A Cell Co-Culture Taste Sensor Using Different Proportions of Caco-2 and SH-SY5Y Cells for Bitterness Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Co-Culture
2.3. Cell Labeling
2.4. Live/Dead Staining
2.5. ECIS Sensor and Detection System
2.6. Statistical Calculations
3. Results and Discussion
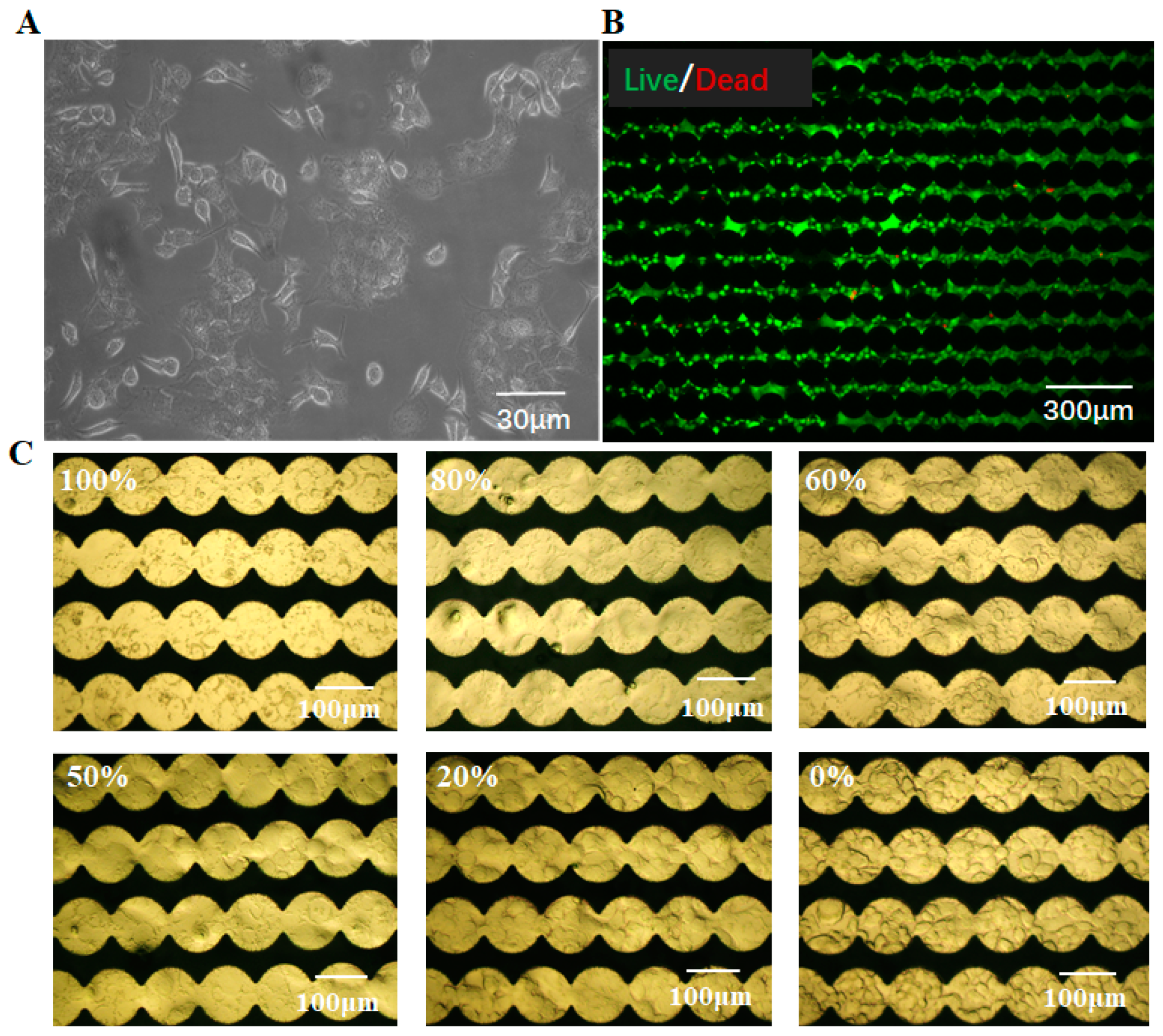
3.1. Cell Co-Culture Taste Sensor Construction
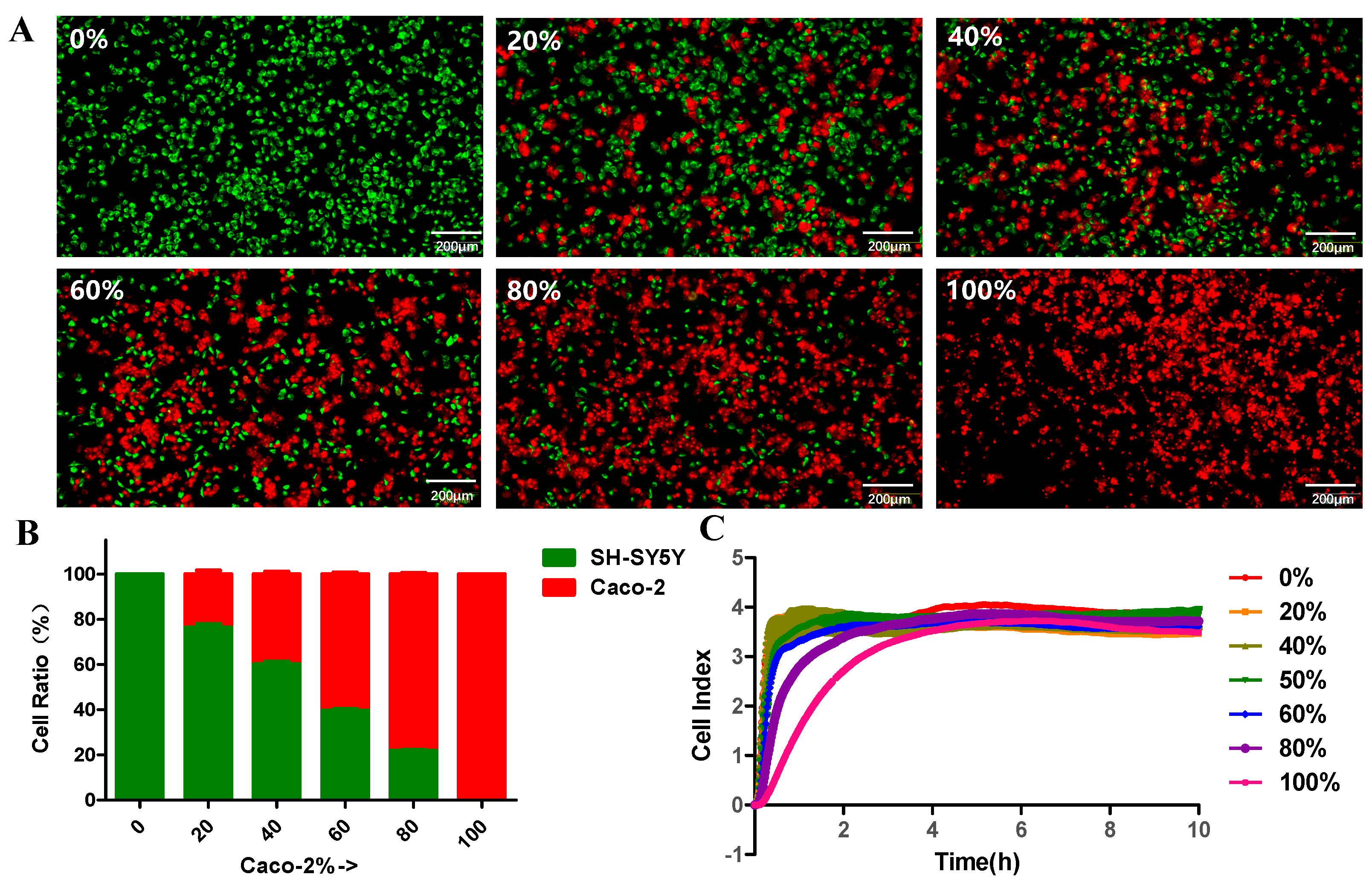
3.2. Caco-2 Cells and SH-SY5Y Cells Co-Culture
3.3. Cell Labeling and Cell Growth Monitoring
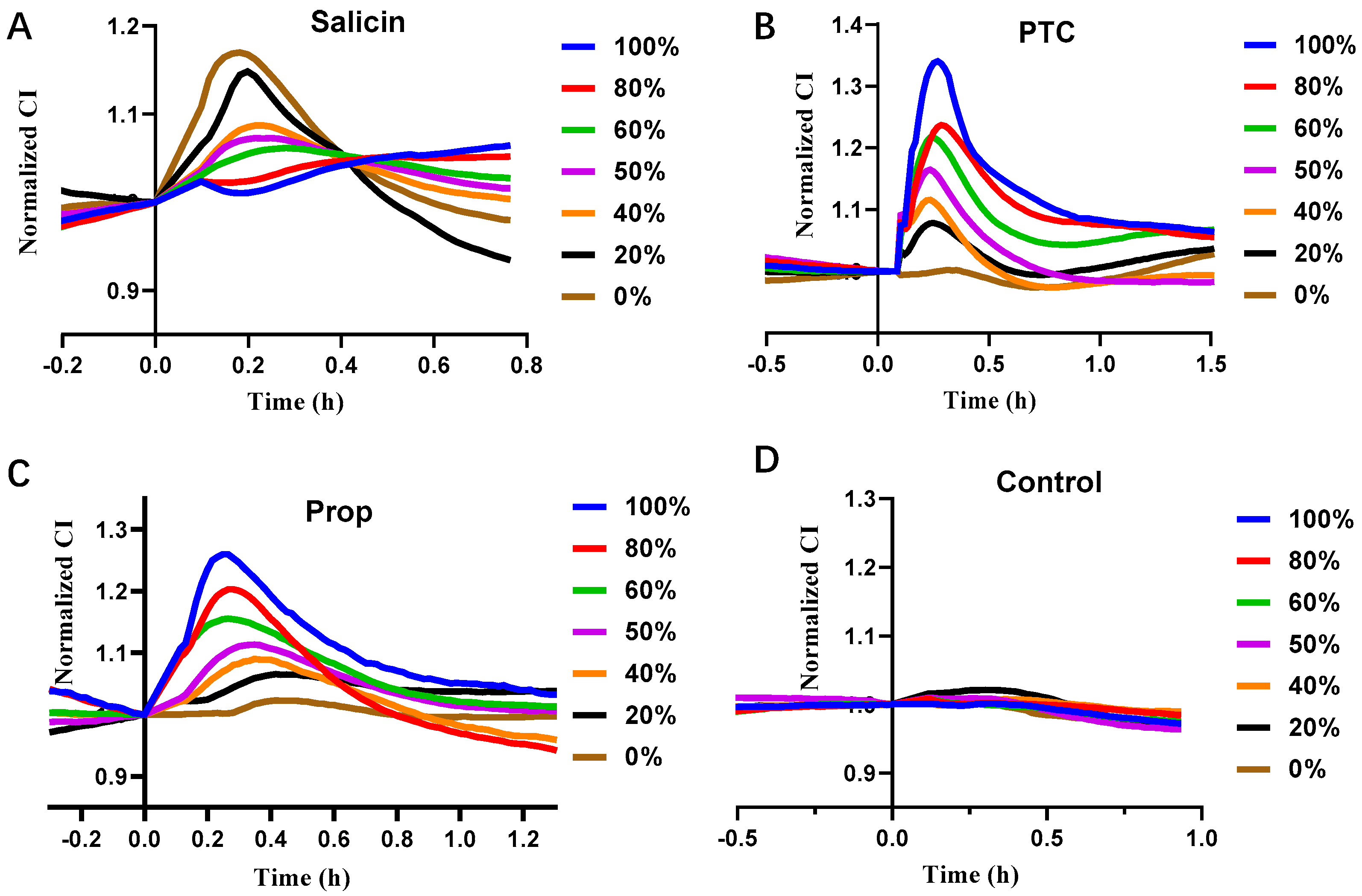
3.4. Cell Co-Culture Taste Sensor for Differentiating T2R38 Ligands from T2R16 Ligands
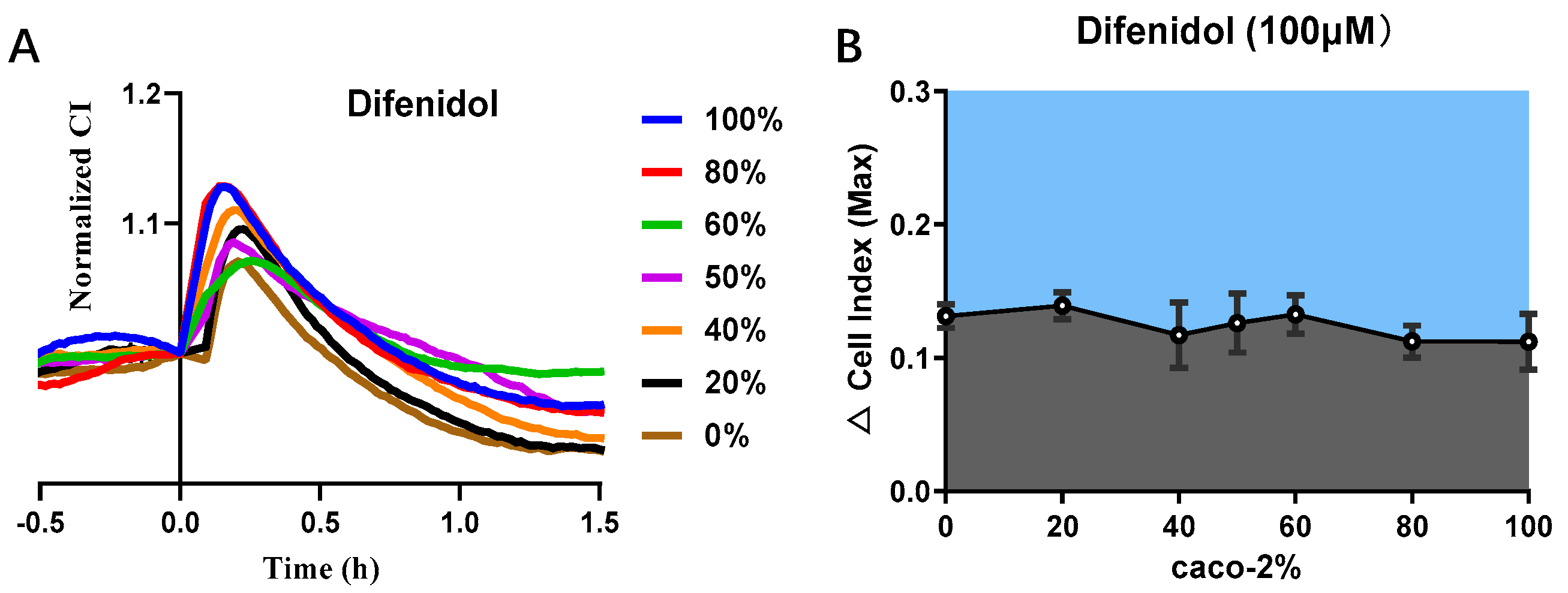
3.5. Cell Co-Culture Taste Sensor for Difenidol Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, R.; Dalziel, J.E. G protein-coupled receptors in taste physiology and pharmacology. Front. Pharmacol. 2020, 11, 587664. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.I.; Zhu, B.; Larson, J.L.; Osborne, T.F. SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J. Clin. Invest. 2008, 118, 3693–3700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Zhang, C.H.; Lifshitz, L.M.; ZhuGe, R. Extraoral bitter taste receptors in health and disease. J. Gen. Physiol. 2017, 149, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, M.C.; Ranciaro, A.; Zinshteyn, D.; Rawlings-Goss, R.; Hirbo, J.; Thompson, S.; Woldemeskel, D.; Froment, A.; Rucker, J.B.; Omar, S.A.; et al. Origin and differential selection of allelic variation at TAS2R16 associated with salicin bitter taste sensitivity in Africa. Mol. Biol. Evol. 2014, 31, 288–302. [Google Scholar] [CrossRef] [Green Version]
- Tuzim, K.; Korolczuk, A. An update on extra-oral bitter taste receptors. J. Transl. Med. 2021, 19, 440. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J. T2Rs function as bitter taste receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Gupta, H.; Sharma, A.; Kumar, S.; Roy, S.K. E-tongue: A tool for taste evaluation. Recent Pat. Drug Deliv. Formul. 2010, 4, 82–89. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef] [Green Version]
- Veloso, A.C.A.; Dias, L.G.; Rodrigues, N.; Pereira, J.A.; Peres, A.M. Sensory intensity assessment of olive oils using an electronic tongue. Talanta 2016, 146, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Ishida, M.; Ide, H.; Arima, K.; Zhao, Z.; Matsui, T.; Toko, K. Identification of the Principle of Taste Sensors to Detect Non-Charged Bitter Substances by 1H-NMR Measurement. Sensors 2022, 22, 2592. [Google Scholar] [CrossRef]
- Fujimoto, H.; Narita, Y.; Iwai, K.; Hanzawa, T.; Kobayashi, T.; Kakiuchi, M.; Ariki, S.; Wu, X.; Miyake, K.; Tahara, Y. Bitterness compounds in coffee brew measured by analytical instruments and taste sensing system. Food Chem. 2021, 342, 128228. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shiino, T.; Tahara, Y.; Ikezaki, H.; Toko, K. Quantification of pharmaceutical bitterness using a membrane electrode based on a hydrophobic tetrakis [3, 5-bis (Trifluoromethyl) phenyl] borate. Chemosensors 2021, 9, 28. [Google Scholar] [CrossRef]
- Qin, C.; Yuan, Q.; Zhuang, L.; Li, R.; Wang, P. Multi-tissue cell sensors based on the expression of bitter taste receptors and their application. Chin. J. Sci. Instrum. 2021, 42, 127–135. [Google Scholar] [CrossRef]
- Qin, C.; Yuan, Q.; Zhang, S.; He, C.; Wei, X.; Liu, M.; Jiang, N.; Huang, L.; Zhuang, L.; Wang, P. Biomimetic in vitro respiratory system using smooth muscle cells on ECIS chips for anti-asthma TCMs screening. Anal. Chim. Acta 2021, 1162, 338452. [Google Scholar] [CrossRef]
- Wu, C.; Du, L.; Mao, L.; Wang, P. A novel bitter detection biosensor based on light addressable potentiometric sensor. J. Innov. Opt. Health Sci. 2012, 5, 1250008. [Google Scholar] [CrossRef]
- Wei, X.; Qin, C.; Gu, C.; He, C.; Yuan, Q.; Liu, M.; Zhuang, L.; Wan, H.; Wang, P.J.B.; Bioelectronics. A novel bionic in vitro bioelectronic tongue based on cardiomyocytes and microelectrode array for bitter and umami detection. Biosens. Bioelectron. 2019, 145, 111673. [Google Scholar] [CrossRef]
- Wang, J.; Kong, S.; Chen, F.; Chen, W.; Du, L.; Cai, W.; Huang, L.; Wu, C.; Zhang, D.-W. A bioelectronic taste sensor based on bioengineered Escherichia coli cells combined with ITO-constructed electrochemical sensors. Anal. Chim. Acta 2019, 1079, 73–78. [Google Scholar] [CrossRef]
- Behrens, M.; Ziegler, F. Structure-function analyses of human bitter taste receptors—Where do we stand? Molecules 2020, 25, 4423. [Google Scholar] [CrossRef]
- Fahey, J.W.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bufe, B.; Hofmann, T.; Krautwurst, D.; Raguse, J.-D.; Meyerhof, W. The human TAS2R16 receptor mediates bitter taste in response to β-glucopyranosides. Nat. Genet. 2002, 32, 397–401. [Google Scholar] [CrossRef]
- Viswanathan, V.J.G.m. Sensing bacteria, without bitterness? Gut Microbes 2013, 4, 91–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wölfle, U.; Haarhaus, B.; Kersten, A.; Fiebich, B.; Hug, M.J.; Schempp, C.M.J.P.R. Salicin from Willow Bark can Modulate Neurite Outgrowth in Human Neuroblastoma SH-SY5Y Cells. Phytother. Res. 2015, 29, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Qin, Z.; Zhao, D.; Pan, Y.; Zhuang, L.; Wan, H.; Di Pizio, A.; Malach, E.; Niv, M.Y.; Huang, L. A bioinspired in vitro bioelectronic tongue with human T2R38 receptor for high-specificity detection of NC= S-containing compounds. Talanta 2019, 199, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Atienza, J.M.; Bernard, J.; Blanc, S.; Zhu, J.; Wang, X.; Xu, X.; Abassi, Y.A. Real-time monitoring of morphological changes in living cells by electronic cell sensor arrays: An approach to study G protein-coupled receptors. Anal. Chem. 2006, 78, 35–43. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W. Vertebrate bitter taste receptors: Keys for survival in changing environments. J. Agric. Food Chem. 2016, 66, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef]
- Dagan-Wiener, A.; Di Pizio, A.; Nissim, I.; Bahia, M.S.; Dubovski, N.; Margulis, E.; Niv, M.Y. BitterDB: Taste ligands and receptors database in 2019. Nucleic Acids Res. 2019, 47, D1179–D1185. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, C.; Zhang, S.; Yuan, Q.; Liu, M.; Jiang, N.; Zhuang, L.; Huang, L.; Wang, P. A Cell Co-Culture Taste Sensor Using Different Proportions of Caco-2 and SH-SY5Y Cells for Bitterness Detection. Chemosensors 2022, 10, 173. https://doi.org/10.3390/chemosensors10050173
Qin C, Zhang S, Yuan Q, Liu M, Jiang N, Zhuang L, Huang L, Wang P. A Cell Co-Culture Taste Sensor Using Different Proportions of Caco-2 and SH-SY5Y Cells for Bitterness Detection. Chemosensors. 2022; 10(5):173. https://doi.org/10.3390/chemosensors10050173
Chicago/Turabian StyleQin, Chunlian, Saisai Zhang, Qunchen Yuan, Mengxue Liu, Nan Jiang, Liujing Zhuang, Liquan Huang, and Ping Wang. 2022. "A Cell Co-Culture Taste Sensor Using Different Proportions of Caco-2 and SH-SY5Y Cells for Bitterness Detection" Chemosensors 10, no. 5: 173. https://doi.org/10.3390/chemosensors10050173
APA StyleQin, C., Zhang, S., Yuan, Q., Liu, M., Jiang, N., Zhuang, L., Huang, L., & Wang, P. (2022). A Cell Co-Culture Taste Sensor Using Different Proportions of Caco-2 and SH-SY5Y Cells for Bitterness Detection. Chemosensors, 10(5), 173. https://doi.org/10.3390/chemosensors10050173







