. Step length estimation with wearable sensors using a switched-gain nonlinear observer. Biomed. Signal Processing Control 2021, 69, 102822. [Google Scholar] [CrossRef]
- Martin, A.; Voix, J. In-Ear Audio Wearable: Measurement of Heart and Breathing Rates for Health and Safety Monitoring. IEEE Trans. Biomed. Eng. 2017, 65, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Bijender; Kumar, A. Flexible and wearable capacitive pressure sensor for blood pressure monitoring. Sens. Bio-Sens. Res. 2021, 33, 100434. [Google Scholar] [CrossRef]
- Kuzubasoglu, B.A.; Sayar, E.; Cochrane, C.; Koncar, V.; Bahadir, S.K. Wearable temperature sensor for human body temperature detection. J. Mater. Sci. Mater. Electron. 2021, 32, 4784–4797. [Google Scholar] [CrossRef]
- Ali, J. Wearable Sweat Sensors-Towards Big Data for Human Health. Electrochem. Soc. Meet. Abstr. 2021, MA2021–01, 1114. [Google Scholar]
- Jun, T.; Xiao, S.; Yu, W.; Liang, H.; Tian, Z. Flexible plasmonic random laser for wearable humidity sensing. Sci. China Inf. Sci. 2021, 64, 222401. [Google Scholar]
- Yu, H.; Sun, J. Sweat detection theory and fluid driven methods: A review. Nanotechnol. Precis. Eng. 2020, 3, 126–140. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klous, L.; Ruiter, C.D.; Alkemade, P.; Daanen, H.; Gerrett, N. Sweat rate and sweat composition following active or passive heat re-acclimation: A pilot study. Temperature 2021, 8, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Nicola, G.; Katy, G.; Bernard, R.; Thomas, V.; Narihiko, K.; George, H. Sweat from gland to skin surface: Production, transport, and skin absorption. J. Appl. Physiol. 2018, 125, 459–469. [Google Scholar]
- Yun, Z.; Qing, Z.; Da, D.; Tiance, A.; Shu, G.; Qian, S.; Wen, C. A Highly Stretchable and Strain-Insensitive Fiber-Based Wearable Electrochemical Biosensor to Monitor Glucose in the Sweat. Anal. Chem. 2019, 91, 6569–6576. [Google Scholar]
- Mohammad, T.Y.; Bin, L.; Yen, N.T. Emerging Strategies in Enhancing Singlet Oxygen Generation of Nano-Photosensitizers toward Advanced Phototherapy. Nano-Micro Letters 2022, 14, 293–341. [Google Scholar]
- Abellan-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallon, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Salzitsa, A.; Blair, C.; Pawel, B.; Vincenzo, C.; Henry, M.; Bruno, R.; Guang, Y. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar]
- Yuta, S.; Daisuke, N.; Yasuyuki, S.; Toshinobu, R.; Hidehiko, I.; Kotaro, M.; Masato, S.; Takatomo, W.; Takeo, N.; Morio, M.; et al. A novel device for detecting anaerobic threshold using sweat lactate during exercise. Sci. Rep. 2021, 11, 4929. [Google Scholar]
- David, K.; Ramesh, G.; Kai, L.; Sriam, M.; Sriram, M.; Shalini, P. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep. 2017, 7, 13312. [Google Scholar]
- Rebeca, M.T.; Jiao, T.; Yi, Y.; Ji, M.; Min, W.; Yu, S.; You, Y.; Chang, X.; Cui, Y.; Waguih, W.I.; et al. Investigation of Cortisol Dynamics in Human Sweat Using a Graphene-Based Wireless mHealth System. Matter 2020, 2, 921–937. [Google Scholar]
- Madhavi, P.; Badrinath, J.; Kai, L.; Sayali, U.; Devangsingh, S.; Sayali, U.; Sriram, M.; Shalini, P. CATCH (Cortisol Apta WATCH): ‘Bio-mimic alarm’ to track Anxiety, Stress, Immunity in human eccrine sweat. Electrochim. Acta 2021, 390, 138834. [Google Scholar]
- Juliane, R.S.; Ahmed, A.K.; Aftab, A.; Andre, N.D.L.E.S.; Abbas, B.; Lu, Y.; Yugender, G.; Mona, A.M.; Eileen, B.; Jennifer, M.; et al. Epidermal Enzymatic Biosensors for Sweat Vitamin C: Toward Personalized Nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar]
- Jiang, Z.; Hnin, Y.Y.N.; Lei, H.; Yuan, L.; Mallika, B.; Christine, H.A.; Wen, J.; Zhi, F.; Ali, J. A Wearable Nutrition Tracke. Adv. Mater. 2020, 33, 2006444. [Google Scholar]
- Ming, L.; Yang, W.; Dong, L.; Rui, Y.; Jing, X.; Hao, H. A stable sandwich-type amperometric biosensor based on poly(3,4-ethylenedioxythiophene)–single walled carbon nanotubes/ascorbate oxidase/nafion films for detection of L-ascorbic acid. Sens. Actuators B Chem. 2011, 159, 277–285. [Google Scholar]
- Tom, M.M.; John, A.C.; Harris, R.L. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar]
- Li-Chia, T.; Wei, G.; Ming, C.; Bariya, M.; Quynh, P.N.; Ziba, S.; Hnin, Y.Y.N.; Hyejin, P.; Jun, S.; Youn, J.; et al. Methylxanthine Drug Monitoring with xWearable Sweat Sensors. Adv. Mater. 2018, 30, 337. [Google Scholar]
- Anjan, P.S.; Sriram, M.V.K.; Shalini, P. A wearable biochemical sensor for monitoring alcohol consumption lifestyle through Ethyl glucuronide (EtG) detection in human sweat. Sci. Rep. 2016, 6, 1562–1578. [Google Scholar]
- Sung, K.; Jahyun, K.; Jangyeol, Y.; Aurelie, H.F.; Boram, L.; Seongbin, J.; Shu, L.; Jungli, C.; Yong, S.O.; Geumbee, L.; et al. Soft, skin-interfaced microfluidic systems with integrated enzymatic assays for measuring the concentration of ammonia and ethanol in sweat. Lab A Chip 2020, 20, 84–92. [Google Scholar]
- Xue, W.; Miao, Z.; Jia, L.; Liu, L.; Jian, Y.; Zhao, L.; Bin, D. Wearable Biosensor for Sensitive Detection of Uric Acid in Artificial Sweat Enabled by a Fiber Structured Sensing Interface. Nano Energy 2021, 85, 106031. [Google Scholar]
- Jayoung, K.; Somayeh, I.; William, R.D.A.; Julian, W.; Gabriela, V.R.; Thiago, P.; Patrick, P.M.; Joseph, W. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar]
- Libu, M.; Saoirse, D.; Ravinder, D. Flexible potentiometric pH sensors for wearable systems. RSC Adv. 2020, 15, 8594–8617. [Google Scholar]
- Yu, Q.; Li, Q.; Pei, Z.; Peng, Z.; Fan, B.; Jia, Z.; Li, G.; Bing, L.; Lei, Z. Phosphoprotein Detection in Sweat Realized by Intercalation Structure 2D@3D g-C3N4@Fe3O4 Wearable Sensitive Motif. Biosensors 2022, 12, 361. [Google Scholar]
- Daniel, P.R.; Michael, E.R.; Daniel, K.G.; Lin, H.; Nancy, K.L.; Rajesh, R.N.; Joshua, A.H.; Ian, P.; Jason, C.H. Adhesive RFID Sensor Patch for Monitoring of Sweat Electrolytes. IEEE Trans. Biomed. Eng. 2015, 62, 1457–1465. [Google Scholar]
- Margaret, M.; Adam, P.; Ruairi, B.; Paddy, W.; Florien, S.; Gordon, W.; Dermot, D. Wearable Platform for Real-time Monitoring of Sodium in Sweat. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2018, 19, 1531–1536. [Google Scholar]
- Verónica, M.G.; Rafael, F.D.O.; Ye, W.; Andrey, B.; Pablo, F.B.; María, B.G.G.; Thomas, M.H.; Davide, B.; Stefano, C.; Paolo, S. Harnessing Selectivity and Sensitivity in Ion Sensing via Supramolecular Recognition: A 3D Hybrid Gold Nanoparticle Network Chemiresistor. Adv. Funct. Mater. 2020, 31, 2170067. [Google Scholar]
- Marc, P.; María, C.; Sara, P.S.; Mina, R.; Niclas, R.; Frank, N.; Gastón, A.C. Wearable All-Solid-State Potentiometric Microneedle Patch for Intradermal Potassium Detection. Anal. Chem. 2019, 91, 1578–1586. [Google Scholar]
- Choi, D.H.; Yi, L.; Cutting, G.R.; Searsonl, P.C. A wearable potentiometric sensor with integrated salt bridge for sweat chloride measurement. Sens. Actuators B. Chem. 2017, 250, 673–678. [Google Scholar] [CrossRef]
- Dong-Hoon, C.; Jin, S.K.; Garry, R.C.; Peter, C.S. Wearable Potentiometric Chloride Sweat Sensor: The Critical Role of the Salt Bridge. Anal. Chem. 2016, 88, 12241–12247. [Google Scholar]
- Guinovart, T.; Bandodkar, A.; Windmiller, J.; Andrade, F.; Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Anal. 2013, 138, 7031–7038. [Google Scholar] [CrossRef]
- Nicola, C.; Marco, G.; Marco, V.; Valeria, L.; Edmondo, B.; Maria, C.; Andrea, Z. Ion selective textile organic electrochemical transistor for wearable sweat monitoring. Org. Electron. 2019, 78, 105579. [Google Scholar]
- Hnin, Y.Y.N.; Wei, G.; Ziba, S.; Sam, E.; Samyuktha, C.; Kevin, C.; Hossain, M.F.; Li-Chia, T.; Hiroki, O.; Ronald, W.D.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar]
- Kim, J.; Araujo, W.R.; Izabela, A.S.; Amay, J.B.; Jia, W.Z.; Brunetii, B.; Thigo, R.L.C.P.; Joseph, W. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 2015, 51, 41–45. [Google Scholar] [CrossRef]
- Wei, G.; Hnin, Y.Y.N.; Ziba, S.; Hossain, M.F.; Kevin, C.; Sam, E.; Yu, G.; Li-Chia, T.; Hiroki, O.; Eric, W.; et al. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar]
- Yi, C.; Si, L.; Sha, Z.; Yan, L.; Zhe, Q.; Ying, C.; Bing, L.; Xin, W.; Xue, F. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar]
- Zheng, L.; Liu, Y.; Zhang, C. A sample-to-answer, wearable cloth-based electrochemical sensor (WCECS) for point-of-care detection of glucose in sweat. Sens. Actuators B Chem. 2021, 343, 130131. [Google Scholar] [CrossRef]
- Wen, H.; Geng, Y.; Woo, K.; Kai, X. Emerging wearable flexible sensors for sweat analysis. Bio Des. Manuf. 2022, 5, 21. [Google Scholar]
- Hemmes, B.; Wert, L.A.D.; Brink, P.R.G.; Oomens, C.W.J.; Bader, D.L.; Poeze, M. Cytokine IL1α and lactate as markers for tissue damage in spineboard immobilisation. A prospective, randomised open-label crossover trial-ScienceDirect. J. Mech. Behav. Biomed. Mater. 2017, 75, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Mónica, C.S.; Feliciano, P.C.; Natacha, T.; Xavier, R.; Bernabé, J.G.; Sanchez, J.C.; Castro, M.D.L.D. Human sweat metabolomics for lung cancer screening. Anal. Bioanal. Chem. 2015, 407, 5381–5392. [Google Scholar]
- Kintz, P.; Samy, N. Chapter 13 Unconventional samples and alternative matrices. Handb. Anal. Sep. 2000, 2, 459–488. [Google Scholar]
- Porucznik, C.A.; Cox, K.J.; Wilkins, D.G.; Anderson, D.J.; Bailey, N.M.; Szczotka, K.M.; Szczotka, K.M. A Preliminary study of biomonitoring for bisphenol-A in human sweat. J. Anal. Toxicol. 2015, 39, 562–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutkiewicz, E.P.; Lin, J.D.; Tseng, T.W.; Wang, Y.S.; Urban, P.L. Hydrogel micropatches for sampling and profiling skin metabolites. Anal. Chem. 2014, 86, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Harshman, S.W.; Pitsch, R.L.; Schaeublin, N.M.; Smith, Z.K.; Strayer, K.E.; Martin, J.A. Metabolomic stability of exercise-induced sweat. J. Chromatogr. B 2019, 1126, 121763. [Google Scholar] [CrossRef]
- Castro, M.; Priego-Capote, F.; Castro, B.; Castro, F.; Capote. The analytical process to search for metabolomics biomarkers. J. Pharm. Biomed. Anal. 2018, 147, 341–349. [Google Scholar] [CrossRef]
- Boeck, K.D.; Vermeulen, F.; Dupont, L. The diagnosis of cystic fibrosis. La Presse Médicale 2017, 46, e97–e108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meo, A.D.; Sohaei, D.; Batruch, I.; Alexandrou, P.; Diamandis, E.P. Proteomic Profiling of the Human Tissue and Biological Fluid Proteome. J. Proteome Res. 2020, 20, 444–452. [Google Scholar] [CrossRef]
- Delgado-Povedano, M.M.; Calderón-Santiago, M.; Priego-Capote, F.; Castro, L.D. Development of a method for enhancing metabolomics coverage of human sweat by gas chromatography–mass spectrometry in high resolution mode. Anal. Chim. Acta 2016, 905, 115–125. [Google Scholar] [CrossRef]
- Ahmed, S.; Zaynab, S.; Abdulsalam, M.H.; Firas, K.; Mohamed, A.F. Sweat metabolome and proteome: Recent trends in analytical advances and potential biological functions. J. Proteom. 2021, 246, 104310. [Google Scholar]
- Patel, P.K. Cystic Fibrosis in Human-A Review. Curr. Trends Biotechnol. Pharm. 2020, 14, 448–457. [Google Scholar] [CrossRef]
- Cui, X.; Su, G.; Zhang, L.; Yi, S.; Yang, P. Integrated omics analysis of sweat reveals an aberrant amino acid metabolism pathway in Vogt-Koyanagi-Harada disease. Clin. Exp. Immunol. 2020, 200, 250–259. [Google Scholar] [CrossRef]
- Ono, E.; Murota, H.; Mori, Y.; Yoshioka, Y.; Nomura, Y.; Munetsugu, T. Sweat glucose and GLUT2 expression in atopic dermatitis: Implication for clinical manifestation and treatment. PLoS ONE 2018, 13, e0195960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, A.N.; Mathiaparanam, S.; Brick, L.; Keenan, K.; Gonska, T.; Pedder, L.; Hill, S.; Britz-McKibbin, P. The Sweat Metabolome of Screen-Positive Cystic Fibrosis Infants: Revealing Mechanisms beyond Impaired Chloride Transport. ACS Cent. Sci. 2017, 3, 904–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, M.; Hernández, G.; Gartner, S. Protocol for the diagnosis and follow up of patients with cystic fifibrosis, Anales Pediatr. An. Pediatr. 2009, 71, 250–264. [Google Scholar]
- Lin, C.; Rui, C.; Su, P.; Jing, X.; Qing, C.; Guan, S.; Chun, Z.; Aize, K.; Pei, Y. Plasma metabolomics study of Vogt-Koyanagi-Harada disease identifies potential diagnostic biomarkers. Exp. Eye Res. 2020, 196, 108070. [Google Scholar]
- Darlenski, R.; Kazandjieva, J.; Hristakieva, E.; Fluhr, J.W. Pic dermatitis as a systemic disease. Clin. Dermatol. 2014, 32, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Murota, H.; Mori, Y.; Yoshioka, Y.; Katayama, I. Metabolomic analysis of sweat revealed glucose as a biomarker of atopic dermatitis. J. Dermatol. Sci. 2017, 86, e30. [Google Scholar] [CrossRef]
- Sabino, F.; Hermes, O.; Auf, U. Body Fluid Degradomics and Characterization of Basic N-Terminome. Methods Enzymol. 2017, 585, 177–199. [Google Scholar]
- Dervisevic, M.; Alba, M.; Beatriz, P.S.; Voelcker, N.H. Skin in the diagnostics game: Wearable biosensor nano- and microsystems for medical diagnostics. Nano Today 2020, 30, 100828. [Google Scholar] [CrossRef]
- Jing, X.; Yang, L.; Lei, S.; Dan, Z.; Xue, Z. Microfluidic Chip-Based Wearable Colorimetric Sensor for Simple and Facile Detection of Sweat Glucose. Anal. Chem. 2019, 91, 14803–14807. [Google Scholar]
- Gang, X.; Jing, H.; Xiao, C.; Yan, Q.; Feng, W.; Qing, X.; Ling, Y. A wearable, cotton thread/paper-based microfluidic device coupled with smartphone for sweat glucose sensing. Cellulose 2019, 26, 4553–4562. [Google Scholar]
- Nadtinan, P.; Pranee, R.; Krisana, S.; Niphaphum, S.; Pranut, P.; Chusak, T.; Juan, P.H.; Nadnudda, R. Non-invasive textile based colorimetric sensor for the simultaneous detection of sweat pH and lactate. Talanta 2019, 192, 424–430. [Google Scholar]
- Ashlesha, B.; Sriram, M.; Amreek, S.; Shalini, P. Simultaneous lancet-free monitoring of alcohol and glucose from low-volumes of perspired human sweat. Sci. Rep. 2018, 8, 6507. [Google Scholar]
- Semih, C. Sodium Polyacrylate Microparticle Containing Multifunctional Skin Patch for Sweat Analysis. Microchem. J. 2020, 159, 105473. [Google Scholar]
- Michele, C.; Claudio, C.; Emanuela, G.; Valerio, R.; Giuseooe, R. Optical monitoring of sweat pH by a textile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sens. Actuators B Chem. 2016, 222, 213–220. [Google Scholar]
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Jin, S.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous capillary-flow sensing of glucose and lactate in sweat with an electrochemical sensor based on functionalized graphene oxide. Sens. Actuators B Chem. 2021, 344, 130253. [Google Scholar] [CrossRef]
- Qing, Z.; Dan, J.; Chang, X.; Yuan, G.; Xiao, L.; Qing, W.; Li, H.; Xue, R.; Cheng, W.; Yi, W. Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens. Actuators B Chem. 2020, 320, 128325. [Google Scholar]
- Tur-García, E.L.; Davis, F.; Collyer, S.D.; Holmes, J.L.; Barr, H.; Higson, S.P.J. Novel flexible enzyme laminate-based sensor for analysis of lactate in sweat. Sens. Actuators B Chem. 2017, 242, 502–510. [Google Scholar] [CrossRef]
- Lin, K.C.; Muthukumar, C.; Prasad, S. Flex-GO (Flexible graphene oxide) sensor for electrochemical monitoring lactate in low-volume passive perspired human sweat. Talanta 2020, 214, 120810. [Google Scholar] [CrossRef]
- Yu, W.; Tsao, P.K.; Rinawati, M.; Kuan-Jung, C.; Kuei-Yuan, C.; Chih-Yu, C.; Yeh, M.H. Designing ZIF-67 derived NiCo layered double hydroxides with 3D hierarchical structure for Enzyme-free electrochemical lactate monitoring in human sweat. Chem. Eng. J. 2022, 427, 131687. [Google Scholar]
- Shitanda, I.; Mitsumoto, M.; Loew, N.; Yoshihara, Y.; Watanabe, H.; Mikawa, T.; Tsujimura, S.; Itagaki, M.; Motosuke, M. Continuous sweat lactate monitoring system with integrated screen-printed MgO-templated carbon-lactate oxidase biosensor and microfluidic sweat collector. Electrochim. Acta 2020, 368, 137620. [Google Scholar] [CrossRef]
- Li, Z.; Jian, L.; Zhen, F.; Li, Q. A Wearable Biosensor Based on Bienzyme Gel-Membrane for Sweat Lactate Monitoring by Mounting on Eyeglasses. J. Nanosci. Nanotechnol. 2020, 20, 1495–1503. [Google Scholar]
- Ren, W.; Qing, Z.; Tiance, A.; Shu, G.; Wen, C. Stretchable gold fiber-based wearable textile electrochemical biosensor for lactate monitoring in sweat. Talanta 2021, 222, 121484. [Google Scholar]
- Azar, A.; Andre, B.; Ralf, L.; Rachel, G.; Jeffrey, A.; Adam, P.; Margaret, M.; Ruairi, B.; Dermot, D. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab A Chip 2018, 18, 2632–2641. [Google Scholar]
- Pirovano, P.; Dorrian, M.; Shinde, A.; Donohoe, A.; Brady, A.J.; Moyna, N.M.; Wallace, G.; Diamond, D.; McCaul, M. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 2020, 219, 121145. [Google Scholar] [CrossRef]
- Vincenzo, M.; Luca, F.; Simone, N.; Gaetano, M.; Fabiana, A. Medium-distance affordable, flexible and wireless epidermal sensor for pH monitoring in sweat. Talanta 2020, 222, 121506. [Google Scholar]
- Yoon, J.H.; Kim, S.M.; Park, H.J.; Kim, Y.K.; Oh, D.X. Highly self-healable and flexible cable-type pH sensors for real-time monitoring of human fluids. Biosens. Bioelectron. 2020, 150, 111946. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Libu, M.; William, T.N.; Leandro, L.; Vincenzo, V.; Ravinder, D. Stretchable wireless system for sweat pH monitoring. Biosens. Bioelectron. 2018, 107, 192–202. [Google Scholar]
- Francesca, M.; Bernardo, P.; Chiara, D.A.; Maria, G.B.; Sonia, C.; Francesco, L.; Giuseppe, A.; Claudia, T.; Antonio, V.; Alan, O.R.; et al. PANI-Based Wearable Electrochemical Sensor for pH Sweat Monitoring. Chemosensors 2021, 9, 169. [Google Scholar]
- Mazzaracchio, V.; Serani, A.; Fiore, L.; Moscone, D.; Arduini, F. All-solid-state ion-selective carbon black-modified printed electrode for sodium detection in sweat. Electrochimica Acta. 2021, 394, 139050. [Google Scholar] [CrossRef]
- Gauglitz, G. ABC Spotlight on paper-based strips analytics. Anal. Bioanal. Chem. 2018, 41, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Amay, J.B.; Denise, M.; Omar, M.; Tomás, G.; Joshua, R.W.; Gabriela, V.R.; Francisco, J.A.; Michael, J.S.; Joseph, W. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar]
- Sekar, M.; Allen, J.A.; Sriramprabha, R.; Pandiaraj, M.; Shekhar, B.; Ponpandian, N.; Viswanathan, C. ZnO Nanorod Integrated Flexible Carbon Fibers for Sweat Cortisol Detection. ACS Publ. 2020, 2, 499–509. [Google Scholar]
- Joong, S.N.; Sharat, C.B.; Md, A.Z.; Md, S.; Hyosang, Y.; Chani, P.; Sangyuk, Y.; Shi, Z.; Jae, Y.P. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2020, 329, 129206. [Google Scholar]
- Chen, C.; Xin, L.; Gang, X.; Yan, L.; Sze, S.L.; Guang, L.; Long, Z.; Cai, L.; Qing, L. Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens. Bioelectron. 2021, 172, 112782. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Wei, S.; Liang, T.; Meng, S.; Meng, J.; Jing, L.; Hai, G.; Chunmei, Y. Preparation of nanostructured PDMS film as flexible immunosensor for cortisol analysis in human sweat. Anal. Chim. Acta 2021, 1184, 339010. [Google Scholar]
- Lu, X.; Zhao, C. Exercise and Type 1 Diabetes. Adv. Exp. Med. Biol. 2020, 1228, 107–121. [Google Scholar]
- Sushmitha, V.; Sushmee, B. Two-Dimensional Metallic NiSe2 Nanoclusters-Based Low-Cost, Flexible, Amperometric Sensor for Detection of Neurological Drug Carbamazepine in Human Sweat Samples. Front. Chem. 2020, 8, 337. [Google Scholar]
- Pavinatto, A.; Imani, S.; Sempionatto, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab A Chip 2017, 17, 1834–1842. [Google Scholar]
- Petar, K.; Marija, S.; Ivana, M.S. Paper-based ion-selective optodes for continuous sensing: Reversible potassium ion monitoring. Talanta 2019, 193, 51–55. [Google Scholar]
- Yu, W.; Xiao, W.; Wei, L.; Qing, Y.; You, Z.; Bo, Y. A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat. Talanta 2019, 198, 62–89. [Google Scholar]
- Zhao, Z.; Li, Q.; Sun, Y.; Zhao, C.; Chen, Y. Highly sensitive and portable electrochemical detection system based on AuNPs@CuO NWs/Cu2O/CF hierarchical nanostructures for enzymeless glucose sensing. Sens. Actuators B Chem. 2021, 345, 130379. [Google Scholar] [CrossRef]
- Yoon, H.; Nah, J.; Kim, H.; Ko, S.; Sharifuzzaman, M.; Barman, S.C.; Xuan, X.; Kim, J.; Park, J.Y. A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection-ScienceDirect. Sens. Actuators B Chem. 2021, 311, 127866. [Google Scholar] [CrossRef]
- Curto, V.F.; Coyle, S.; Byrne, R.; Angelov, N.; Diamond, D.; Benito-Lopez, F. Concept and development of an autonomous wearable micro-fluidic platform for real time pH sweat analysis. Sens. Actuators B Chem. 2012, 175, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Yu, L.; You, H.; Li, T.; Guo, Z. Gold nanostructure-programmed flexible electrochemical biosensor for detection of glucose and lactate in sweat. J. Electroanal. Chem. 2021, 882, 115029. [Google Scholar]
- Seung, Y.O.; Soo, Y.H.; Yu, R.J.; Junyeong, Y.; Heun, P.; Sang, W.J.; Geumbee, L.; Ju, H.O.; Hanchan, L.; Sang-Soo, L.; et al. Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and pH Detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar]
- Rafael, H.; Pascal, B.; Francisco, J.A. IonSens: A Wearable Potentiometric Sensor Patch for Monitoring Total Ion Content in Sweat. Electroanalysis 2018, 30, 1536–1544. [Google Scholar]
- Gamella, M.; Campuzano, S.; Manso, J.; González de Rivera, G.; López-Colino, F.; Reviejo, A.J.; Pingarrón, J.M. A novel non-invasive electrochemical biosensing device for in situ determination of the alcohol content in blood by monitoring ethanol in sweat. Anal. Chim. Acta 2014, 806, 1–7. [Google Scholar] [CrossRef]
- Sánchez, M.; González, A.; Sabio, L.; Zou, W.; Dominguez-Vera, J.M. Photochromic polyoxometalate–based enzyme-free reusable sensors for real-time colorimetric detection of alcohol in sweat and saliva. Mater. Today Chem. 2021, 21, 100491. [Google Scholar] [CrossRef]
- Hussain, M.H.; Fook, L.P.; Putri, M.; Tan, H.L.; Bakar, N.; Radacsi, N. Advances on ultra-sensitive electrospun nanostructured electrochemical and colorimetric sensors for diabetes mellitus detection. Nano Mater. Sci. 2021, 3, 321–343. [Google Scholar] [CrossRef]
- Frank, W.B.; Christian, K.R.; Matthew, J.L. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2021, 2, 1143–1211. [Google Scholar]
- Ahyeon, K.; Daeshik, K.; Ye, X.; Seungmin, L.; Rafal, M.P.; Jeonghyun, K.; Taehwan, H.; Seunghwan, M.; Anthony, B.; Philippe, B.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 165. [Google Scholar]
- Matthew, G.; Peter, E. Dietary Vitamin C in Human Health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar]
- Gabriel, H.R.; Qiang, L.; Tao, Y.; Cong, C.; Alex, I.; Tian, J.; Dimitri, P.O.; Erin, B.; Sylvane, D.; Barbara, R.; et al. Association of Gray Matter and Personality Development With Increased Drunkenness Frequency During Adolescence. JAMA Psychiatry 2019, 77, 409–419. [Google Scholar]
- Güneş, A.; Berna, H. Determination of ethyl glucuronide (EtG) in blood samples using partial least squares discriminant analysis applied to surface-enhanced Raman spectroscopy. Vib. Spectrosc. 2020, 106, 103012. [Google Scholar]
- Emilie, E.A.; Andreas, L.; Anton, L.; Peter, A.; Ilona, K.; Sven, A.; Claes-Göran, Ö.; Anna-Karin, D. Alcohol and type 2 diabetes: The role of socioeconomic, lifestyle and psychosocial factors. Scand. J. Public Health 2018, 47, 408–416. [Google Scholar]
- Chun, C.; Yuan, W.; Shui, D.; Cheng, H.; Zi, W. A novel sensitive and selective electrochemical sensor based on integration of molecularly imprinted with hollow silver nanospheres for determination of carbamazepine. Microchem. J. 2019, 147, 191–197. [Google Scholar] [CrossRef]
- Shu, L.; Bo, W.; Wen, Y.; Kait, C.; Clastillo, H.; Xuan, C.; Yi, Z.; Yu, G.; Zhao, W.; Hai, L.; et al. Design Framework and Sensing System for Noninvasive Wearable Electroactive Drug Monitoring. ACS Sens. 2020, 5, 265–273. [Google Scholar]
- Subhas, C.M. Wearable Sensors for Human Activity Monitoring: A Review. IEEE Sens. J. 2021, 15, 1321–1330. [Google Scholar]
- Jing, N.; Yan, L.; Yi, H.; Yuan, W.; Steghen, X.; Matthias, P.; Xiao, J. SPIDERS+: A light-weight, wireless, and low-cost glasses-based wearable platform for emotion sensing and bio-signal acquisition. Pervasive Mob. Comput. 2021, 75, 101724. [Google Scholar]
- Yi, Y.; Yu, S.; Xiang, B.; Ji, M.; On, S.P.; Lai, Z.; Min, W.; Jiao, T.; Adam, K.; Hai, Z.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar]
- Jaehong, L.; Byron, L.Z.; Janghoon, W.; Kukro, Y.; Taeyoon, L. Recent Advances in 1D Stretchable Electrodes and Devices for Textile and Wearable Electronics: Materials, Fabrications, and Applications. Adv. Mater. 2019, 32, 1902532. [Google Scholar]
- Tiance, A.; David, V.A.; Shu, G.; Lim, W.Y.; Fenge, L.; Ren, W.; Mehmet, R.Y.; Wen, C. Self-powered gold nanowire tattoo triboelectric sensors for soft wearable human-machine interface. Nano Energy 2020, 77, 105295. [Google Scholar]
- Jun, S.; Lin, G.; Ying, L.; Pei, W.; Xiao, D.; Xing, Y.; Kong, Q.; Lu, L. MXene/tissue paper composites for wearable pressure sensors and 2thermotherapy electronics. Thin Solid Film. 2022, 743, 139054. [Google Scholar]
- Hao, D.; Yu, X.; Jun, W. Nanomaterial-sensors for herbicides detection using electrochemical techniques and prospect applications. TrAC Trends Anal. Chem. 2021, 135, 116178. [Google Scholar]
- Fatemeh, H.; Leila, N.; Majid, A. Comparative study of electrochemically-grown vanadium pentoxide nanostructures synthesized using differential pulse voltammetry, cyclic voltammetry, and chronoamperometry methods as the hole transport layer. J. Alloys Compd. 2022, 900, 163501. [Google Scholar]
- Pan, M.; Lin, W.; Wang, H.; Hsieh, S.; Hsieh, P.; Su, Y.; Huang, P. Determination of carbamazepine: A comparison of the differential pulse voltammetry (DPV) method and the immunoassay method in a clinical trial. J. Anal. Chem. 2014, 69, 57–61. [Google Scholar] [CrossRef]
- Yi, D.; Alireza, M.; Kevin, A.; Chung, L. A Cuprous Oxide Thin Film Non-Enzymatic Glucose Sensor Using Differential Pulse Voltammetry and Other Voltammetry Methods and a Comparison to Different Thin Film Electrodes on the Detection of Glucose in an Alkaline Solution. Biosensors 2018, 8, 4. [Google Scholar]
- Yan, Z.; Li, C.; Xin, Y.; Fei, L.; Yi, X.; Xin, L.; Shao, W. Dual-mode biosensor for femtomolar miRNA-155 detection by electrochemiluminescence and adsorptive stripping voltammetry. Microchem. J. 2021, 165, 106091. [Google Scholar]
- Grégoire, H.; Valerio, B. Stripping voltammetry at micro-interface arrays: A review. Anal. Chim. Acta 2013, 769, 10–21. [Google Scholar]
- Jorge, H.A.; Mario, V.; José, C.C. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2016, 221, 1371–1381. [Google Scholar]
- John, C.H.; Megan, A.M.; Lawrence, A.B. Diagnostic Criteria for the Characterization of Electrode Reactions with Chemical Reactions Following Electron Transfer by Cyclic Square Wave Voltammetry. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2016, 17, 2596–2606. [Google Scholar]
- Balwant, K.S.; Aasiya, S.; Rajiv, O.D.; Smrutiranjan, P. Copper oxide nanosheets and nanowires grown by one-step linear sweep voltammetry for supercapacitor application. J. Energy Storage 2020, 31, 101631. [Google Scholar]
- Yuki, U.; Enno, K.; Richard, G.C. Linear sweep voltammetry with non-triangular waveforms: New opportunities in electroanalysis. J. Electroanal. Chem. 2018, 818, 140–148. [Google Scholar]
- Ajay, P.V.S.; Printo, J.; Kiruba, D.; Lakshmanan, S.; Takatoshi, K.; Sivakumar, M. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar]
- Yan, L.; Liang, F. Progress in Paper-based Colorimetric Sensor Array. Chin. J. Anal. Chem. 2020, 48, 1448–1457. [Google Scholar]
- Jing, S.; Yue, L.; Liu, H.; Jia, P.; Feng, Y.; Yue, L. Colorimetric sensor array based on gold nanoparticles: Design principles and recent advances. TrAC Trends Anal. Chem. 2020, 122, 115754. [Google Scholar]
- Nina, M.; Marco, H.; Frédéric, O.; Jong-Sook, L.; Vanesa, R.; Emilio, N.; Steffen, S.; Gareth, H.; Rinaldo, R.; Miran, G.; et al. Application of electrochemical impedance spectroscopy to commercial Li-ion cells: A review. J. Power Sources 2020, 480, 228742. [Google Scholar]
- Jia, H.; Ying, Z.; Jayne, W. Review of Non-invasive Continuous Glucose Monitoring Based on Impedance Spectroscopy. Sens. Actuators A Phys. 2020, 311, 112103. [Google Scholar]
- Jose, M.; Raquel, M.; Mireia, B. Trends in Electrochemical Impedance Spectroscopy involving nanocomposite transducers: Characterization, architecture surface and bio-sensing. Trac Trends Anal. Chem. 2017, 97, 201–205. [Google Scholar]
- Burakhta, V.A.; Sataeva, S.S. A Gallium Arsenide Sensor for the Potentiometric Titration of Silver, Copper, Lead, and Cadmium Ions. J. Anal. Chem. 2020, 75, 1080–1085. [Google Scholar] [CrossRef]
- Somaiyeh, K.K.; Xuan, A.N.; Hamid, A.; Robert, B.; Sarah, C.; Marcel, M.; Nichola, M.; Yorck-Michael, N.; Graeme, P. Activity-based Analysis of Potentiometric pH Titrations. Anal. Chim. Acta 2019, 1075, 49–56. [Google Scholar]
- Zhong, Z.; David, W.F. Modified potentiometric titration method to distinguish and quantify oxygenated functional groups on carbon materials by pK a and chemical reactivity. Carbon 2020, 166, 436–445. [Google Scholar] [CrossRef]
- Jin, H.; Andreas, S.; Philippe, B. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar]
- Jing, W.; Hong, Z. Recent advances in electrochemical techniques for characterizing surface properties of minerals. Adv. Colloid Interface Sci. 2021, 288, 102346. [Google Scholar]
- Sam, E.; Gao, W.; Eric, W.; Zoe, A.D.; Hnin, Y.Y.N.; Samyuktha, C.; Sean, P.R.; Hossain, M.; Kevin, C.; Ziba, S.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar]
- Dong, L.; Wen, L.; Yi, Z.; Wei, H. Printable Transparent Conductive Films for Flexible Electronics. Adv. Mater. 2018, 30, 1704738. [Google Scholar]
- Wei, G.; Hiroki, O.; Daisuke, K.; Kuniharu, T.; Ali, J. Flexible Electronics toward Wearable Sensing. Acc. Chem. Res. 2019, 52, 523–533. [Google Scholar]
- Byeong, U.H.; Ju-Hyuck, L.; Tran, Q.T.; Eun, R.; Do-Il, K.; Sang-Woo, K.; Nae-Eung, L. Transparent Stretchable Self-Powered Patchable Sensor Platform with Ultrasensitive Recognition of Human Activities. ACS Nano 2015, 9, 8801–8810. [Google Scholar]
- Tanmoy, D.; Bhupendra, K.S.; Ajit, K.K.; Jong, H.A. Graphene-based flexible and wearable electronics. J. Semicond. 2018, 39, 90–108. [Google Scholar]
- Yin, M.; Hai, L.; Si, C.; Yan, M.; Jia, C.; Xue, F. Skin-Like Electronics for Perception and Interaction: Materials, Structural Designs, and Applications. Adv. Intell. Syst. 2020, 3, 1–17. [Google Scholar]
- Shun, M.; Tian, W.; Xin, C.; Yin, W.; Hong, T.; Yu, Y.; Yan, W.; Jian, D.; Jing, W.; Zheng, S.; et al. An artificial neural network chip based on two-dimensional semiconductor. Sci. Bull. 2022, 67, 270–277. [Google Scholar]
- Abdelrahman, B.; Hong, Z. Nanocellulose-Graphene Hybrids: Advanced Functional Materials as Multifunctional Sensing Platform. Nano-Micro Lett. 2021, 13, 231–267. [Google Scholar]
- Jiao, H.; Rui, L.; Ye, S. An overview of healthcare monitoring by flexible electronics. Sci. China Phys. Mech. Astron. 2018, 61, 5–22. [Google Scholar]
- Shi, C.; Zhi, H.; Feng, W.; Kun, Z.; Yu, C.; Yin, M.; Xue, F. Review on flexible photonics/electronics integrated devices and fabrication strategy. Sci. China Inf. Sci. 2018, 61, 5–31. [Google Scholar]
- Yong, H.; Chen, Z.; Wen, X.; Yu, W.; Yong, J.; Lei, Q.; Dong, G.; Chao, H.; Shan, J.; Zhao, Y.; et al. Flexible smart sensing skin for “Fly-by-Feel” morphing aircraft. Sci. China Technol. Sci. 2022, 65, 1–29. [Google Scholar]
- Naveen, T.; Subhodeep, C.; Kuldeep, K.; Jun, C.; Kai, F.; Zong, L. Recent advancements in sampling, power management strategies and development in applications for non-invasive wearable electrochemical sensors. J. Electroanal. Chem. 2022, 907, 116064. [Google Scholar]
- Chang, W.; Zheng, L. Proteomic Profiling of Sweat Exosome Suggests its Involvement in Skin Immunity. J. Investig. Dermatol. 2018, 138, 89–97. [Google Scholar]
- Vani, S.R.; Tabea, M.S.; Margaret, V.W. Protein Kinase C-Mediated Cardiac Troponin I S43/45 Phosphorylation Causes Contractile Dysfunction in Human Heart Failure and in Rodents-ScienceDirect. Biophys. J. 2019, 116, 29a. [Google Scholar]
- Yasmine, F.M.; Nichollas, E.S.; Antonio, M.; Carole, C.; Ximena, O.; Ganjana, L.; Michael, M.T.; Heather, G.; Andrew, M.J.; David, D.; et al. A general protein O-glycosylation machinery conserved in Burkholderia species improves bacterial fitness and elicits glycan immunogenicity in humans. J. Biol. Chem. 2019, 36, 13248–13268. [Google Scholar]
- Alejandra, Z.M.; Ramsés, G.W.; Roxana, C.; Luis-Ángel, N.; Mabel, B. The regulation of protein acetylation influences the redox homeostasis to protect the heart. Life Sci. 2021, 277, 119599. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).