Acetylcholinesterase Immobilization on ZIF-8/Graphene Composite Engenders High Sensitivity Electrochemical Sensing for Organophosphorus Pesticides
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Instruments and Measurements
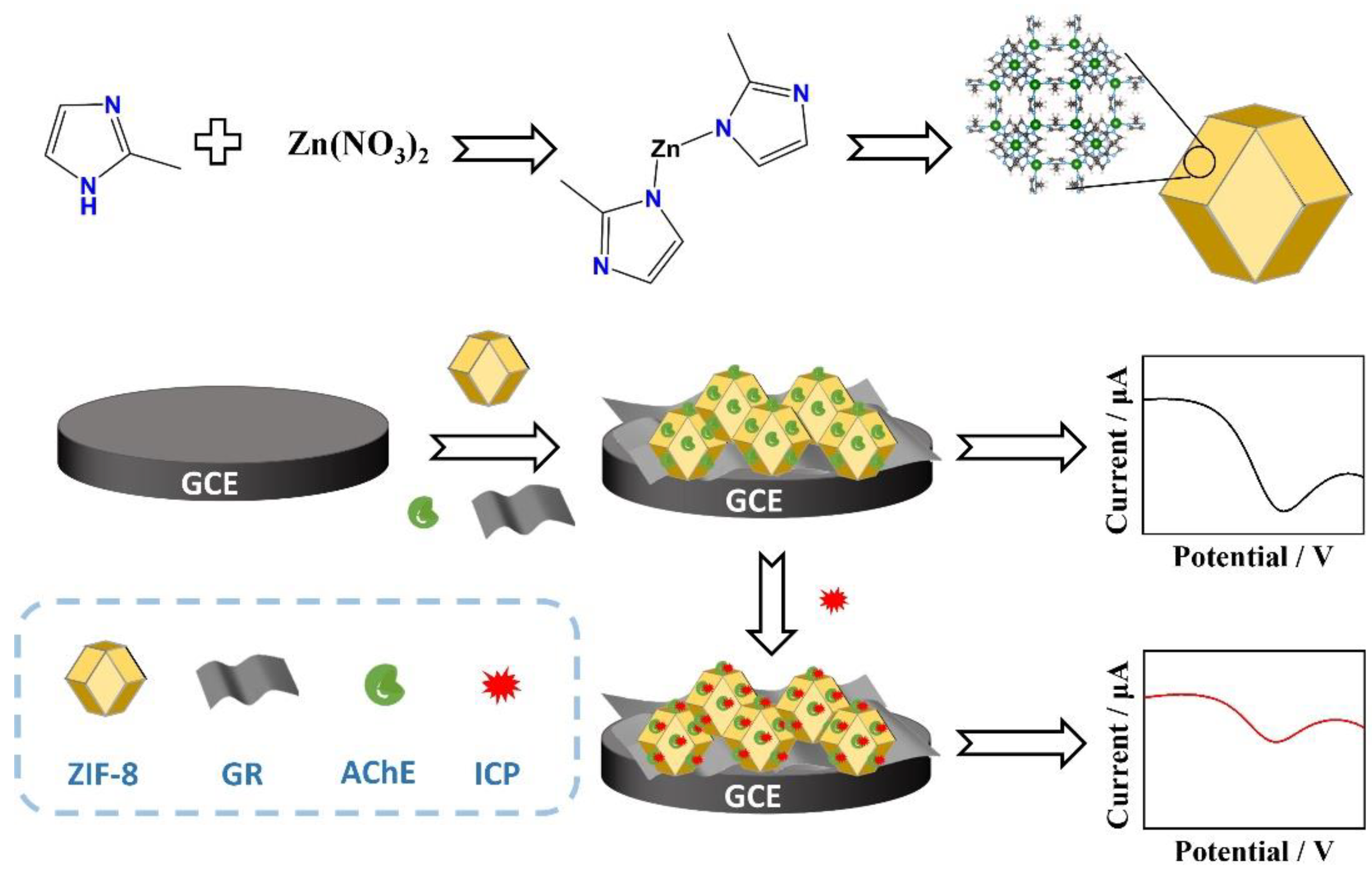
2.3. Synthesis of AChE-CS/GR/ZIF-8 Composites
2.4. Fabrication of Electrochemical Enzyme Biosensor
2.5. Electrochemical Measurement
2.6. Detection of Isocarbophos in Real Samples
2.7. Limit of Detection
3. Results and Discussion
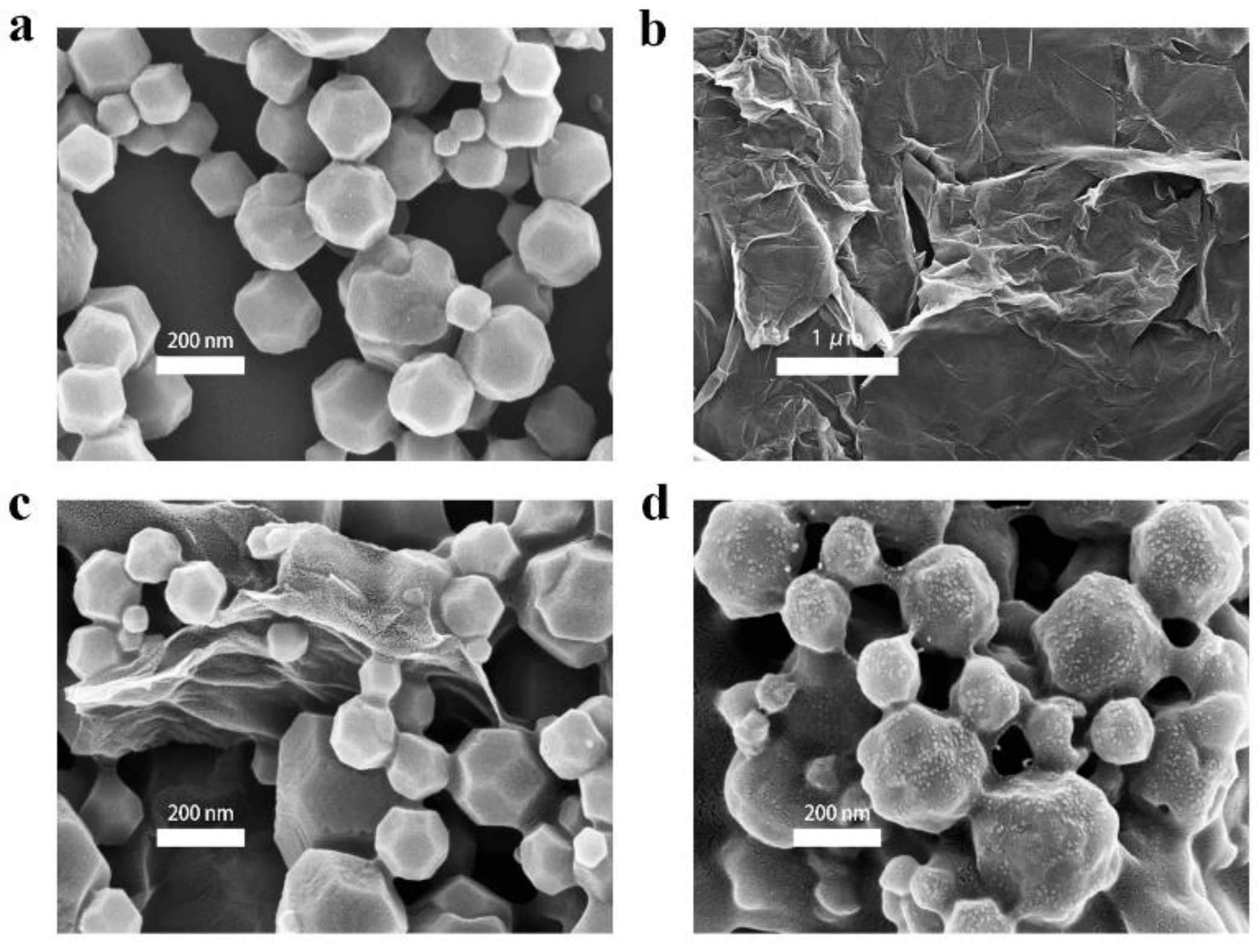
3.1. Characterization of Nanomaterials
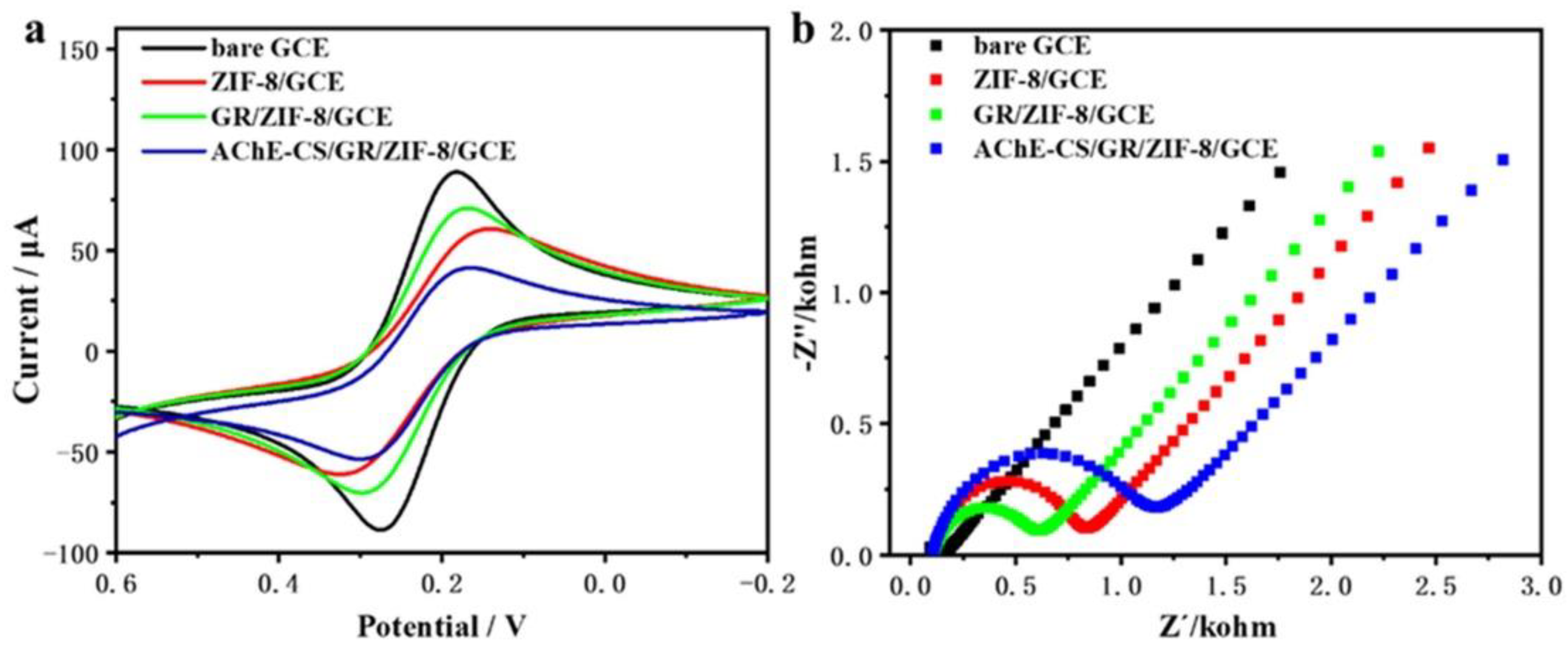
3.2. Electrochemical Characterization of Enzyme Biosensor
3.3. Study on Substrate Response of Different Enzyme Electrodes
3.4. Optimization of Detection Conditions of Enzyme Biosensor
3.5. The Performance of AChE-CS/GR/ZIF-8/GCE Electrochemical Biosensor
3.6. Anti-Interference and Reproducibility of Enzyme Sensor
3.7. Real Samples Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, H.; Thiermann, H. Poisoning by organophosphorus nerve agents and pesticides: An overview of the principle strategies and current progress of mass spectrometry-based procedures for verification. J. Mass Spectrom. Adv. Clin. Lab 2021, 19, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Vale, A.; Marrs, T.C.; Rice, P. Chemical terrorism and nerve agents. Medicine 2016, 44, 106–108. [Google Scholar] [CrossRef]
- Catalá-Icardo, M.; Lahuerta-Zamora, L.; Torres-Cartas, S.; Meseguer-Lloret, S. Determination of organothiophosphorus pesticides in water by liquid chromatography and post-column chemiluminescence with cerium (IV). J. Chromatogr. A 2014, 1341, 31–40. [Google Scholar] [CrossRef]
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. In situ ionic liquid dispersive liquid-liquid microextraction coupled to gas chromatography-mass spectrometry for the determination of organophosphorus pesticides. J. Chromatogr. A 2018, 1559, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Wang, X.; Zhang, H.; Wang, X.; Xu, H.; Wang, Q. Rapid Enantioseparation and Determination of Isocarbophos Enantiomers in Orange Pulp, Peel, and Kumquat by Chiral HPLC-MS/MS. Food Anal. Methods 2015, 8, 531–538. [Google Scholar] [CrossRef]
- Liu, M.; Wei, J.; Wang, Y.; Ouyang, H.; Fu, Z. Dopamine-functionalized upconversion nanoparticles as fluorescent sensors for organophosphorus pesticide analysis. Talanta 2019, 195, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Kordasht, H.K.; Pazhuhi, M.; Pashazadeh-Panahi, P.; Hasanzadeh, M.; Shadjou, N. Multifunctional aptasensors based on mesoporous silica nanoparticles as an efficient platform for bioanalytical applications: Recent advances. TrAC Trends Anal. Chem. 2020, 124, 115778. [Google Scholar] [CrossRef]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants—Trends and perspective. TrAC Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Panda, A.K. Recent advances in nano-engineered approaches used for enzyme immobilization with enhanced activity. J. Mol. Liq. 2021, 338, 116602. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme immobilized nanomaterials as electrochemical biosensors for detection of biomolecules. Enzyme Microb. Technol. 2022, 156, 110006. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Nie, M.; Li, Y.; Zhu, H.; Shi, G. Design of composite nanosupports and applications thereof in enzyme immobilization: A review. Colloids Surf. B Biointerfaces 2022, 217, 112602. [Google Scholar] [CrossRef]
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 2015, 348, 6238. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, Y.; Liu, S.; Wu, D.; Su, Z.; Chen, G.; Liu, J.; Li, G. Recent advances in enzyme immobilization based on novel porous framework materials and its applications in biosensing. Coord. Chem. Rev. 2022, 459, 214414. [Google Scholar] [CrossRef]
- Daniel, M.; Mathew, G.; Anpo, M.; Neppolian, B. MOF based electrochemical sensors for the detection of physiologically relevant biomolecules: An overview. Coord. Chem. Rev. 2022, 468, 214627. [Google Scholar] [CrossRef]
- Mohamud, M.A.; Yurtcan, A.B. Zeolotic imidazolate frameworks (ZIFs) derived porous carbon: A review from crystal growth & green synthesis to oxygen reduction reaction activity. Int. J. Hydrog. Energy 2021, 46, 33782–33800. [Google Scholar] [CrossRef]
- Gao, L.; Chen, Q.; Gong, T.; Liu, J.; Li, C. Recent advancement of imidazolate framework (ZIF-8) based nanoformulations for synergistic tumor therapy. Nanoscale 2019, 11, 21030–21045. [Google Scholar] [CrossRef]
- Park, K.S.; Zheng, N.; Cté, A.; Choi, J.Y.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, X.; Huang, L.; Zhang, Z.; Dong, S. GOx@ZIF-8(NiPd) Nanoflower: An Artificial Enzyme System for Tandem Catalysis. Angew. Chem. 2017, 56, 16082–16085. [Google Scholar] [CrossRef]
- Ouyang, G.; Chen, G.; Huang, S.; Kou, X.; Wei, S.; Huang, S.; Jiang, S.; Shen, J.; Zhu, F. A Convenient and Versatile Amino-Acid-Boosted Biomimetic Strategy for the Nondestructive Encapsulation of Biomacromolecules within Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 1463–1467. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, C.; Zhang, Y.; He, F.; Liu, M.; Li, X. Preparation of graphene nano-sheet bonded PDA/MOF microcapsules with immobilized glucose oxidase as a mimetic multi-enzyme system for electrochemical sensing of glucose. J. Mater. Chem. B 2016, 4, 3695–3702. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Dai, H.; Li, Z.; Fu, Y.; Li, Y. Biomineralization-mimetic preparation of robust metal-organic frameworks biocomposites film with high enzyme load for electrochemical biosensing. J. Electroanal. Chem. 2018, 823, 40–46. [Google Scholar] [CrossRef]
- Wei, T.H.; Wu, S.H.; Huang, Y.D.; Lo, W.S.; Williams, B.P.; Chen, S.Y.; Yang, H.C.; Hsu, Y.S.; Lin, Z.Y.; Chen, X.H.; et al. Rapid mechanochemical encapsulation of biocatalysts into robust metal-organic frameworks. Nat. Commun. 2019, 10, 5002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Qiao, S.; Zheng, Y.; Andaloussi, Y.H.; Li, X.; Zhang, Z.; Li, A.; Cheng, P.; Ma, S.; Chen, Y. Fabricating covalent organic framework capsules with commodious microenvironment for enzymes. J. Am. Chem. Soc. 2020, 142, 6675–6681. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Tran, U.; Le, K.; Phan, N. Expanding Applications of Metal−Organic Frameworks: Zeolite Imidazolate Framework ZIF-8 as an Efficient Heterogeneous Catalyst for the Knoevenagel Reaction. ACS Catal. 2011, 1, 120–127. [Google Scholar] [CrossRef]
- Zhai, C.; Guo, Y.; Sun, X.; Zheng, Y.; Wang, X. An acetylcholinesterase biosensor based on graphene–gold nanocomposite and calcined layered double hydroxide. Enzym. Microb. Technol. 2014, 58, 8–13. [Google Scholar] [CrossRef]
- Du, D.; Wang, M.; Cai, J.; Qin, Y.; Zhang, A. One-step synthesis of multiwalled carbon nanotubes-gold nanocomposites for fabricating amperometric acetylcholinesterase biosensor. Sens. Actuators B Chem. 2010, 143, 524–529. [Google Scholar] [CrossRef]
- Song, D.; Li, Y.; Lu, X.; Sun, M.; Liu, H.; Yu, G.; Gao, F. Palladium-copper nanowires-based biosensor for the ultrasensitive detection of organophosphate pesticides. Anal. Chim. Acta 2017, 982, 168–175. [Google Scholar] [CrossRef]
- Yu, G.; Wu, W.; Zhao, Q.; Wei, X.; Lu, Q. Efficient immobilization of acetylcholinesterase onto amino functionalized carbon nanotubes for the fabrication of high sensitive organophosphorus pesticides biosensors. Biosens. Bioelectron. 2015, 68, 288–294. [Google Scholar] [CrossRef]
- Shi, Q.; Teng, Y.; Zhang, Y.; Liu, W. Rapid detection of organophosphorus pesticide residue on Prussian blue modified dual-channel screen-printed electrodes combing with portable potentiostat. Chin. Chem. Lett. 2018, 29, 1379–1382. [Google Scholar] [CrossRef]





| Method | Target | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|
| GN-AuNPs/CLDH-AChE/GCE | Chlorpyrifos | 0.1426–427.87 μM | 142.6 nM | [26] |
| AChE-MWCNTs-Au-CHIT/GCE | Malathion | 3.027–3027 nM | 1.8 nM | [27] |
| AChE-Cs/Pd-Cu NWs/GCE | Malathion | 15 pM--9 μM | 4.5 pM | [28] |
| AChE/CNT-NH2/GCE | Paraoxon | 0.2–1 nM, 1–30 nM | 0.08 nM | [29] |
| Nafion-AChE/PB/DSPE | Isocarbophos | 0.35–17.3 μM | 1.73 μM | [30] |
| AChE-CS/GR/ZIF-8/GCE | Isocarbophos | 1.73–345.7 nM | 0.62 nM | This work |
| Sample | Added (μg/mL) | Found (μg/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Cabbage | 0.001 0.01 0.1 | 0.00106 0.00918 0.11427 | 106.1 91.7 114.3 | 2.2 5.6 2.7 |
| Tap | 0.001 | 0.00096 | 95.7 | 3.9 |
| water | 0.01 | 0.00881 | 88.1 | 1.7 |
| 0.1 | 0.12237 | 122.4 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Wang, N.; Liu, Z.; Tao, C.-a.; Zou, X.; Wang, F.; Wang, J. Acetylcholinesterase Immobilization on ZIF-8/Graphene Composite Engenders High Sensitivity Electrochemical Sensing for Organophosphorus Pesticides. Chemosensors 2022, 10, 418. https://doi.org/10.3390/chemosensors10100418
Wen L, Wang N, Liu Z, Tao C-a, Zou X, Wang F, Wang J. Acetylcholinesterase Immobilization on ZIF-8/Graphene Composite Engenders High Sensitivity Electrochemical Sensing for Organophosphorus Pesticides. Chemosensors. 2022; 10(10):418. https://doi.org/10.3390/chemosensors10100418
Chicago/Turabian StyleWen, Long, Ning Wang, Zhuoliang Liu, Cheng-an Tao, Xiaorong Zou, Fang Wang, and Jianfang Wang. 2022. "Acetylcholinesterase Immobilization on ZIF-8/Graphene Composite Engenders High Sensitivity Electrochemical Sensing for Organophosphorus Pesticides" Chemosensors 10, no. 10: 418. https://doi.org/10.3390/chemosensors10100418
APA StyleWen, L., Wang, N., Liu, Z., Tao, C.-a., Zou, X., Wang, F., & Wang, J. (2022). Acetylcholinesterase Immobilization on ZIF-8/Graphene Composite Engenders High Sensitivity Electrochemical Sensing for Organophosphorus Pesticides. Chemosensors, 10(10), 418. https://doi.org/10.3390/chemosensors10100418











