Luminescent Sensors Based on the Assembly of Coinage Metal Nanoclusters
Abstract
:1. Introduction
2. Assembly Mechanism of Coinage Metal NCs
2.1. Van der Waals Interaction
2.2. Electrostatic Interactions
2.3. Hydrogen Bonding Interactions
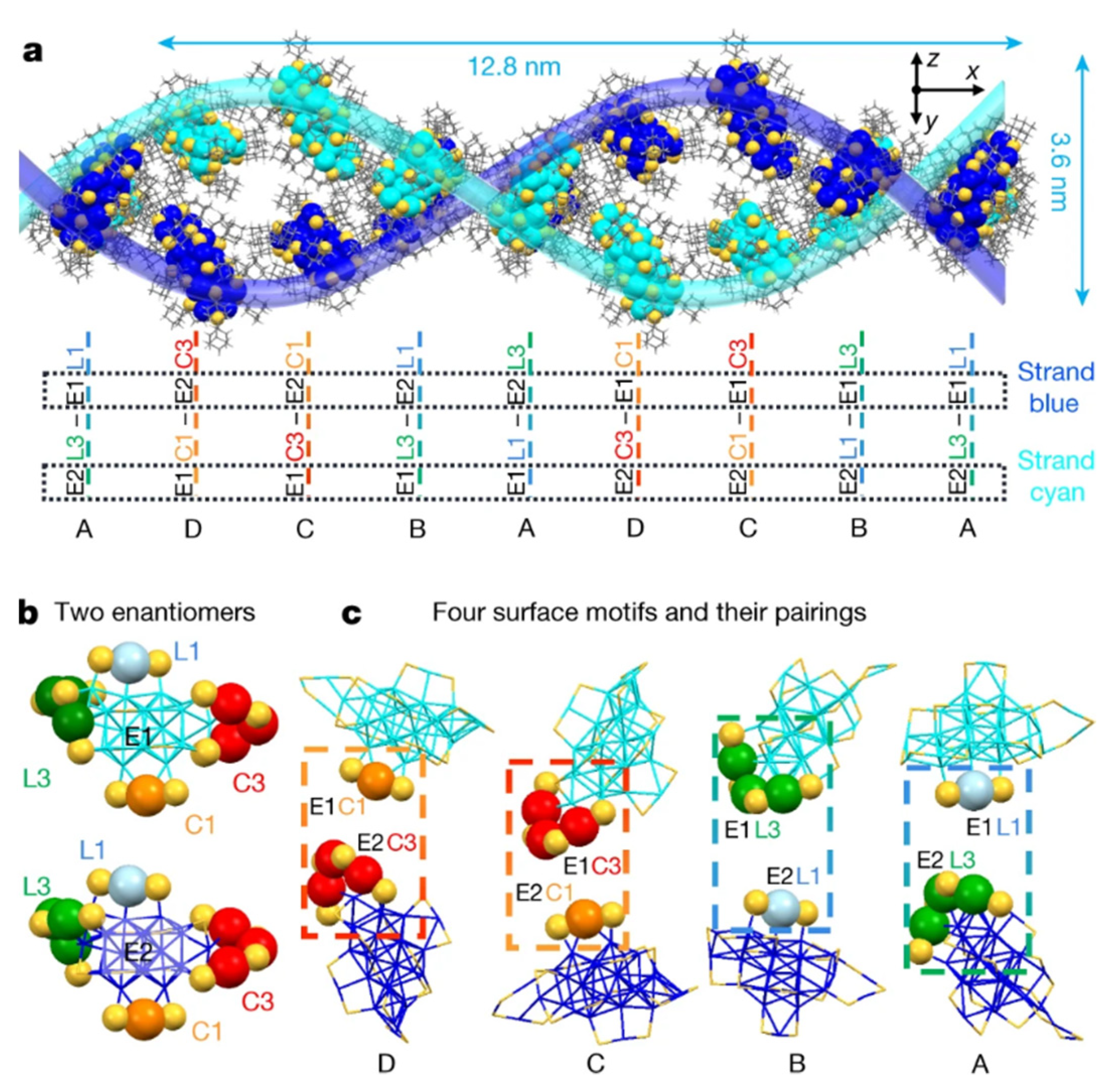
2.4. C-H···π/π···π Interactions
2.5. Metallophilic Interactions
3. Sensing Application
3.1. Metal Ions
3.2. Biomolecules
3.3. Small Organic Molecule
3.4. Gas
3.5. Temperature Sensing
3.6. pH
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.; Chen, Z.; Mei, X. Fluorescence enhancement for noble metal nanoclusters. Adv. Colloid Interface Sci. 2017, 250, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, Z.; Liu, Z.; Lin, Y.; Yuan, X.; Xie, J. Molecular reactivity of thiolate-protected noble metal nanoclusters: Synthesis, self-assembly, and applications. Chem. Sci. 2021, 12, 99–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hong, G.; Luo, Z.; Chen, J.; Chang, J.; Gong, M.; He, H.; Yang, J.; Yuan, X.; Li, L.; et al. Atomic-Precision Gold Clusters for NIR-II Imaging. Adv. Mater. 2019, 31, 1901015. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Yu, Y.; Wang, Y.; Zhang, L.; Lin, B.; Guo, M.; Cao, Y. A novel universal nanoplatform for ratiometric fluorescence biosensing based on silver nanoclusters beacon. Chem. Eng. J. 2020, 391, 123526. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, Z. New immunoprobes based on bovine serum albumin-stabilized copper nanoclusters with triple signal amplification for ultrasensitive electrochemical immunosensing for tumor marker. Sens. Actuators B Chem. 2017, 241, 849–854. [Google Scholar] [CrossRef]
- Higaki, T.; Li, Y.; Zhao, S.; Li, Q.; Li, S.; Du, X.; Yang, S.; Chai, J.; Jin, R. Atomically Tailored Gold Nanoclusters for Catalytic Application. Angew. Chem. Int. Ed. 2019, 58, 8291–8302. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Fedorenko, S.V.; Mustafina, A.R.; Kholina, K.V.; Nizamee, I.R.; Strekalova, S.O.; Grinenko, V.V.; Gryaznova, T.V.; Zairov, R.R.; Mazzaro, R.; et al. Silica-Supported Silver Nanoparticles as an Efficient Catalyst for Aromatic C-H Alkylation and Fluoroalkylation. Dalton Trans. 2018, 47, 9608. [Google Scholar]
- Xie, J.; Zheng, Y.; Ying, J. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, Q.; Yin, J.; Xu, N. Polyethyleneimine protected silver nanoclusters luminescence probe for sensitive detection of cobalt (II) in living cells. J. Photochem. Photobiol. B Biol. 2017, 173, 508–513. [Google Scholar]
- Basu, K.; Paul, S.; Jana, R.; Datta, A.; Banerjee, A. Red-Emitting Copper Nanoclusters: From Bulk-Scale Synthesis to Catalytic Reduction. ACS Sustain. Chem. Eng. 2018, 7, 1998–2007. [Google Scholar] [CrossRef]
- Halawa, M.I.; Lai, J.; Xu, G. Gold nanoclusters: Synthetic strategies and recent advances in fluorescent sensing. Mater. Today Nano 2018, 3, 9–27. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lou, X.; Liang, F.; Yang, Y. Surface-functionalized gold and silver nanoparticles for colorimetric and fluorescent sensing of metal ions and biomolecules. Coord. Chem. Rev. 2022, 459, 214461. [Google Scholar] [CrossRef]
- Chen, P.; Periasamy, A.P.; Harroun, S.G.; Wu, W.; Chang, H. Photoluminescence sensing systems based on copper, gold and silver nanomaterials. Coord. Chem. Rev. 2016, 320–321, 129–138. [Google Scholar] [CrossRef]
- Rehman, A.; Zeng, X. Interfacial composition, structure, and properties of ionic liquids and conductive polymers for the construction of chemical sensors and biosensors: A perspective. Curr. Opin. Electrochem. 2020, 23, 47–56. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Zhang, C.; Chen, J.; Mao, W.; Yu, C. Dual-type responsive electrochemical biosensor for the detection of alpha2,6-sialylated glycans based on AuNRs-SA coupled with c-SWCNHs/S-PtNC nanocomposites signal amplification. Biosens. Bioelectron. 2019, 130, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, Z.; Bi, Y.; Sun, D.; Zhao, T.; Zhao, F.; Wang, W.; Xin, X. Self-Assembly-Driven Aggregation-Induced Emission of Silver Nanoclusters for Light Conversion and Temperature Sensing. ACS Appl. Nano Mater. 2020, 3, 2038–2046. [Google Scholar] [CrossRef]
- Xue, F.; Qu, F.; Han, W.; Xia, L.; You, J. Aggregation-induced emission enhancement of gold nanoclusters triggered by silicon nanoparticles for ratiometric detection of protamine and trypsin. Anal. Chim. Acta 2019, 1046, 170–178. [Google Scholar] [CrossRef]
- Pan, S.; Liu, W.; Tang, J.; Yang, Y.; Feng, H.; Qian, Z.; Zhou, J. Hydrophobicity-guided self-assembled particles of silver nanoclusters with aggregation-induced emission and their use in sensing and bioimaging. J. Mater. Chem. B 2018, 6, 3927–3933. [Google Scholar] [CrossRef]
- Shen, J.; Bi, Y.; Zhang, H.; Xu, L.; Feng, J.; Qi, W. A sensitive chemosensor for nitro-containing compounds based on Au nanoclusters/Ba2+ co-assembly system: The crucial role of ligands to metal charge transfer. Colloids Surf. A Physicochem. Eng Asp. 2021, 627, 127160. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Sun, D.; Li, S.; Deng, Q.; Xin, X. Supramolecular Self-Assembly of Atomically Precise Silver Nanoclusters with Chiral Peptide for Temperature Sensing and Detection of Arginine. Nanomaterials 2022, 12, 424. [Google Scholar] [CrossRef]
- Han, X.; Han, M.; Ma, L.; Qu, F.; Kong, R.; Qu, F. Self-assembled gold nanoclusters for fluorescence turn-on and colorimetric dual-readout detection of alkaline phosphatase activity via DCIP-mediated fluorescence resonance energy transfer. Talanta 2019, 194, 55–62. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Z.; Sun, D.; Liu, G.; Yuan, S.; Kurmoo, M.; Xin, X. Self-assembly of water-soluble silver nanoclusters: Superstructure formation and morphological evolution. Nanoscale 2017, 9, 19191–19200. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Paul, A.; Chattopadhyay, A. Zinc mediated crystalline assembly of gold nanoclusters for expedient hydrogen storage and sensing. J. Mater. Chem. A 2016, 4, 1218–1223. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhuang, J.; Lynch, J.; Chen, O.; Wang, Z.; Wang, X.; LaMontagne, D.; Wu, H.; Wang, Z.; Cao, Y.C. Self-Assembled Colloidal Superparticles from Nanorods. Scinece 2012, 338, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, R.; Shi, L.; Zhang, C.; Zhang, Y.; Zhou, Y.; Dong, C.; Li, G.; Shuang, S. Aggregation/assembly induced emission based on silk fibroin-templated fluorescent copper nanoclusters for “turn-on” detection of S2−. Sens. Actuators B Chem. 2019, 279, 361–368. [Google Scholar] [CrossRef]
- An, M.; Li, H.; Su, M.; Gao, S.; Wang, M.; Shen, S.; Gao, Z.; Dong, J. Cu2+ enhanced fluorescent Ag nanoclusters with tunable emission from red to yellow and the application for Ag+ sensing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 252, 119484. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Z.; Xia, C.; Sun, D.; Yuan, S.; Xin, X. Amphiphilicity Regulation of Ag(I) Nanoclusters: Self-Assembly and Its Application as a Luminescent Probe. Chem. Eur. J. 2019, 25, 4713–4721. [Google Scholar] [CrossRef]
- Ghosh, D.; Ganayee, M.A.; Som, A.; Srikrishnarka, P.; Murali, N.; Bose, S.; Chakraborty, A.; Mondal, B.; Ghosh, P.; Pradeep, T. Hierarchical Assembly of Atomically Precise Metal Clusters as a Luminescent Strain Sensor. ACS Appl. Mater. Interfaces 2021, 13, 6496–6504. [Google Scholar] [CrossRef]
- Liu, J.; Tian, Y.; Ai, L.; Wu, Z.; Yao, D.; Liu, Y.; Yang, B.; Zhang, H. Self-Assembly of Au Nanoclusters into Helical Ribbons by Manipulating the Flexibility of Capping Ligands. Langmuir 2020, 36, 14614–14622. [Google Scholar] [CrossRef]
- Chen, T.; Yang, S.; Li, Q.; Song, Y.; Li, G.; Chai, J.; Zhu, M. A double helical 4H assembly pattern with secondary hierarchical complexity in an Ag70 nanocluster crystal. Nanoscale Horiz. 2021, 6, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Y.; Liu, J.; Lu, Z.; Zhang, H.; Yang, B. Colloidal self-assembly of catalytic copper nanoclusters into ultrathin ribbons. Angew. Chem. Int. Ed. 2014, 53, 12196–12200. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sun, P.; Wang, Z.; Li, H.; Yu, L.; Sun, D.; Chen, M.; Bi, Y.; Xin, X.; Hao, J. Metal-Organic Gels from Silver Nanoclusters with Aggregation-Induced Emission and Fluorescence-to-Phosphorescence Switching. Angew. Chem. Int. Ed. 2020, 59, 9922–9927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, A.; Hou, W.; Li, T.; Wang, K.; Zhang, Q.; Fuente, J.M.; Jin, W.; Cui, D. Mimicking Pathogenic Invasion with the Complexes of Au22(SG)18-Engineered Assemblies and Folic Acid. ACS Nano 2018, 12, 4408–4418. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Kang, X.; Yuan, Q.; Qin, C.; Jin, S.; Wang, S.; Zhu, M. Capture of Cesium Ions with Nanoclusters: Effects on Inter- and Intramolecular Assembly. Chem. Mater. 2019, 31, 4945–4952. [Google Scholar] [CrossRef]
- Xu, M.; Jia, T.; Li, B.; Ma, W.; Chen, X.; Zhao, X.; Zang, S. Tuning the properties of atomically precise gold nanoclusters for biolabeling and drug delivery. ChemComm 2020, 56, 8766–8769. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Z.; Dong, M.; Wang, L.; Dong, S.; Hao, J. Self-Assembly of Amphiphilic Copper Nanoclusters Driven by Cationic Surfactants. Langmuir 2021, 37, 6613–6622. [Google Scholar] [CrossRef]
- AbdulHalim, L.G.; Bootharaju, M.S.; Tang, Q.; Del Gobbo, S.; AbdulHalim, R.G.; Eddaoudi, M.; Jiang, D.E.; Bakr, O.M. Ag29(BDT)12(TPP)4: A Tetravalent Nanocluster. J. Am. Chem. Soc. 2015, 137, 11970–11975. [Google Scholar] [CrossRef]
- Li, Q.; Russell, J.C.; Luo, T.; Roy, X.; Rosi, N.L.; Zhu, Y.; Jin, R. Modulating the hierarchical fibrous assembly of Au nanoparticles with atomic precision. Nat. Commun. 2018, 9, 3871. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Zang, S.; Mak, T.C.W. Spontaneous Resolution of Chiral Multi-Thiolate-Protected Ag30Nanoclusters. ACS Cent. Sci. 2020, 6, 1971–1976. [Google Scholar] [CrossRef]
- Wu, Z.; Du, Y.; Liu, J.; Yao, Q.; Chen, T.; Cao, Y.; Zhang, H.; Xie, J. Aurophilic Interactions in the Self-Assembly of Gold Nanoclusters into Nanoribbons with Enhanced Luminescence. Angew. Chem. Int. Ed. 2019, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Maity, S.; Sengupta, T.; Bista, D.; Reber, A.C.; Patra, A.; Khanna, S.N.; Mandal, S. One-Dimensional Silver-Thiolate Cluster-Assembly: Effect of Argentophilic Interactions on Excited-State Dynamics. J. Phys. Chem. Lett. 2021, 12, 2154–2159. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, J.; Gao, Y.; Liu, H.; Li, T.; Zou, H.; Wang, Z.; Zhang, K.; Wang, Y.; Zhang, H.; et al. Assembly-Induced Enhancement of Cu Nanoclusters Luminescence with Mechanochromic Property. J. Am. Chem. Soc. 2015, 137, 12906–12913. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhu, J.; Gong, L.; Nong, L.; Liu, J. Amphiphilic Block Copolymer-Guided in Situ Fabrication of Stable and Highly Controlled Luminescent Copper Nanoassemblies. J. Am. Chem. Soc. 2019, 141, 2852–2856. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-Assembly at All Scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef]
- Winterton, R.H.S. Van der Waals forces. Contemp. Phys. 1970, 11, 559–574. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Song, Y.; Higaki, T.; Wang, H.; Jin, R. Double-helical assembly of heterodimeric nanoclusters into supercrystals. Nature 2021, 594, 380–384. [Google Scholar] [CrossRef]
- Tsuzuki, S. CH/π interactions. Annu. Rep. Sect. “C” (Phys. Chem.) 2012, 108, 69–95. [Google Scholar] [CrossRef]
- Sculfort, S.; Braunstein, P. Intramolecular d10-d10 interactions in heterometallic clusters of the transition metals. Chem. Soc. Rev. 2011, 40, 2741–2760. [Google Scholar] [CrossRef]
- Wang, H.; Bai, H.; Wang, Y.; Gan, T.; Liu, Y. Highly selective fluorimetric and colorimetric sensing of mercury(II) by exploiting the self-assembly-induced emission of 4-chlorothiophenol capped copper nanoclusters. Mikrochim. Acta 2020, 187, 185. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Sun, P.; Zhang, N.; Zhou, S.; Xin, X. Self-assembly of silver nanoclusters and phthalic acid into hollow tubes as a superior sensor for Fe3+. J. Mol. Liq. 2021, 323, 115032. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, X.; Yin, C.; Zhang, X.; Zhang, J.; Wang, X.; Xin, J. An electrostatic self-assembly route to prepare C-dots/gold nanoclusters for dual-emission ratiometric optical thermometry in living cells. Anal. Methods 2019, 11, 3974–3980. [Google Scholar] [CrossRef]
- Shen, J.; Xiao, Q.; Sun, P.; Feng, J.; Xin, X.; Yu, Y.; Qi, W. Self-Assembled Chiral Phosphorescent Microflowers from Au Nanoclusters with Dual-Mode pH Sensing and Information Encryption. ACS Nano 2021, 15, 4947–4955. [Google Scholar] [CrossRef]
- Han, B.; Yan, Q.; Xin, Z.; Yan, Q.; Jiang, J. Ionic Liquids-Assisted Highly Luminescent Copper Nanoclusters with Triangle Supramolecular Nanostructures. Chin. J. Chem. 2021, 39, 1867–1870. [Google Scholar] [CrossRef]
- Wang, H.; Mao, A.; Gan, T.; Liu, Y. A turn-on fluorescence strategy for cellular glutathione determination based on the aggregation-induced emission enhancement of self-assembled copper nanoclusters. Analyst 2020, 145, 7009–7017. [Google Scholar] [CrossRef]
- Nag, A.; Chakraborty, P.; Thacharon, A.; Paramasivam, G.; Mondal, B.; Bodiuzzaman, M.; Pradeep, T. Atomically Precise Noble Metal Cluster-Assembled Superstructures in Water: Luminescence Enhancement and Sensing. J. Phys. Chem. C 2020, 124, 22298–22303. [Google Scholar] [CrossRef]
- Araujo, J.E.; Lopez-Fernandez, H.; Diniz, M.S.; Baltazar, P.M.; Pinheiro, L.C.; da Silva, F.C.; Carrascal, M.; Videira, P.; Santos, H.M.; Capelo, J.L. Dithiothreitol-based protein equalization technology to unravel biomarkers for bladder cancer. Talanta 2018, 180, 36–46. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Li, D.; Li, L.; Lou, X.; Liu, H. Self-Nucleation and Self-Assembly of Highly Fluorescent Au5 Nanoclusters for Bioimaging. Chem. Mater. 2018, 30, 5507–5515. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, X. Cytidine-stabilized gold nanocluster as a fluorescence turn-on and turn-off probe for dual functional detection of Ag+ and Hg2+. Anal. Chim. Acta 2015, 870, 1–7. [Google Scholar] [CrossRef]
- Packirisamy, V.; Pandurangan, P. Heterocyclic thiol protected supramolecular self-assembly of silver nanoclusters for ultrasensitive detection of toxic Hg (II) ions in nanomolar range. J. Mol. Liq. 2021, 344, 117769. [Google Scholar] [CrossRef]
- Sieg, H.; Ellermann, A.L.; Maria Kunz, B.; Jalili, P.; Burel, A.; Hogeveen, K.; Bohmert, L.; Chevance, S.; Braeuning, A.; Gauffre, F.; et al. Aluminum in liver cells—The element species matters. Nanotoxicology 2019, 13, 909–922. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Z.; Sun, D.; Xia, C.; Yuan, S.; Sun, P.; Xin, X. pH-Responsive Nanovesicles with Enhanced Emission Co-Assembled by Ag(I) Nanoclusters and Polyethyleneimine as a Superior Sensor for Al3+. ACS Appl. Mater. Interfaces 2018, 10, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.S.; Clucas, D.B.; McColl, G.; Hall, L.T.; Simpson, D.A. Re-examining ferritin-bound iron: Current and developing clinical tools. Clin. Chem. Lab. Med. 2021, 59, 459–471. [Google Scholar] [CrossRef]
- Hu, W.; Niu, Y.; Zhu, H.; Dong, K.; Wang, D.; Liu, F. Remediation of zinc-contaminated soils by using the two-step washing with citric acid and water-soluble chitosan. Chemosphere 2021, 282, 131092. [Google Scholar] [CrossRef] [PubMed]
- Kuppan, B.; Maitra, U. Instant room temperature synthesis of self-assembled emission-tunable gold nanoclusters: Million-fold emission enhancement and fluorimetric detection of Zn2+. Nanoscale 2017, 9, 15494–15504. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wang, T.; Yu, S.; Yi, P.; Li, L. Influence of UV lamp, sulfur(IV) concentration, and pH on bromate degradation in UV/sulfite systems: Mechanisms and applications. Water Res. 2017, 111, 288–296. [Google Scholar] [CrossRef]
- Cui, J.; Shao, Y.; Zhang, H.; Zhang, H.; Zhu, J. Development of a novel silver ions-nanosilver complementary composite as antimicrobial additive for powder coating. Chem. Eng. J. 2021, 420, 127633. [Google Scholar] [CrossRef]
- Berndt, C.; Lillig, C.H. Glutathione, Glutaredoxins, and Iron. Antioxid. Redox Signal. 2017, 27, 1235–1251. [Google Scholar] [CrossRef]
- Siller, A.F.; Whyte, M.P. Alkaline Phosphatase: Discovery and Naming of Our Favorite Enzyme. J. Bone Miner. Res. 2018, 33, 362–364. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, F.; Zhao, R.; Liu, S.; Li, W.; He, F.; Gai, S.; Yang, P. A novel “off-on-off” fluorescent sensor based on inner filter effect for ultrasensitive detection of protamine/trypsin and subcellular colocalization. Sens. Actuators B Chem. 2021, 340, 129930. [Google Scholar] [CrossRef]
- Du, J.; O’Reilly, R.K. Advances and challenges in smart and functional polymer vesicles. Soft Matter 2009, 5, 3544–3561. [Google Scholar] [CrossRef]
- Liao, H.; Lin, J.; Liu, Y.; Huang, P.; Jin, A.; Chen, X. Self-assembly mechanisms of nanofibers from peptide amphiphiles in solution and on substrate surfaces. Nanoscale 2016, 8, 14814–14820. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Yuan, X.; Yu, Y.; Yu, Y.; Xie, J.; Lee, J. Introducing amphiphilicity to noble metal nanoclusters via phase-transfer driven ion-pairing reaction. J. Am. Chem. Soc. 2015, 137, 2128–2136. [Google Scholar] [CrossRef]
- Gershman, S.J.; Uchida, N. Believing in dopamine. Nat. Rev. Neurosci. 2019, 20, 703–714. [Google Scholar] [CrossRef]
- Obara, I.; Telezhkin, V.; Alrashdi, I.; Chazot, P.L. Histamine, histamine receptors, and neuropathic pain relief. Br. J. Pharmacol. 2020, 177, 580–599. [Google Scholar] [CrossRef]
- Han, A.; Xiong, L.; Hao, S.; Yang, Y.; Li, X.; Fang, G.; Liu, J.; Pei, Y.; Wang, S. Highly Bright Self-Assembled Copper Nanoclusters: A Novel Photoluminescent Probe for Sensitive Detection of Histamine. Anal. Chem. 2018, 90, 9060–9067. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Xu, Z. Fluorescent probes for biothiols based on metal complex. Coord. Chem. Rev. 2021, 429, 213638. [Google Scholar] [CrossRef]
- Han, A.; Luo, X.; Hao, S.; Yang, Y.; Chen, J.; Fang, G.; Liu, J.; Wang, S. Synthesis of red photoluminescent nickel doped self-assembled copper nanoclusters and their application in biothiol sensing. Sens. Actuators B Chem. 2021, 349, 130777. [Google Scholar] [CrossRef]
- Gao, X.; Wu, J.; Wu, D. Rational design of the beta-galactosidase from Aspergillus oryzae to improve galactooligosaccharide production. Food Chem. 2019, 286, 362–367. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, H.; Liu, W.; Zhang, S.; Tang, C.; Chen, J.; Qian, Z. Cation-driven luminescent self-assembled dots of copper nanoclusters with aggregation-induced emission for beta-galactosidase activity monitoring. J. Mater. Chem. B 2017, 5, 5120–5127. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, L.; Wang, Y.; Hao, J. Stimuli-Responsive Fluorescent Nanoswitches: Solvent-Induced Emission Enhancement of Copper Nanoclusters. Chem. Eur. J. 2020, 26, 3545–3554. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.W.; Jarrad, A.M.; Cooper, M.A.; Blaskovich, M.A.T. Nitroimidazoles: Molecular Fireworks That Combat a Broad Spectrum of Infectious Diseases. J. Med. Chem. 2017, 60, 7636–7657. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yan, B.; Wang, J.; Xi, X.; Wang, Z.; Zang, S. Layer-sliding-driven crystal size and photoluminescence change in a novel SCC-MOF. ChemComm 2018, 54, 5361–5364. [Google Scholar] [CrossRef]
- Dong, X.; Huang, H.; Wang, J.; Li, H.; Zang, S. A Flexible Fluorescent SCC-MOF for Switchable Molecule Identification and Temperature Display. Chem. Mater. 2018, 30, 2160–2167. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, Q.; Li, H.; Dong, X.; Wang, S.; Zang, S. Fabrication of silver chalcogenolate cluster hybrid membranes with enhanced structural stability and luminescence efficiency. ChemComm 2019, 55, 14677–14680. [Google Scholar] [CrossRef]
- Ding, H.; Chang, J.; He, F.; Gai, S.; Yang, P. Hydrogen Sulfide: An Emerging Precision Strategy for Gas Therapy. Adv. Healthc. Mater. 2022, 11, 2101984. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, K.; Yu, H.; Zhu, H.; Sun, M.; Hayat, T.; Alsaedi, A.; Wang, S. Sensitive detection of sulfide based on the self-assembly of fluorescent silver nanoclusters on the surface of silica nanospheres. Talanta 2017, 174, 387–393. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q. Metal-Nanoparticle-Catalyzed Hydrogen Generation from Formic Acid. Acc. Chem. Res. 2017, 50, 1449–1458. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation-A review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Basu, S.; Bhandari, S.; Pan, U.N.; Paul, A.; Chattopadhyay, A. Crystalline nanoscale assembly of gold clusters for reversible storage and sensing of CO2 via modulation of photoluminescence intermittency. J. Mater. Chem. C 2018, 6, 8205–8211. [Google Scholar] [CrossRef]
- Huang, R.; Wei, Y.; Dong, X.; Wu, X.; Du, C.; Zang, S.; Mak, T.C.W. Hypersensitive dual-function luminescence switching of a silver-chalcogenolate cluster-based metal-organic framework. Nat. Chem. 2017, 9, 689–697. [Google Scholar] [CrossRef]
- Dong, X.; Si, Y.; Yang, J.; Zhang, C.; Han, Z.; Luo, P.; Wang, Z.; Zang, S.; Mak, T.C.W. Ligand engineering to achieve enhanced ratiometric oxygen sensing in a silver cluster-based metal-organic framework. Nat. Commun. 2020, 11, 3678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, D.; Wang, H.; Li, J.; Nienhaus, G.U.; Su, Z.; Wei, G.; Shang, L. Supramolecular Self-Assembly Bioinspired Synthesis of Luminescent Gold Nanocluster-Embedded Peptide Nanofibers for Temperature Sensing and Cellular Imaging. Bioconjug. Chem. 2017, 28, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, Y.; Lin, H.; Xu, Z.; Wu, J.; Humphrey, M.G.; Zhang, C. Gold nanoclusters based dual-emission hollow TiO2 microsphere for ratiometric optical thermometry. RSC Adv. 2015, 5, 61586–61592. [Google Scholar] [CrossRef]










| Interactions | Strength of the Bonds (kJ/mol) | Nature | Advantages | Disadvantages | Application |
|---|---|---|---|---|---|
| Van der Waals interaction | 2–20 | The van der Waals forces are distance-dependent weak electrostatic attractions or repulsions that arise due to the fluctuating electromagnetic polarization of nearby atoms, molecules or particles. | The accumulation of Van der Waals interactions enables topological macromolecules to preserve their stable functional shape for biological activity. Van der Waals interactions are also very important for initiating NCs periodic assembly in the long range. | Van der Waals forces are not very strong. | Catalytic [32]. Detection [51]. |
| Electrostatic force | 20–40 | Electrostatic interactions are generally strong attractions or repulsions between two oppositely charged ions, mostly positive and negative charges. | Electrostatic attraction has no direction, and the interaction between the anions and cations can be in any direction. | Self-assembly of two counter-charged NCs is difficult, owing to the instability of cationic NCs. | Imaging and drug delivery [36]. Light-emitting diodes [37]. Detection [17,21,52] Temperature [53], pH sensing [54,55] |
| Hydrogen bond | 25–40 | The essence of hydrogen bond is the electrostatic force between hydrogen nucleus on the strong polar bond and electronegative atom containing lone electron pair with partial negative charge. | Sophisticated networks of hydrogen bonds with flexible and structure-directing properties. | The conditions for forming the most stable hydrogen bonds are harsh. | Temperature sensing [33] pH sensing [34] Detection [56,57]. |
| C-H···π/π···π | 3-8/8–12 | The weak attraction between the C–H bond and the delocalized π electrons system is called and that between the delocalized π electrons is known as π···π interaction. | The C–H···π interactions play an important role in the nanoscale assemblies and crystal packing of and were further extended to explain the crystallization of larger NCs. | The C–H···π attraction is often considered the weakest hydrogen bonding interaction occurring between the soft acids and bases. | Electrical Transport [39]. Separation of the racemates [40]. Detection [58]. |
| Metallophilic interactions | 25–30 (aurophilic interactions) | Weak interactions between metal ions in closed shells are often referred to as metallophilic interactions. | Metallophilic interactions are weak; they will be very prominent in the presence of particular solvents. | Metallophilic interactions are weak. | Light-emitting diodes [43]. Detection [59]. Temperature sensing [16,20] |
| NCs | Fluorescent | Detection Analyte | Detection Limit | Detection Range | The Practical Application |
|---|---|---|---|---|---|
| TSA-AgNCs [18] | Red | Hg2+ | 91.3 nM | 0.3–2.2 μM | No |
| SA-AgNCs [61] | Orange | Hg2+ | 3.1 nM | 3.5–100 nM | Tap water, Lactating mother’s milk |
| CuNCs [51] | Red | Hg2+ | 0.3 nM | 1–500 nM | Tap water, Lake water, wastewater |
| Ag6NCs [63] | Blue | Al3+ | 3.13 μM | No | No |
| (NH4)9[Ag9(MBA)9] [52] | Red | Fe3+ | 0.611 μM | 0–6 μM | No |
| AuNCs [66] | Yellow/green | Zn2+ | 9 nM | 0.2–1 μM | No |
| SF@CuNCs [26] | Blue | S2- | 0.286 μM | 5.0–110.0 μM | Tap water, River waste |
| DPA@Ag NCs [27] | Red to yellow | Ag+ | 0.03 μM | 0.05–800 μM | Tap water, Post-sewage water, Lake water |
| Poly-Au5 [59] | Red | GSH | 0.56 μM | 0–10 μM | HeLa cells |
| CuNCs [56] | Red | GSH | 300 nM | 1–100 μM | Three kinds of human cell lines |
| GSH-AuNCs [21] | Yellow | ALP | 0.2 U/L | 0.5–80 U/L | Human serum |
| GSH-AuNCs [17] | Yellow | Protamine and trypsin | 0.07 μg/mL 4.50 ng/mL | 0.15–3 μg/mL, 10–100 ng/mL | Human serum |
| (NH4)6[Ag6(mna)6] [28] | Yellow | Arginine | 28 μM | 0–200 μM | No |
| Ag29LA12 [57] | Red | Dopamine | 10 nM | 0–100 nM | No |
| Cu3NCs [77] | Yellow | Histamine | 60 nM | 0.1–10 μM | Fish, Shrimp, Red wine |
| Cu4(TTP)3 [79] | Red | Biothiols | 0.01 μM 0.01 μM 0.1 μM | 0.1–100 μM 1–100 μM 1–1000 μM | Fetal bovine serum |
| GSH-CuNCs [81] | Red | β-galactosidase | 0.7 U L−1 | 2.3 U L−1–96.0 U L−1 | No |
| [Ag6(mna)6]6− [22] | Blue | Dithiothreit-ol | 0.11 mM | No | No |
| TBA-AuNCs [19] | Yellow | 2-methyl-4-nitroimidazole | 13.8 μM | 0–400 μM | No |
| GSH-AgNCs [88] | Blue | H2S | 32 nM | 0–3 μM | No |
| [Ag9(mba)9] [16] | Orange-red | Temperature | No | 20–100 °C | No |
| [Ag9(mba)9] [20] | Orange-red | Temperature | No | −160 °C–(−80 °C) 80–260 °C | No |
| Peptide-AuNCs [94] | Red | Temperature | No | 10–45 °C | HeLa cells |
| GSH-AuNCs [53] | Red | Temperature | No | 20–80 °C | Living cells |
| Cys-AuNCs [54] | Red | pH | No | 6.7–10.5 | No |
| CuNCs@MMI [55] | Blue | pH | No | 4–12 | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, C.; Shu, T.; Du, X.; Yang, L.; Su, L.; Zhang, X. Luminescent Sensors Based on the Assembly of Coinage Metal Nanoclusters. Chemosensors 2022, 10, 253. https://doi.org/10.3390/chemosensors10070253
Ren C, Shu T, Du X, Yang L, Su L, Zhang X. Luminescent Sensors Based on the Assembly of Coinage Metal Nanoclusters. Chemosensors. 2022; 10(7):253. https://doi.org/10.3390/chemosensors10070253
Chicago/Turabian StyleRen, Chenyu, Tong Shu, Xin Du, Linzhi Yang, Lei Su, and Xueji Zhang. 2022. "Luminescent Sensors Based on the Assembly of Coinage Metal Nanoclusters" Chemosensors 10, no. 7: 253. https://doi.org/10.3390/chemosensors10070253
APA StyleRen, C., Shu, T., Du, X., Yang, L., Su, L., & Zhang, X. (2022). Luminescent Sensors Based on the Assembly of Coinage Metal Nanoclusters. Chemosensors, 10(7), 253. https://doi.org/10.3390/chemosensors10070253









