Electrochemical Immunosensor Modified with Nitrogen-Doped Reduced Graphene Oxide@Carboxylated Multi-Walled Carbon Nanotubes/Chitosan@Gold Nanoparticles for CA125 Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Synthesis of N-rGO@CMWCNTs
2.4. Synthesis of CS@AuNPs
2.5. Analytical Procedure
2.6. Collection of Serum Samples
3. Results and Discussion
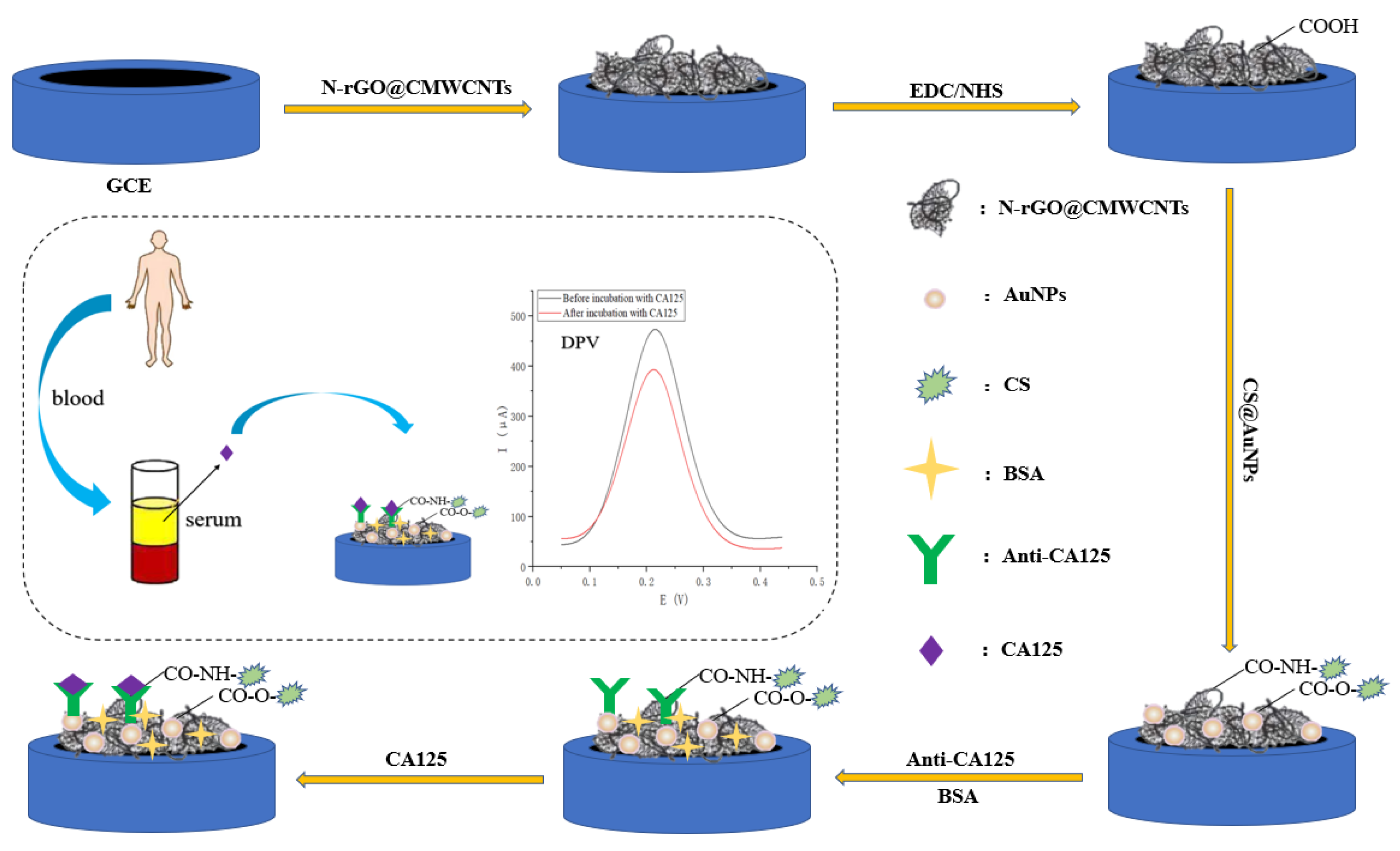
3.1. Sensing Schemes
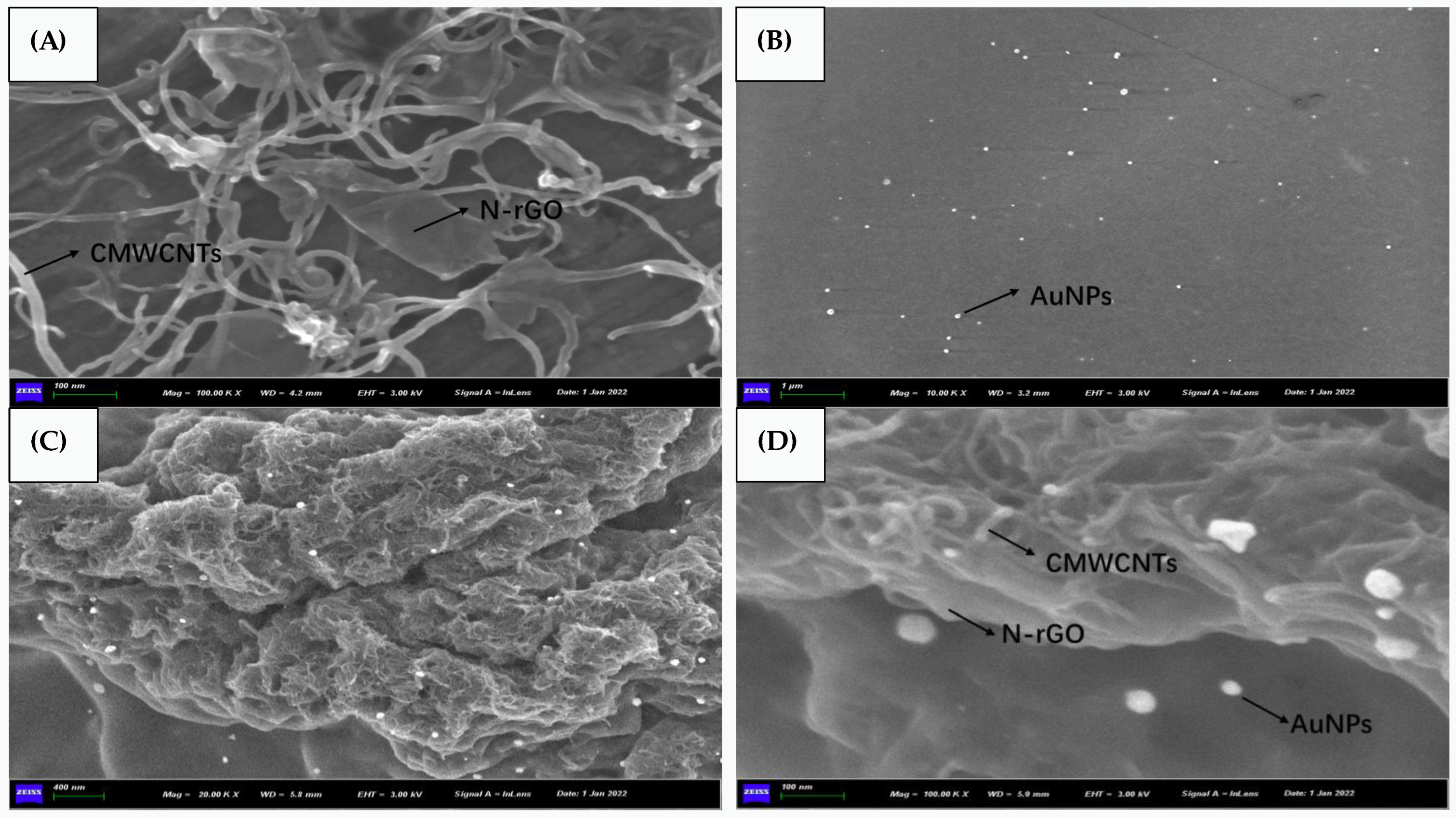
3.2. Morphology Characterization
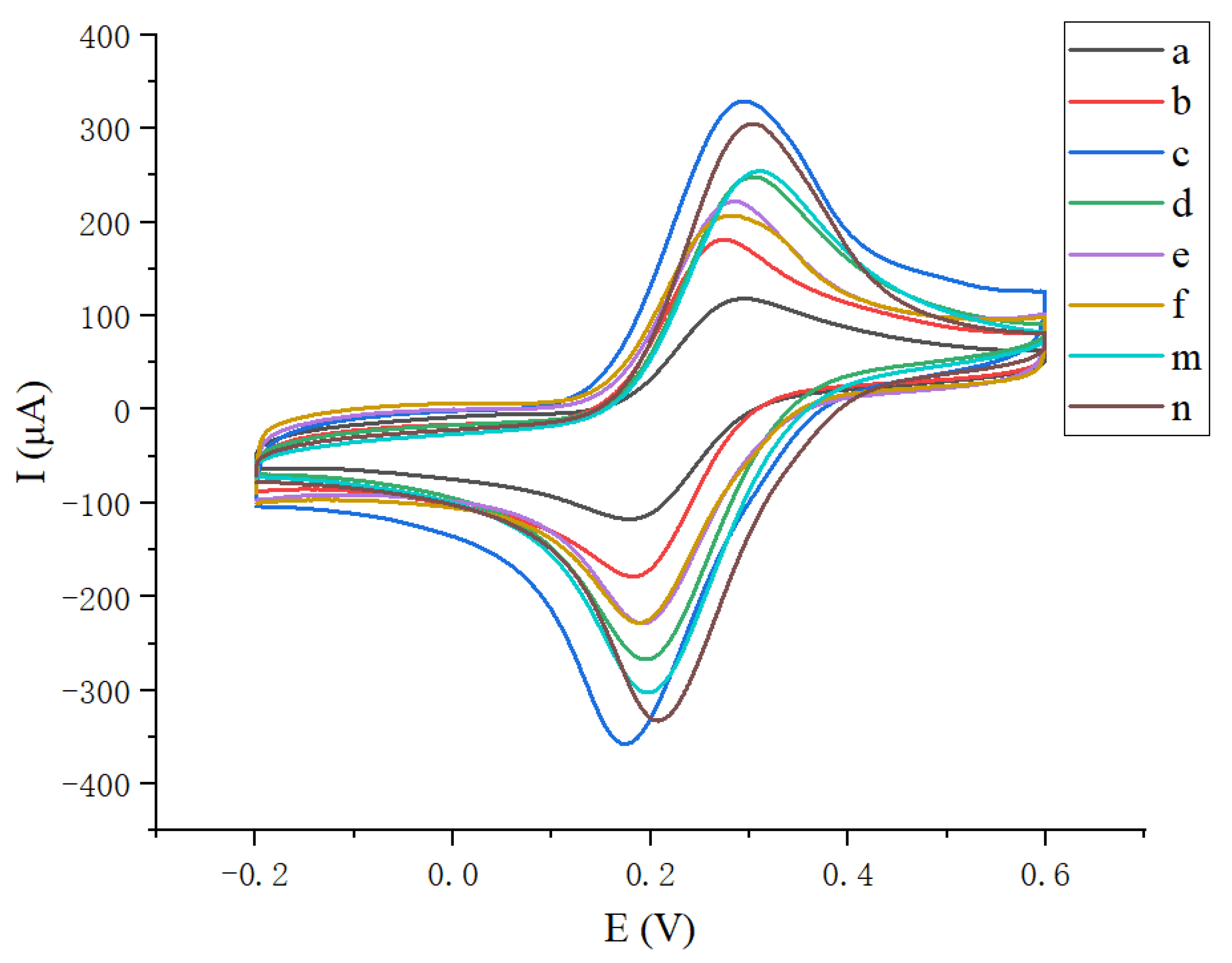
3.3. Electrochemical Characterization
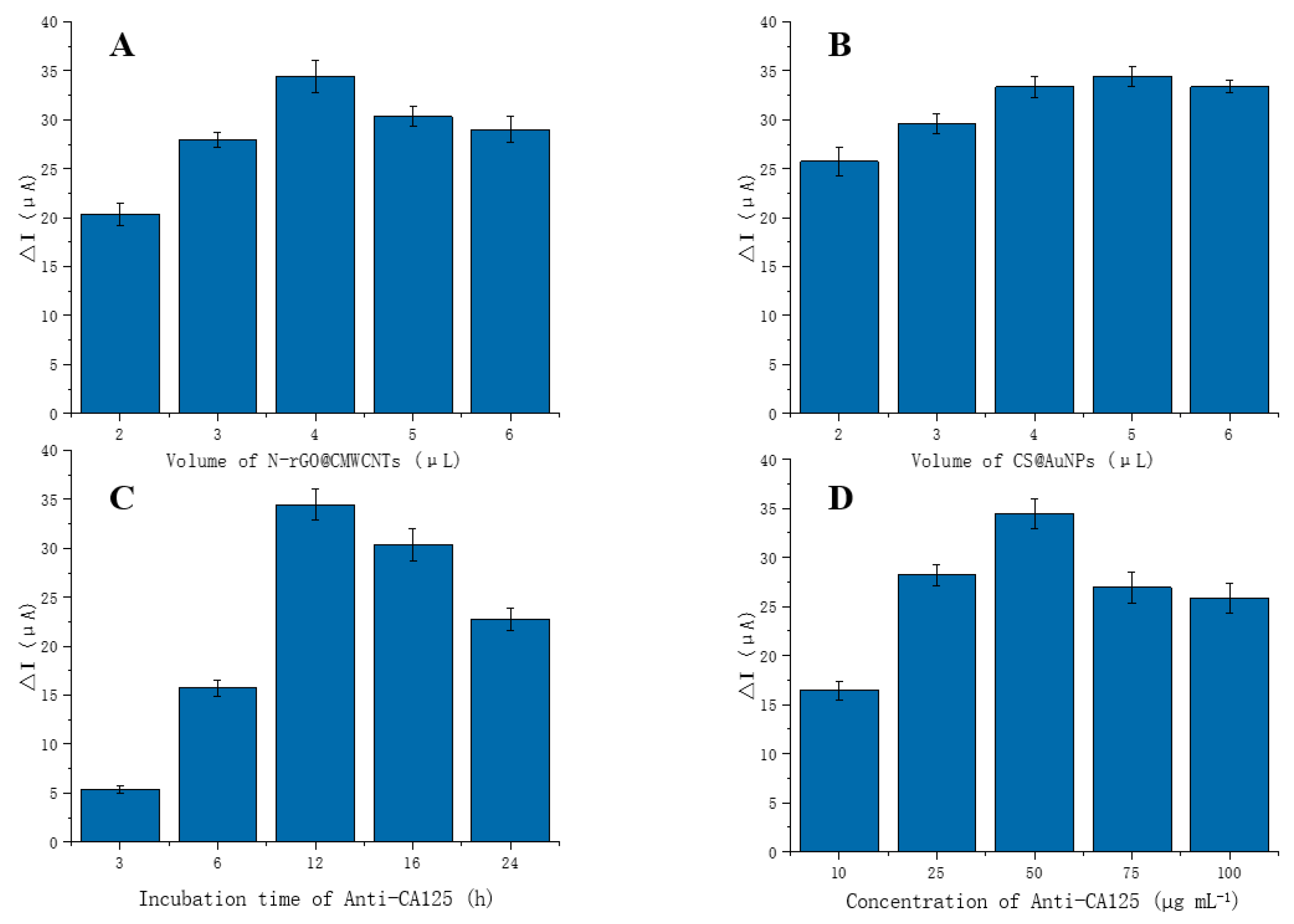
3.4. Optimization of Experimental Conditions
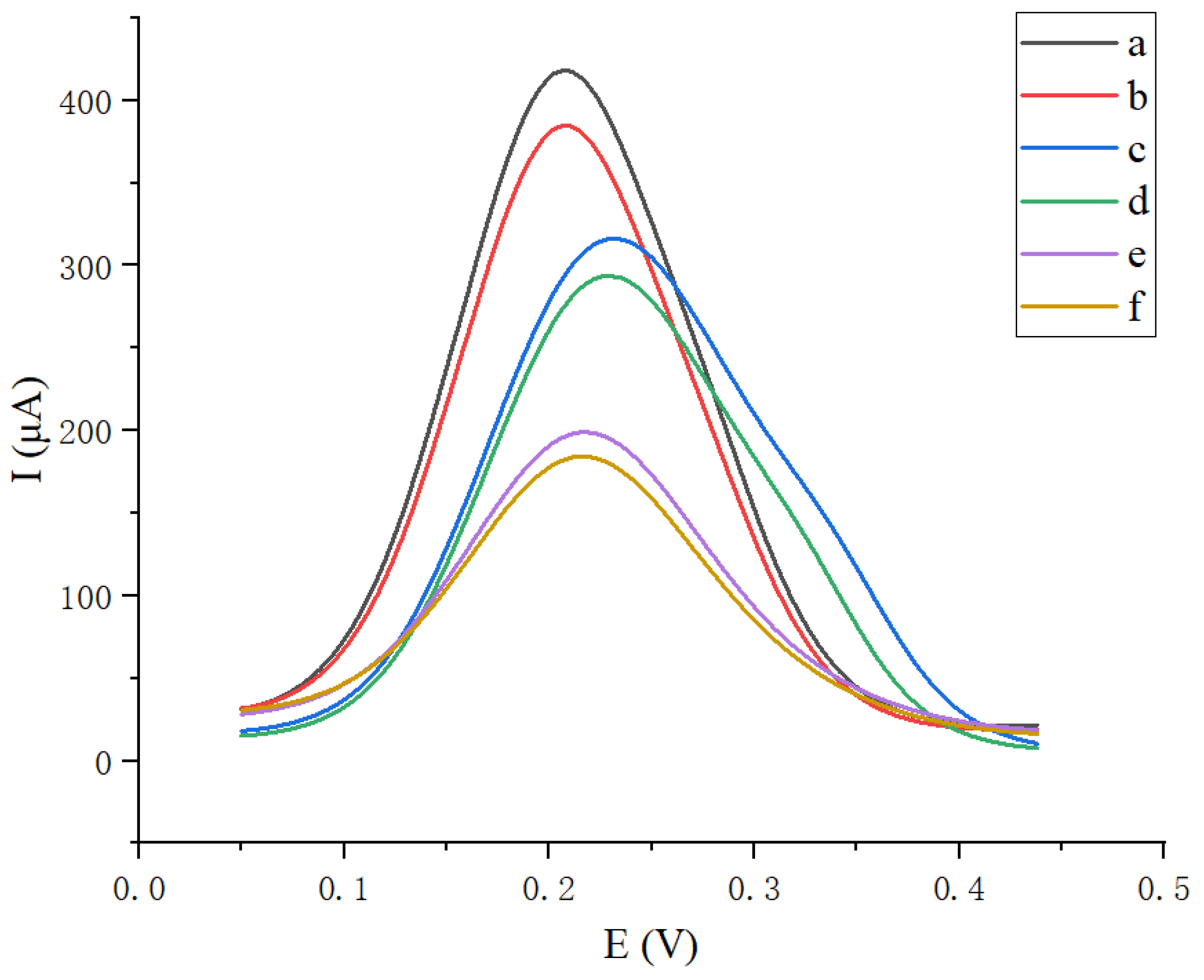
3.5. Linear Range and Detection Limit
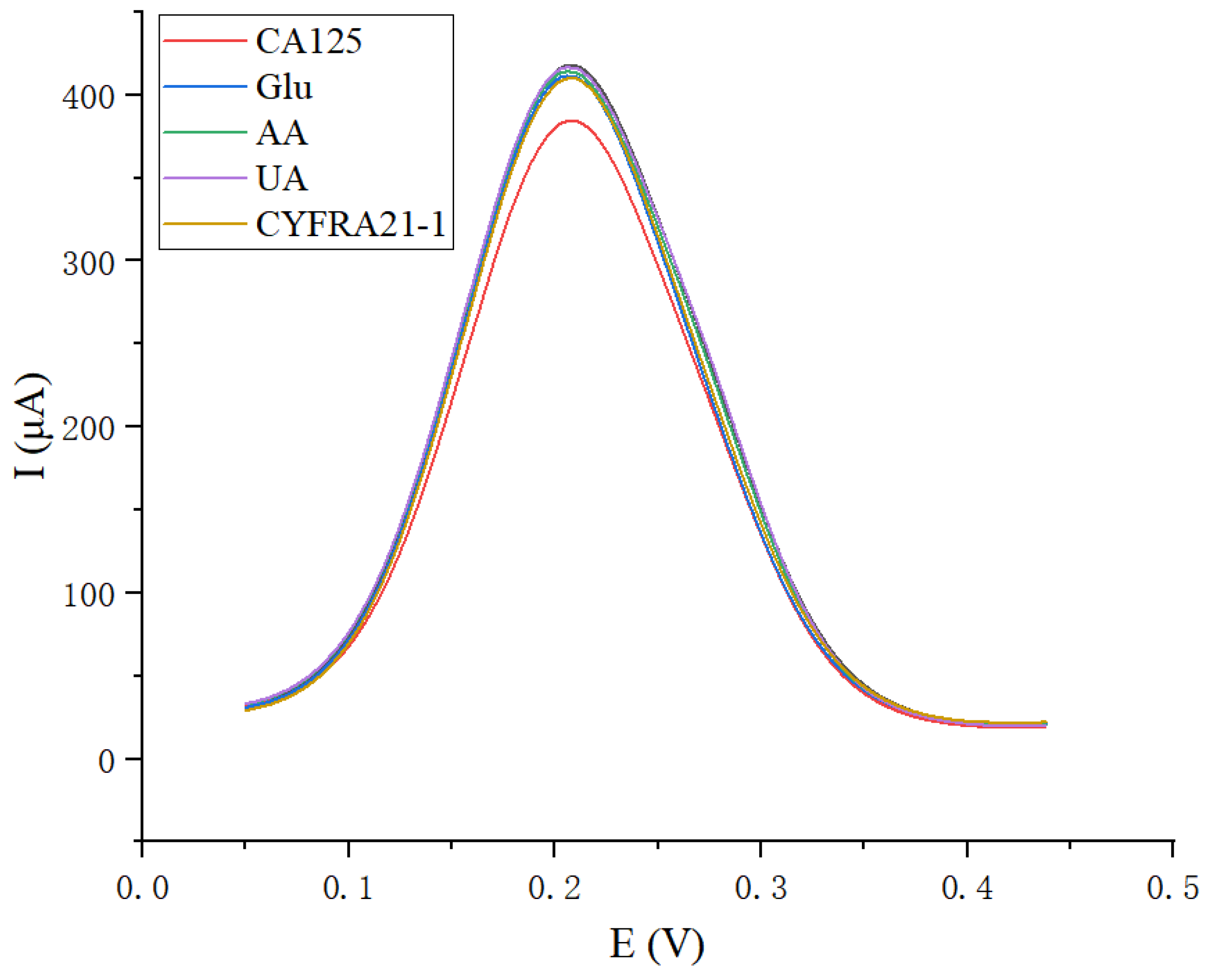
3.6. Reproducibility, Stability, and Selectivity
3.7. Analysis of Real Serum Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, E.T.; Agnihotram, V.R.; Eduardo, L.F. Lung cancer screening: Review and performance comparison under different risk scenarios. Lung 2014, 192, 55–63. [Google Scholar]
- Rastogi, A.; Yadav, K.; Mishra, A.; Singh, M.S.; Chaudhary, S.; Manohar, R.; Parmar, A.S. Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems. Nanotechnol. Rev. 2022, 11, 544–574. [Google Scholar] [CrossRef]
- Wulfkuhle, J.D.; Liotta, L.A.; Petricoin, E.F. Proteomic applications for the early detection of cancer. Nat. Rev. Cancer. 2003, 3, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Wu, R.; Kathleen, R.C.; Dafydd, G.T.; Gabrielle, G.; Rebecca, J.L.; Thomas, J.G.; Kerby, A.S.; David, E.M.; David, M.L. Differential protein mapping of ovarian serous adenocarcinomas: Identification of potential markers for distinct tumor stage. J. Proteome Res. 2009, 8, 1452–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujol, J.L.; Boher, J.M.; Grenier, J.; Quantin, X. Cyfra 21-1, neuron specific enolase and prognosis of non-small cell lung cancer: Prospective study in 621 patients. Lung Cancer 2001, 31, 221–231. [Google Scholar] [CrossRef]
- Amy, E.L.; Mary, E.F.; Geri, H.; Paige, H.; Amanda, O.; Amin, A. Understanding the value of tumor markers in pediatric ovarian neoplasms. J. Pediatr. Surg. 2019, 55, 122–125. [Google Scholar]
- Parvin, S.P.; Marziyeh, F.; Hossein, G.; Reza, S.; Yadollah, O. A novel electrochemical immunosensor for ultrasensitive detection of CA125 in ovarian cancer. Biosens. Bioelectron. 2020, 153, 112029. [Google Scholar]
- He, Z.; Ning, G.; Jin, W. Determination of tumor marker CA125 by capillary electrophoretic enzyme immunoassay with electrochemical detection. Anal. Chim. Acta 2003, 497, 75–81. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.C.; Luo, L.; Mu, C.Y.; Xu, J.; Feng, Q.; Li, S.B.; Gu, B.; Ma, P.; Lan, T. The clinical value of the combined detection of sEGFR, CA125 and HE4 for epithelial ovarian cancer diagnosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 604–610. [Google Scholar]
- Ge, L.; Wang, P.P.; Ge, S.G.; Li, N.Q.; Yu, J.H.; Yan, M.; Huang, J.D. Photoelectrochemical lab-on-paper device based on an integrated paper supercapacitor and internal light source. Anal. Chem. 2013, 85, 3961–3970. [Google Scholar] [CrossRef]
- Samaneh, R.; Abolfazl, M.K.; Shahla, C.; Ali-Akbar, D.; Leila, A.; Mahin, A.P.; Sepideh, K.; Ibrahim, A. The diagnostic accuracy of combined enolase/Cr, CA125, and CA19-9 in the detection of endometriosis. Biomed. Res. Int. 2020, 2020, 5208279. [Google Scholar]
- Jie, W.; Fu, Z.; Feng, Y.; Ju, H. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. Trends Anal. Chem. 2007, 26, 679–688. [Google Scholar]
- Chandrakala, G.; Kumarasamy, J.; Archana, D.; Savita, K.; Meera, V.; Sharmila, B.; Rajan, M.G.R. Comparison of serum thyroglobulin levels in differentiated thyroid cancer patients using in-house developed radioimmunoassay and immunoradiometric procedures. Indian J. Clin. Biochem. 2019, 34, 465–471. [Google Scholar]
- Fabiana, S.F.; Lúcio, A. Electrochemical immunosensors: A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar]
- Lai, Y.X.; Wang, L.J.; Liu, Y.; Yang, G.J.; Tang, C.L.; Deng, Y.; Li, S. Immunosensors based on nanomaterials for detection of tumor markers. J. Biomed. Nanotechnol. 2018, 14, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.L.; Yang, T.; Ma, S.S.; Peng, F.; Yi, M.H.; Wan, M.M.; Mao, C.; Shen, J. Label-free immunosensor based on hyperbranched polyester for specific detection of α-fetoprotein. Biosens. Bioelectron. 2017, 92, 1–7. [Google Scholar] [CrossRef]
- Kesarwani, S.; Verma, R.K. A critical review on synthesis, characterization and multifunctional applications of reduced graphene oxide (rGO)/composites. Nano 2021, 16, 2130008. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, F.; Liu, Y.; Pang, F.; Zhang, X. Green synthesis of silver nanoparticles on nitrogen-doped graphene for hydrogen peroxide detection. Electrochim. Acta 2014, 146, 646–653. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Chen, H.; Ye, H.R.; Chen, Z.X.; Nicole, J.R.; Guo, Z.Z. An ultrasensitive aptamer-antibody sandwich cortisol sensor for the noninvasive monitoring of stress state. Biosens. Bioelectron. 2021, 190, 113451. [Google Scholar] [CrossRef]
- Alle, M.; Gangapuram, B.R.; Maragoni, V.; Guttena, V.; Dudde, A.K.; Sumathi, N.; Ming-Yeh, Y.; Anren, H.; Surya, S.S. Efficient pH dependent drug delivery to target cancer cells by gold nanoparticles capped with carboxymethyl chitosan. Int. J. Mol. Sci. 2014, 15, 8216–8234. [Google Scholar]
- Singh, A.; Sinsinbar, G.; Choudhary, M.; Kumar, V.; Pasricha, R.; Verma, H.N.; Surinder, P.S.; Arora, K. Graphene oxide-chitosan nanocomposite based electrochemical DNA biosensor for detection of typhoid. Sens. Actuators B Chem. 2013, 185, 675–684. [Google Scholar] [CrossRef]
- Parvin, S.P.; Hossein, G.; Reza, S.; Yadollah, O. Electrochemical immunosensor based on chitosan-gold nanoparticle/carbon nanotube as a platform and lactate oxidase as a label for detection of CA125 oncomarker. Biosens. Bioelectron. 2018, 122, 68–74. [Google Scholar]
- Gisane, G.; Costa, J.P.C.; Costa, P.I.; Zaghete, M.A.; Mazon, T. Electrochemical immunosensor based on ZnO nanorods-Au nanoparticles nanohybrids for ovarian cancer antigen CA-125 detection. Mater. Sci. Eng. C 2017, 76, 1240–1247. [Google Scholar]
- Ge, S.G.; Yu, F.; Ge, L.; Yan, M.; Yu, J.H.; Chen, D.R. Disposable electrochemical immunosensor for simultaneous assay of a panel of breast cancer tumor markers. Analyst 2012, 137, 4727. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Lu, J.J.; Ge, L.; Ge, S.G.; Yan, M.; Song, X.R.; Yu, J.H. Triple catalysis amplification strategy for simultaneous multiplexed electrochemical immunoassays based on cactus-like MnO2 functionalized nanoporous gold. Sens. Actuators B Chem. 2013, 186, 545–549. [Google Scholar] [CrossRef]
- Tang, D.P.; Hou, L.; Reinhard, N.; Xu, M.D.; Gao, Z.Q.; Dietmar, K. Multiplexed electrochemical immunoassay of biomarkers using metal sulfide quantum dot nanolabels and trifunctionalized magnetic beads. Biosens. Bioelectron. 2013, 46, 37–43. [Google Scholar] [CrossRef]
- Maalavika, S.L.; Wang, F.M.; Ilangovan, R. Electrochemical detection of CA125 using thionine and gold nanoparticles supported on heteroatom-doped graphene nanocomposites. Appl. Nanosci. 2021, 11, 2167–2180. [Google Scholar]
- Omer, F.E.; Hilal, K.; Omruye, O.; Sebahattin, C.; Arif, K. A novel electrochemical sensor for monitoring ovarian cancer tumor protein CA125 on benzothiophene derivative based electrodes. J. Electroanal. Chem. 2022, 904, 115854. [Google Scholar]
- Chen, Z.H.; Li, B.B.; Liu, J.B.; Li, H.J.; Li, C.P.; Xuan, X.W.; Li, M.J. A label-free electrochemical immunosensor based on a gold–vertical graphene/TiO2 nanotube electrode for CA125 detection in oxidation/reduction dual channels. Microchim. Acta 2022, 189, 257. [Google Scholar] [CrossRef]
- Wu, M.D.; Liu, S.M.; Qi, F.F.; Qiu, R.; Feng, J.; Ren, X.S.; Rong, S.Z.; Ma, H.K.; Chang, D.; Pan, H.Z. A label-free electrochemical immunosensor for CA125 detection based on CMK-3(Au/Fc@MgAl-LDH)n multilayer nanocomposites modification. Talanta 2022, 241, 123254. [Google Scholar] [CrossRef]
- Zhang, F.L.; Fan, L.F.; Liu, Z.G.; Han, Y.J.; Guo, Y.J. A label-free electrochemical aptasensor for the detection of cancer antigen 125 based on nickel hexacyanoferrate nanocubes/polydopamine functionalized graphene. J. Electroanal. Chem. 2022, 918, 116424. [Google Scholar] [CrossRef]
- Ren, X.; Wang, H.; Wu, D.; Fan, D.W.; Zhang, Y.; Du, B.; Wei, Q. Ultrasensitive immunoassay for CA125 detection using acid site compound as signal and enhancer. Talanta 2015, 144, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Hu, W.B.; Wei, J.; Yu, F.; Wu, L.; Wang, C.M.; Wang, W.; Zuo, S.Y.; Shang, B.; Chen, Q.H. An electrochemical aptasensing platform for carbohydrate antigen 125 based on the use of flower-like gold nanostructures and target-triggered strand displacement amplification. Microchim. Acta 2019, 388, 186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chai, Y.Q.; Zhuo, Y.; Yuan, R. Ultrasensitive simultaneous detection of four biomarkers based on hybridization chain reaction and biotin-streptavidin signal amplification strategy. Biosens. Bioelectron. 2015, 68, 42–48. [Google Scholar] [CrossRef] [PubMed]


| Type of Immunosensor | Method | Linear Range | LOD | Reference |
|---|---|---|---|---|
| PAMAM/AuNP-3DrGO- MWCNT nanocomposite modified GCE | SWV a | 0.5 mU mL−1–75 U mL−1 | 6 μU mL−1 | [7] |
| ZnO nanorods-Au nanoparticles nanohybrids modified GCE | CV b | - | 2.5 μg mL−1 | [23] |
| AuNPs/GR modified SPCE | LSV c | 1 mU mL−1–100 U mL−1 | 0.34 mU mL−1 | [24] |
| Cactus-like MnO2 functionalized nanoporous gold modified GCE | SWV | 10 mU mL−1–50 U mL−1 | 3.5 mU mL−1 | [25] |
| Metal sulfide quantum dot nanolabels and trifunctionalized magnetic beads modified GCE | SWASV d | 10 mU mL−1–50 U mL−1 | 5 mU mL−1 | [26] |
| Thionine and gold nanoparticles supported on heteroatom-doped graphene nanocomposites modified GCE | DPV e | 3.2 mU mL−1–10 U mL−1 | 0.28 U mL−1 | [27] |
| Benzothiophene derivative modified GCE | DPV | 1 ng mL−1–100 ng mL−1 | 9.6 pg mL−1 | [28] |
| Gold-vertical graphene/TiO2 nanotube modified GCE | DPV | 0.01 mU mL−1–1 U mL−1 | 0.1 μU mL−1 | [29] |
| CMK-3(Au/Fc@MgAl-LDH)n multilayer nanocomposites modified GCE | DPV | 10 mU mL−1–1000 U mL−1 | 4 mU mL−1 | [30] |
| Nickel hexacyanoferrate nanocubes/polydopamine functionalized graphene modified GCE | DPV | 0.1 pg mL−1–1 μg mL−1 | 0.076 pg mL−1 | [31] |
| FA, H-PANI and CS-HCl modified GCE | SWV | 1 pg mL−1–25 ng mL−1 | 0.25 pg mL−1 | [32] |
| Electrochemical aptasensing platform based on combination of target-triggered SDA and aptamer recognition | SWV | 50 pg mL−1–50 ng mL−1 | 5 pg mL−1 | [33] |
| Hybridization chain reaction and biotin-streptavidin signal amplification strategy | DPV | 0.2 pg mL−1–1 ng mL−1 | 0.08 pg mL−1 | [34] |
| N-rGO@CMWCNTs/ CS@AuNPs modified GCE | DPV | 0.1 pg mL−1–100 ng mL−1 | 0.04 pg mL−1 | This work |
| No | Added (pg mL−1) | Found (pg mL−1) | Recovery | RSD (N = 3) |
|---|---|---|---|---|
| 1 | 10 | 10.39 | 103.9% | 7.07% |
| 2 | 100 | 107.72 | 107.7% | 4.17% |
| 3 | 1000 | 945.16 | 94.5% | 3.88% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Gong, G.; Jiang, Y.; Qin, J.; Mei, Y.; Han, J. Electrochemical Immunosensor Modified with Nitrogen-Doped Reduced Graphene Oxide@Carboxylated Multi-Walled Carbon Nanotubes/Chitosan@Gold Nanoparticles for CA125 Detection. Chemosensors 2022, 10, 272. https://doi.org/10.3390/chemosensors10070272
Gu Y, Gong G, Jiang Y, Qin J, Mei Y, Han J. Electrochemical Immunosensor Modified with Nitrogen-Doped Reduced Graphene Oxide@Carboxylated Multi-Walled Carbon Nanotubes/Chitosan@Gold Nanoparticles for CA125 Detection. Chemosensors. 2022; 10(7):272. https://doi.org/10.3390/chemosensors10070272
Chicago/Turabian StyleGu, Yingying, Guoao Gong, Yuting Jiang, Jiangyang Qin, Yong Mei, and Jun Han. 2022. "Electrochemical Immunosensor Modified with Nitrogen-Doped Reduced Graphene Oxide@Carboxylated Multi-Walled Carbon Nanotubes/Chitosan@Gold Nanoparticles for CA125 Detection" Chemosensors 10, no. 7: 272. https://doi.org/10.3390/chemosensors10070272
APA StyleGu, Y., Gong, G., Jiang, Y., Qin, J., Mei, Y., & Han, J. (2022). Electrochemical Immunosensor Modified with Nitrogen-Doped Reduced Graphene Oxide@Carboxylated Multi-Walled Carbon Nanotubes/Chitosan@Gold Nanoparticles for CA125 Detection. Chemosensors, 10(7), 272. https://doi.org/10.3390/chemosensors10070272







