3.1. Characteristics of Nanocrystalline In2O3
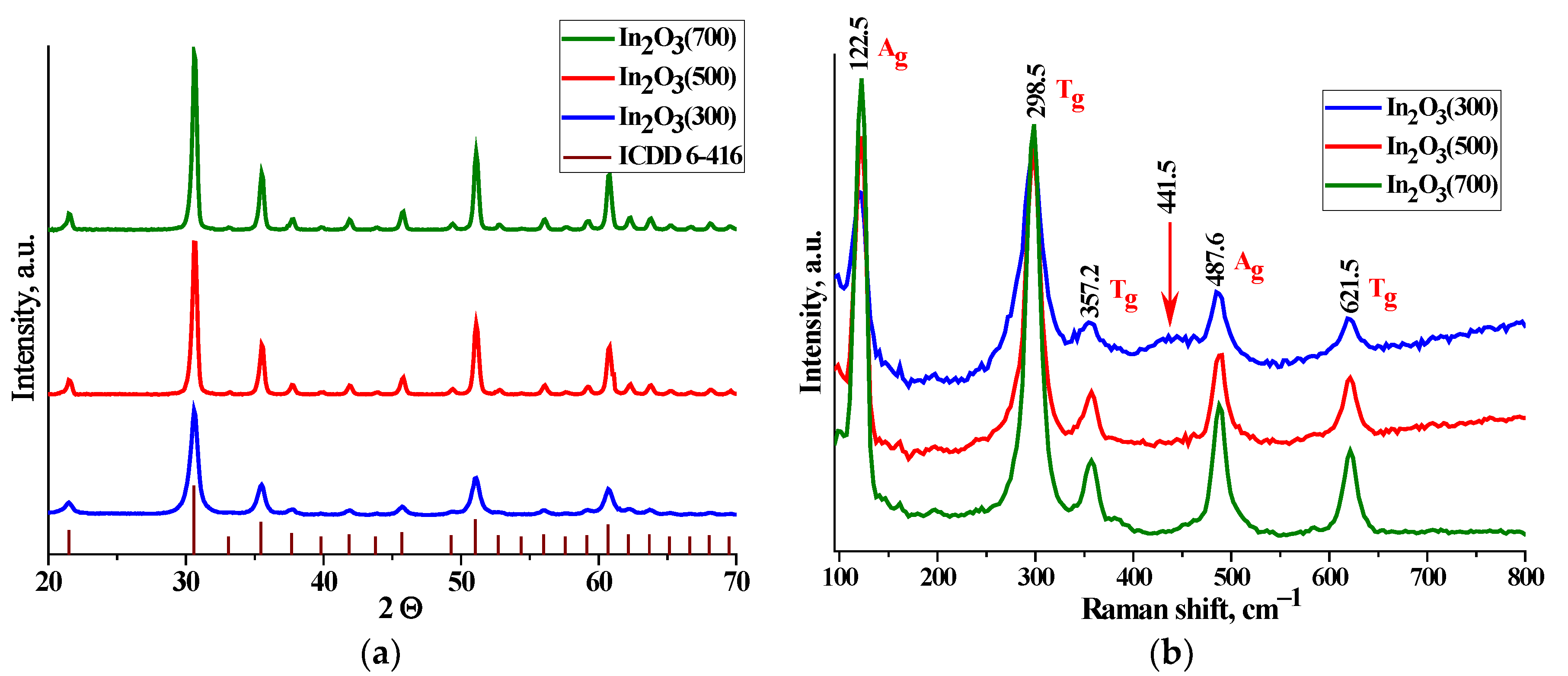
Figure 2a shows X-ray diffraction patterns of nanocrystalline In
2O
3 samples annealed at temperatures of 300, 500 and 700 °C. According to the results of X-ray phase analysis and ICDD PDF-2 database, the obtained indium oxide powders are single-phase corresponding to a cubic bixbyite structure. It was found that increasing the annealing temperature leads to an increase in crystallite size (d
XRD) and a decrease in specific surface area (S
surf) (
Table 2).
Figure 2b represents the Raman spectra of nanocrystalline In
2O
3 samples annealed at temperatures of 300, 500 and 700 °C. The main characteristic Raman modes for the body-centered cubic lattice of In
2O
3 were observed. The peak at 122.5 cm
−1 is associated with the vibrations of the In-O bond of the [InO
6], and the peaks at 298.5 cm
−1, 487.6 and 621.5 cm
−1 are due to the deformation and stretching vibration of the [InO
6] octahedrons, respectively. The stretching vibrations of In-O-In bonds were corresponded at 357.2 cm
−1 [
28,
29,
30]. However, the In
2O
3(300) sample contains a broad band at 441.5 cm
−1, which corresponds to a superposition of surface modes. These vibration modes are characteristic of nanosized powders and indicate a highly defective surface layer [
31]. It should also be noted that with an increase in the annealing temperature, the background of the Raman spectra descends and becomes horizontal. The defect structure leads to luminescence of the sample under the influence of a powerful radiation source, which leads to the rise in the baseline at higher values of the Raman shift. This statement confirms the defect structure of the surface of the samples annealed at lower temperatures.
The curves of the TPR-H
2 method are represented in
Figure 3a. Two regions of hydrogen consumption can be distinguished—low-temperature (up to 400 °C) and high-temperature (above 400 °C). The absorption of hydrogen at low temperatures is due to the reduction of surface OH groups and various forms of chemisorbed oxygen:
In the high-temperature region, the H
2 consumption is due to the reduction of In
2O
3 to metallic indium:
The amount of consumed hydrogen was calculated from the peak area, and the calibration was performed using a standard Ag
2O sample. The calculation results of the H
2 consumption for two temperature ranges are presented in
Table 3. In the low-temperature region, the amount of absorbed hydrogen is 2 times higher in the case of the most dispersed sample (In
2O
3(300)), which indicates a greater number of surface OH groups and chemisorbed O
2. A shift in the maximum H
2 consumption peak and an increase in its amount with an increase in the annealing temperature of the sample can be observed in the high-temperature region. This is associated with an increase in the particle size and crystallinity degree of In
2O
3, which increases the crystal lattice energy, and therefore its destruction during bulk reduction requires large amounts of thermal energy. The total amount of hydrogen consumed during the reduction of In
2O
3 ranges from n = 3.0 to 3.3 mol of H
2 per mol of In
2O
3, which is in good agreement with the theoretical value (n = 3 mol) of the reduction of indium oxide to metal (reaction (5)).
FTIR spectra of the samples are shown in
Figure 3b. The spectra contain peaks corresponding to the vibrations of bonds in the crystal lattice of In-O (400–600 cm
−1), adsorbed water (1628 cm
−1) and hydroxyl groups (3000–3670 cm
−1). With an increase in the annealing temperature, a decrease in OH groups on the surface is observed, while the In
2O
3(300) sample contains the largest number of OH groups, which is in good agreement with the TPR-H
2 results.
The electrophysical properties of the In
2O
3(300), In
2O
3(500) and In
2O
3(700) samples were investigated in the temperature range of 300–25 °C in a dry air flow. The temperature dependence of the conductivity has a complicated form for all samples, which may be associated with the chemisorption of oxygen from air. Therefore, in order to study the electrophysical properties, the temperature dependence of the conductivity was plotted in the Mott coordinates [
32] (6):
where G
M and T
M are the Mott characteristic parameters.
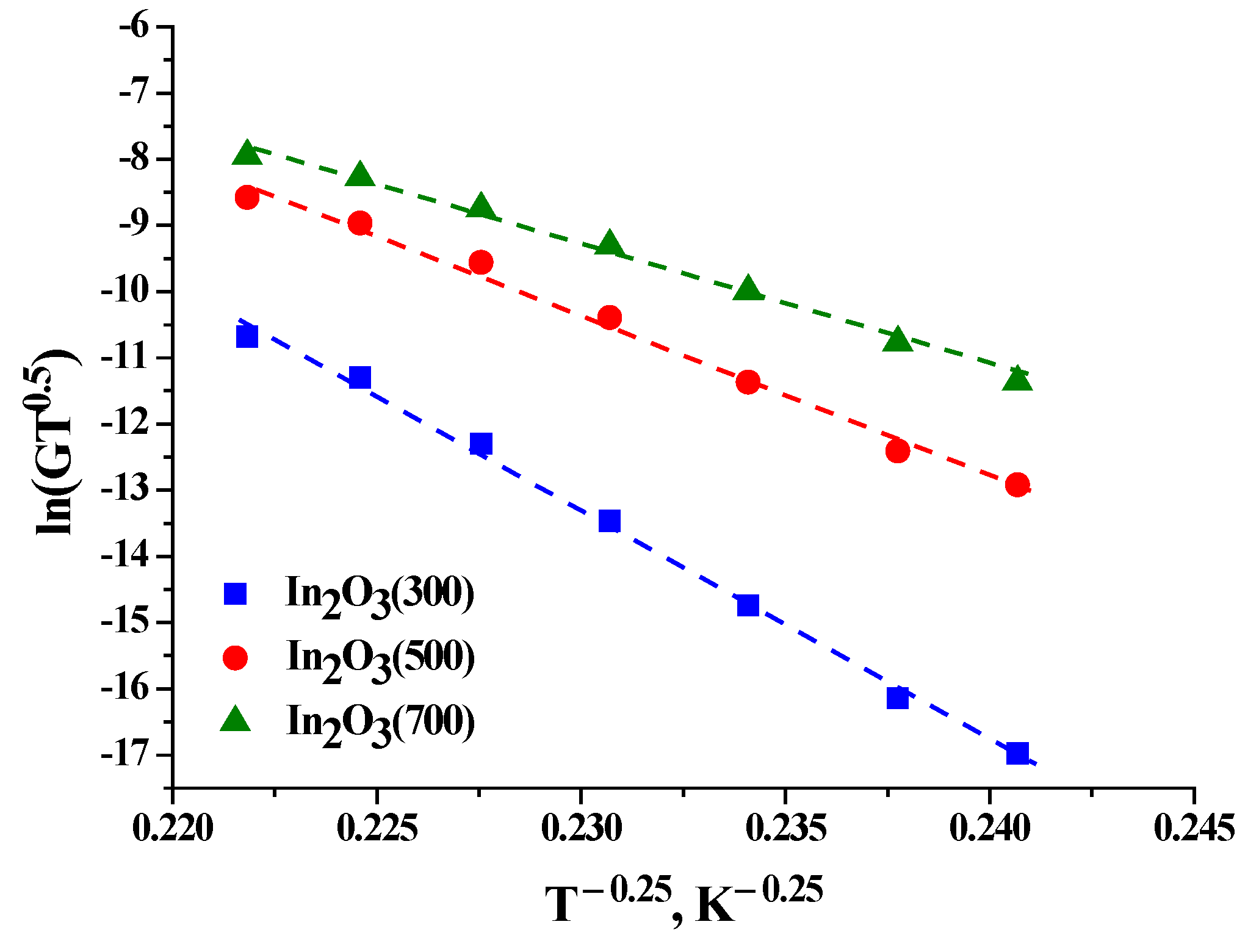
This dependence is well linearized in the temperature range of 140–25 °C. The value of T
M was calculated from the slope of the straight line ln(GT
0.5) = f(T
−0.25) (
Figure 4). Then, using equations (7–9), the following characteristic parameters were calculated: N(E
F), R
hop and W
hop, where N(E
F) is the density of localized states near the Fermi level, R
hop is the electron “jump” length, W
hop is the “jump” energy electron, α is a quantity that describes the spatial degree of localization of the wave function, and is equal to 1.24 nm
−1 and k
B is the Boltzmann constant.
Table 4 shows the parameters characterizing the conductivity of the studied samples according to the Mott model [
32]. The Mott model assumes the activation character of conductivity, which indicates the presence of barriers, and the height of the barrier is directly proportional to the resistance of the material. In this case, the resistance value correlates with the grain size in the range of In
2O
3(300) > In
2O
3(500) > In
2O
3(700). The calculated values of the electron “jump” energy or activation energy (W
hop) for the temperature range of 25–140 °C also changes monotonically, and may be associated with an increase in the crystallite size, which leads both to a decrease in the height of intergrain barriers and to a shift of the Fermi level to the edge of the conduction band.
3.2. Characteristics of Hybrid Materials
The results of a quantitative analysis of the composition of hybrid materials obtained by X-ray fluorescence analysis (XRF) are presented in
Table 5. It can be observed from the table that the ruthenium content in the hybrids is close to the theoretically specified value [Ru]/([Ru] + [In]) = 0.5 wt.%. These values are averaged and taken from 4 different areas of the sample.
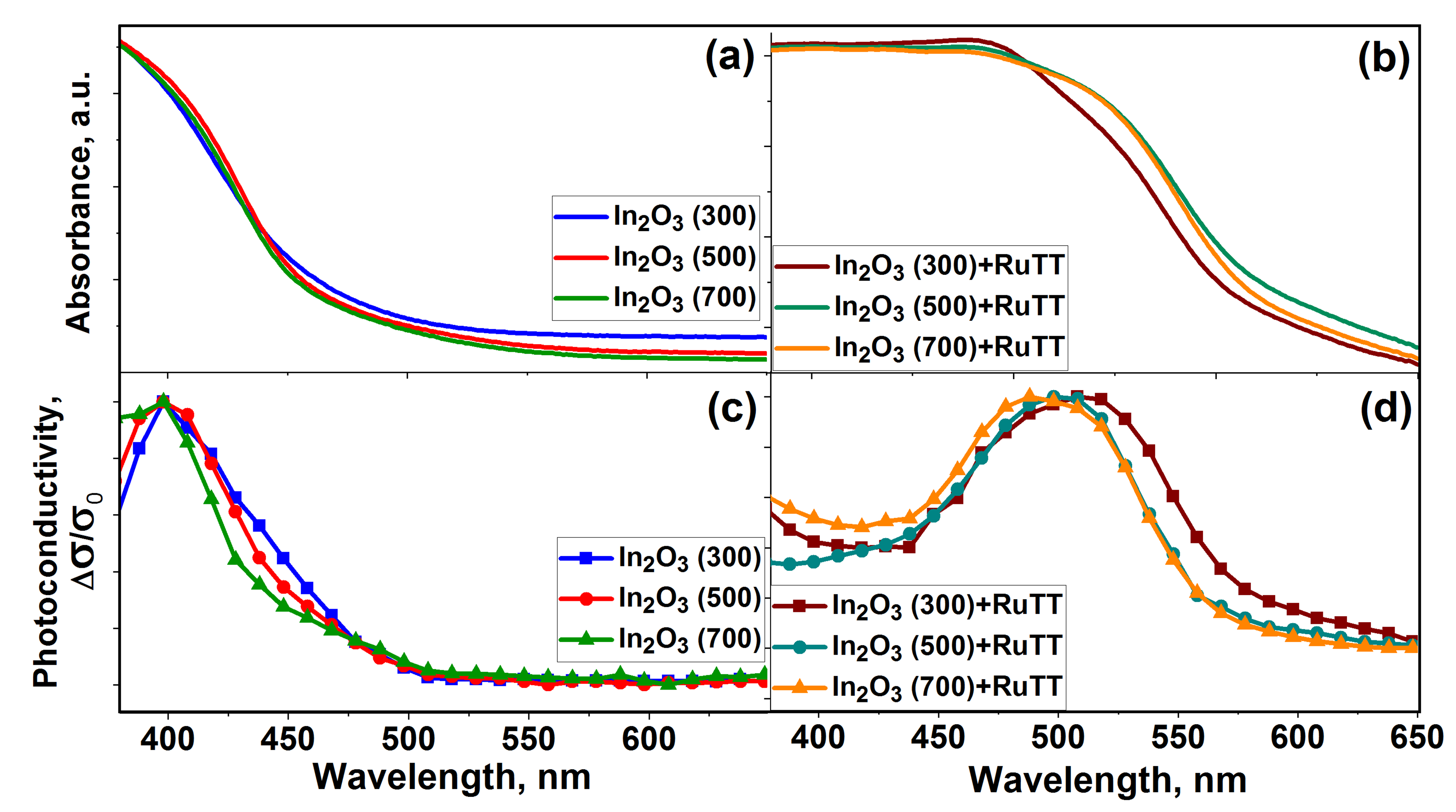
The optical absorption spectra (a,b) and normalized spectral dependences of the photoconductivity (c,d) of the sensitized composites and pure In
2O
3 samples are shown in
Figure 5.
As can be observed from the figures, unmodified In
2O
3-based samples are transparent in the visible spectral range. All spectra exhibit an absorption band in the UV region, which corresponds to a direct transition from the valence band to the conduction band, as the energy of this transition exceeds the semiconductor band gap. The curves of the absorption spectra coincide and are overlapped on the curves of the spectral dependence of the photoconductivity for all samples. However, some features can be noticed. In particular, in
Figure 5c, a shoulder is observed in the blue region of the spectrum, while its intensity increases with a decrease in the size of indium oxide crystallites. This may be related to excitation of the impurities within the bandgap, because the In
2O
3(300) sample has a more defective structure.
An absorption edge is observed in the visible region of the spectrum in hybrid materials due to the presence of organic complexes in their composition. Moreover, the maximum of the absorption edge for In2O3(500)+RuTT and In2O3(700)+RuTT samples is shifted to longer wavelengths by about 10 nm; for these composites, a bathochromic shift is observed. This shift may be associated with the stabilization effect of the excited states of Ru(II) heteroleptic complexes by the solid phase of the semiconductor oxide. The increase in photoconductivity for hybrid materials in the visible range of the spectrum indicates the transfer of photoexcited electrons from the Ru(II) heteroleptic complex to the conduction band of the semiconductor oxide.
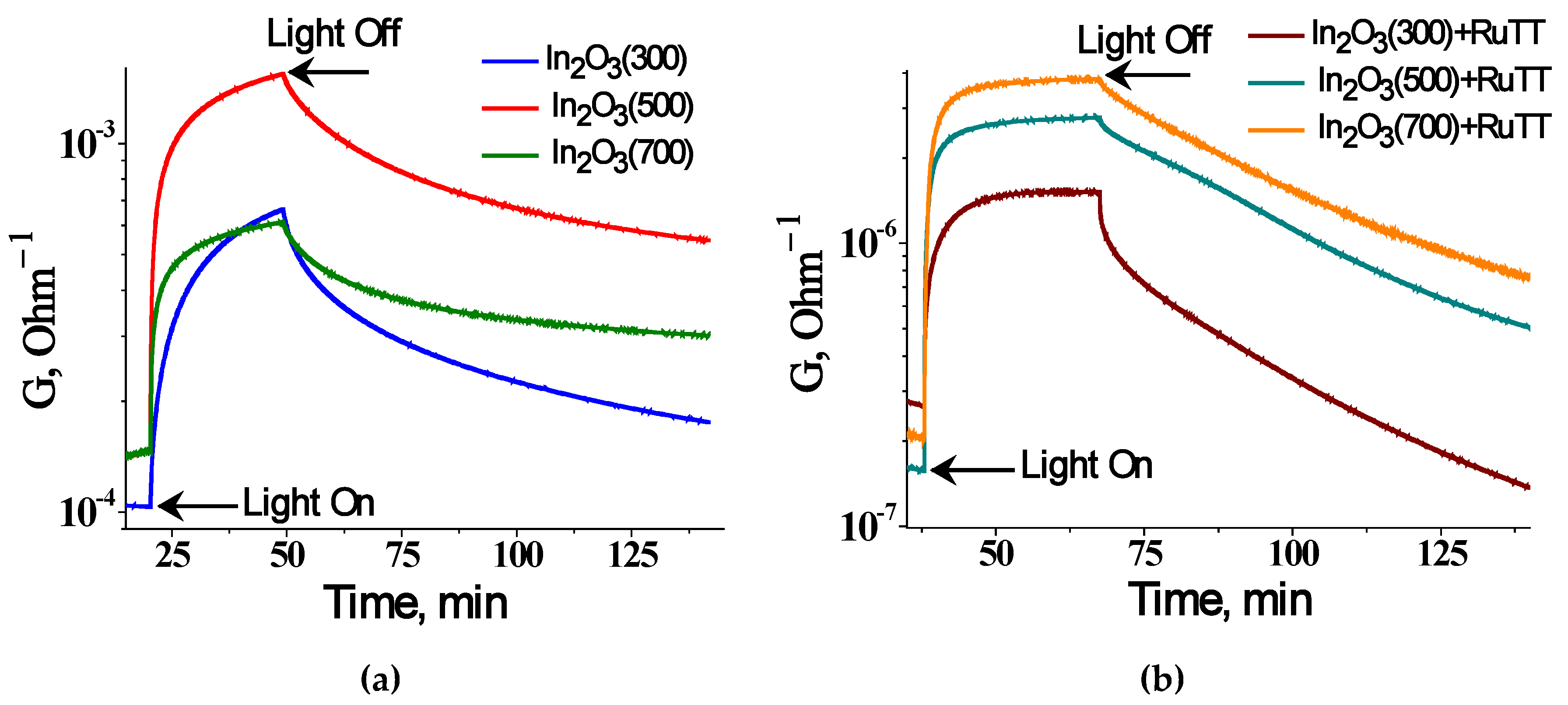
The photoconductivity of nanocrystalline In
2O
3 and hybrid materials was studied under constant illumination with blue LED (λ
max = 470 nm). The curves of changes in the conductivity of the studied samples under illumination are shown in
Figure 6. When the light is turned on, the electrical conductivity of materials increases; turning off the LED leads to relaxation of the conductivity. The effect of the annealing temperature of In
2O
3 on the behavior of photoconductivity under the influence of blue LED radiation is nonmonotonic. The maximum value of the photoresponse, calculated as the ratio of the conductivity under illumination to the conductivity under dark conditions, was achieved by the In
2O
3(500) sample. In this case, the change in conductivity can be associated with the presence of acceptor levels in the band gap of the semiconductor, as the value of the quantum energy corresponding to the emission of a blue LED is less than the band gap of indium oxide.
As can be observed, the difference in photoresponse between the hybrid material and the corresponding matrix increases with the increasing annealing temperature (
Figure 7). The In
2O
3 annealed at 300 °C has the largest number of structural defects that can act as charge carrier traps. An increase in the annealing temperature leads to ordering of the system and structural perfection, leading to a decrease in the concentration of traps.
3.3. Investigation of the Gas Sensor Properties of Hybrid Materials in Interaction with Nitrogen Dioxide in Dry Air
The illumination of the sensor was carried out in a pulsed mode with periods of 2 min by switching on and off the LED, as a result of which the change in the resistance of the sensor with time also acquires a periodic character and is an alternation of the curves of the decay and rise of the photoconductivity. The repeated cycle of turning on and off the light leads to the change in the resistance of the sensor in each of the subsequent cycles close to the previous one. In this steady state, the minimum resistance R
light is achieved during the illumination of the sensor and the maximum resistance R
dark is achieved in the dark conditions. The ratio (10) varies with the composition of the atmosphere and has been used as a “sensor signal” for this measurement technique.
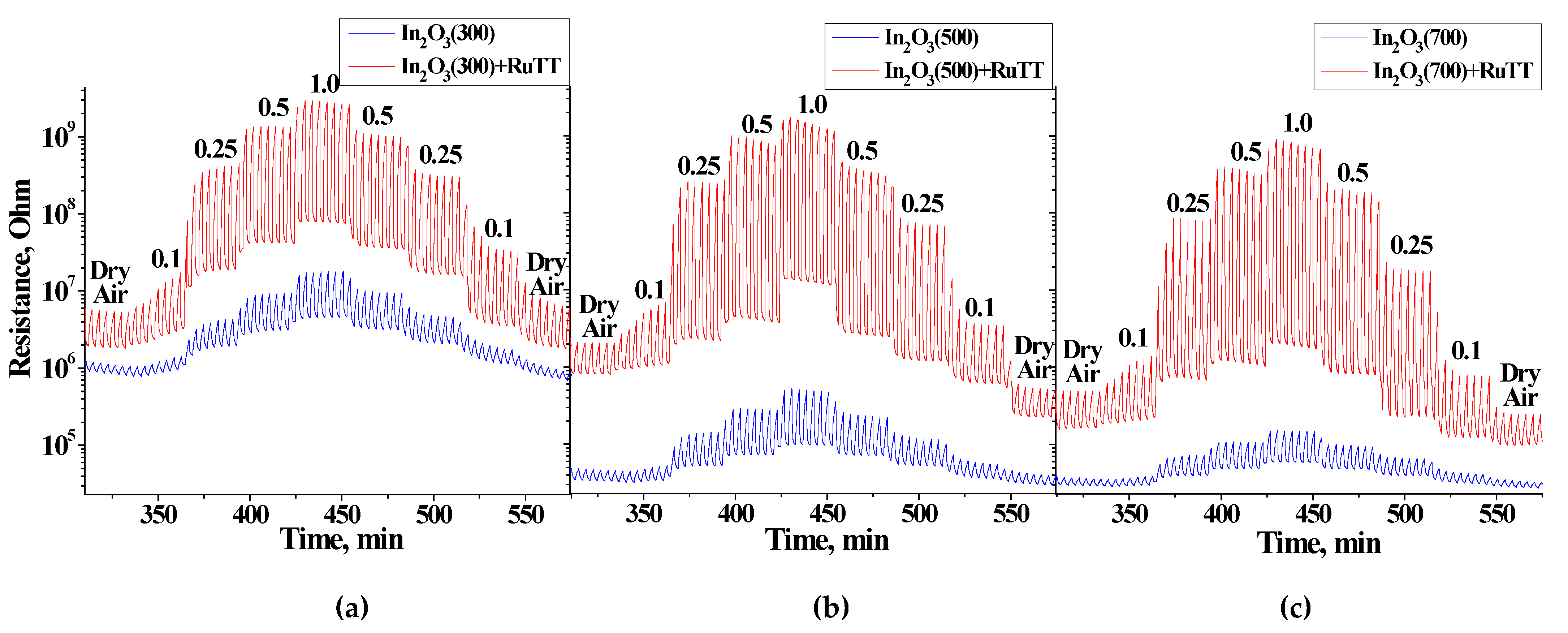
Figure 8 shows the change in the resistance of sensors based on pure oxides and hybrid materials under periodic blue LED illumination in the presence of various concentrations of NO
2 (0.1–0.25–0.5–1.0 ppm). As can be observed, the sensors show a periodic change in resistance under the influence of pulsed illumination, both in air and in the presence of NO
2. On the logarithmic scale of resistance, it can be observed that with an increase in the concentration of NO
2, the ratio of the photoresponse (11) increases for all sensors sensitized with Ru(II) heteroleptic complex. Moreover, with an increase in the concentration of NO
2, the average resistance of the sensors increases. The sensor resistances at the same NO
2 concentrations, obtained both with an increase and a decrease in the detected gas in air, are very close, which indicates a good ability of these sensors to operate reversibly.
Table 6 represents the average values of the sensor resistances under dark conditions and constant light illumination in dry air. The resistances were measured during 1 h for each step, in order to obtain a relatively stable state. The first observation is that the resistance of all sensors under the LED illumination is much less, which is due to the photodesorption of oxygen from the surface that was adsorbed in the air flow in dark conditions. It can also be noticed that the higher the annealing temperature of the sample (resulting in larger In
2O
3 grain size), the lower the baseline resistance during measurements. This can be associated with the height of potential barriers at the grain boundary: the smaller the crystallite size, the higher the barrier.
In our previous works, it was demonstrated that the energy of the blue LED (λ
max = 470 nm) excitation is equal to the absorption band of the Ru(II) heteroleptic complex in the visible range of the spectrum [
8,
9]. During light-activation, the electron-hole pairs are generated in it and then electrons can be transferred to the In
2O
3 conduction band facilitating to increase conductivity. NO
2 being a stronger electron acceptor than O
2, can attract the electrons from the conduction band of the semiconductor:
On the other hand, the chemisorbed particles (both
) can be converted into physically sorbed particles by interaction with photogenerated holes:
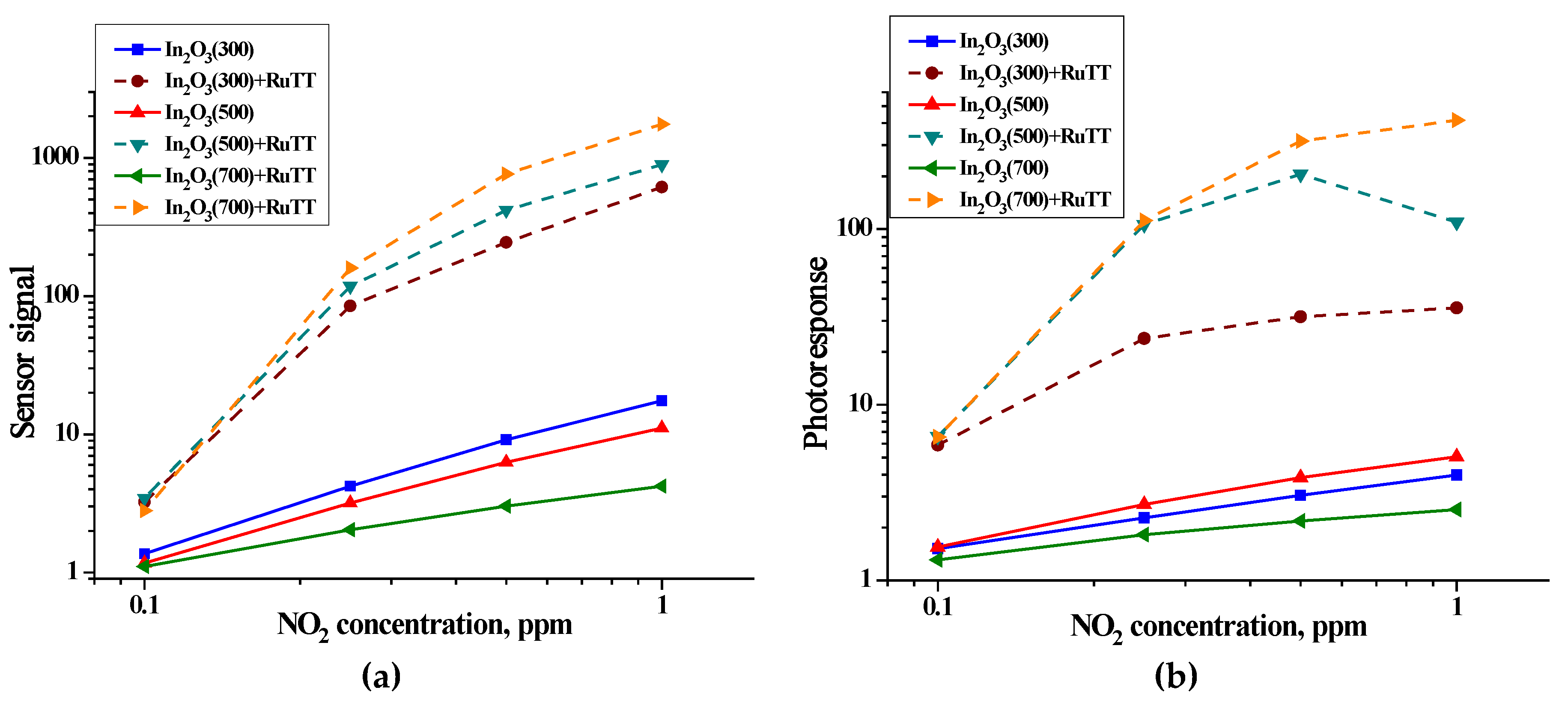
The values of the sensor signal were calculated using formula (10) and the photoresponse—using formula (11). The concentration dependences of the sensor signal and photoresponse of semiconductor oxides and hybrids are shown in
Figure 9.
The obtained data show that the introduction of a photosensitizer into nanocrystalline oxides, In2O3, leads to an increase in sensor signal and photoresponse by 2–3 orders. For pure oxides, the sensor signal correlates with a specific surface area and is inversed with crystallite size. For hybrid materials, both the sensor signal and photoresponse increase with increasing matrix crystallinity. In this case, the main role is played by traps of nonequilibrium charge carriers, which are structural defects in the matrix. An increase in the annealing temperature decreases their concentration, which makes it possible to accelerate the kinetics of photoconductivity.
On the one hand, nanocrystalline In
2O
3 has a significant advantage over the sample with a large crystallite size, because nanosized crystalline grains have a large specific surface area, which leads to a more intense interaction of the semiconductor with the gas phase and, as a result, to an increase in the sensor signal. This was observed for pure oxides in NO
2 detection. However, a consequence of a decrease in nanocrystals is an increase in the height of potential barriers at the grain boundary, which in turn can lead to a decrease in the mobility of free electrons and the probability of their tunneling [
25,
26]. The consequence of this was an increase in resistance with a decrease in the size of crystallites (
Figure 8 and
Table 6). The obtained result corresponds to the calculated values of the activation energy (W
hop) for the samples at room temperature: In
2O
3(300) (0.55 eV) > In
2O
3(500) (0.38 eV) > In
2O
3(700) (0.29 eV). On the other hand, the crystal structure of nanosized materials is more defective, and that has been proven above using various characterization methods. Acting as traps, these defects can reduce the number of nonequilibrium charge carriers and, as a result, increase the time of relaxation and recovery to a steady state of resistance in dark conditions compared to the photoresponse time. Moreover, the photogenerated electrons from the heterocyclic Ru(II) complex can also be trapped by this type of defect.
The humidity effect was studied for the samples based on In
2O
3 annealed at 300 and 700 °C.
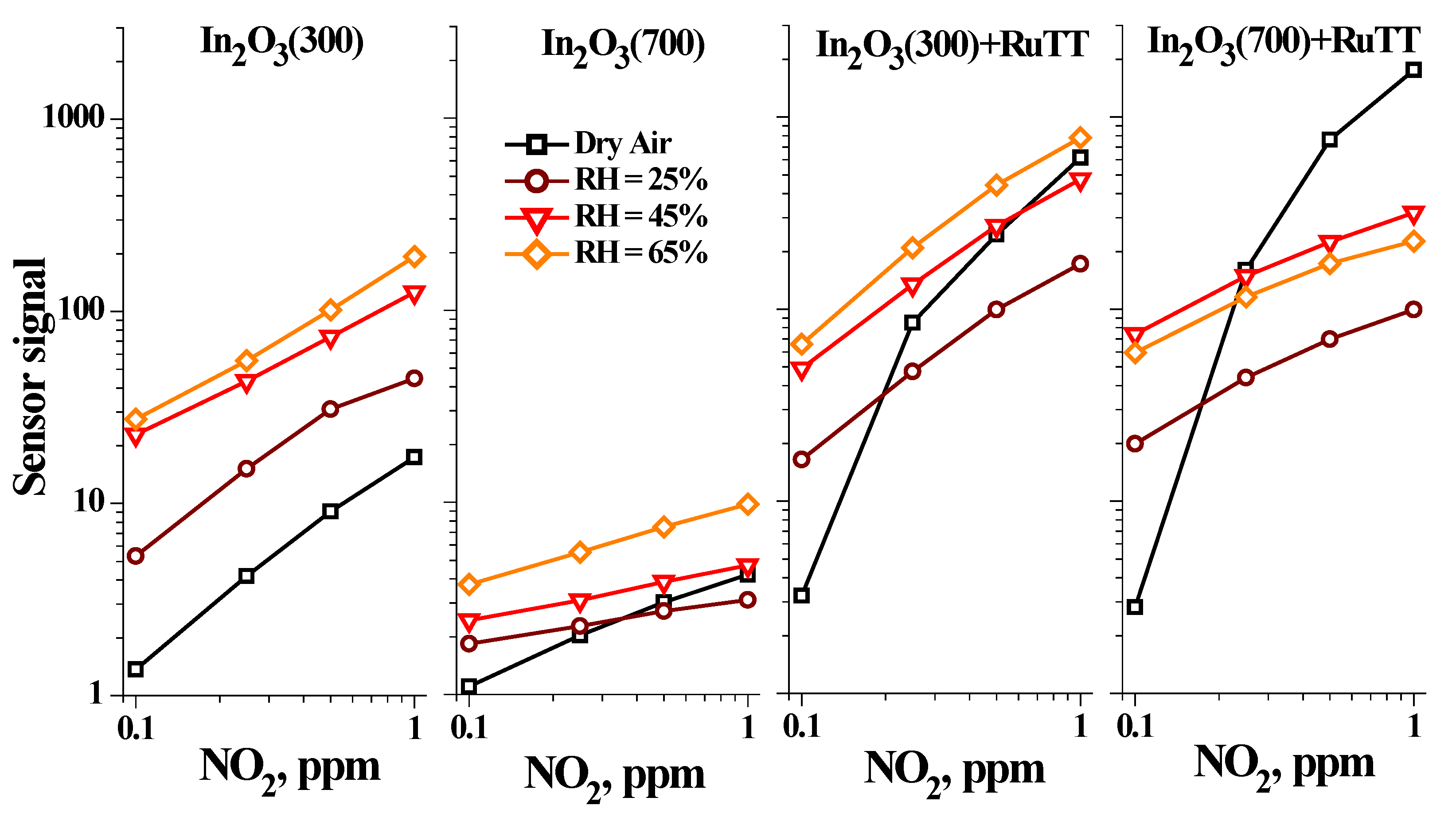
Figure 10 shows the dependence of the sensor signal on the NO
2 concentration at different air humidity (relative humidity at 25 °C RH = 25, 45, 65%).
As can be noticed, a monotonous increase in sensor signal is observed with increasing humidity for pure oxides. It was demonstrated that in humid atmosphere the conductivity of the n-type metal oxides increases, as dissociative adsorption of H
2O molecules or replacement of the chemisorbed oxygen ions can lead to release of the additional electrons into the conduction band [
21,
27]. The increase in the concentration of the electrons can stimulate the adsorption of the NO
2 molecules [
6]. Sample In
2O
3(300) has the largest specific surface area and, consequently, more adsorption sites; therefore, this effect is more significant for it compared to sample In
2O
3(700).
The hybrid materials have specific interactions with NO2 at the humid atmosphere. For the NO2 concentration below 0.25 ppm, the sensor signal increases with increasing relative humidity because of additional electrons released by the interaction with water vapor. However, for higher NO2 concentrations, the change in sensor signal in humid atmosphere is negligible comparing with the sensor signal in dry air. In this case, vacant surface active sites play vital roles as, due to their limited amount, the surface is saturated with adsorbed water and the proportion of “water electrons” becomes limited. Moreover, certain sites are already occupied by molecules of the Ru(II) heteroleptic complex in hybrid samples. Therefore, water and nitrogen dioxide molecules can compete for the same remaining adsorption sites. As the In2O3(700) sample has the smallest specific surface area, a very limited number of water molecules will be able to adsorb onto the free sites. This is additionally confirmed by the fact that with an increase in relative humidity up to 65%, the sensor signal begins to decrease and, compared to the sample In2O3(300), the sensor signal in a humid atmosphere is less.
Table 7 illustrates a comparative analysis of materials used for the detection of NO
2 gas by photoactivation. Using illumination at room temperature instead of heating for activation of physical and chemical processes on the surface of the sensitive layer will significantly reduce the power consumption of sensors in the case of their practical application. An analysis of the literature data demonstrated that UV sources are mainly used for photoactivated gas sensors. The use of low-power LEDs with radiation in the visible region of the spectrum, unlike UV sources, will further reduce energy consumption and allows one to embed such sensors into compact electronic devices. The periodic illumination with a duty light cycle of 50% used in this work will further reduce the power consumption by half. The highest sensor signal is established for CdSe QD@In
2O
3 composite material [
5] and here the authors also used a periodic illumination approach. However, the specific power was higher (20 mW/cm
2 vs. 8 mW/cm
2) and the response differs by one order (10
6 vs. 1.75 × 10
5). Hereby, the hybrid materials based on nanocrystalline In
2O
3 and Ru(II) heteroleptic complex have good prospects, also like the CdSe QD@In
2O
3 composites, for further research and applications as low power consumption gas sensors.


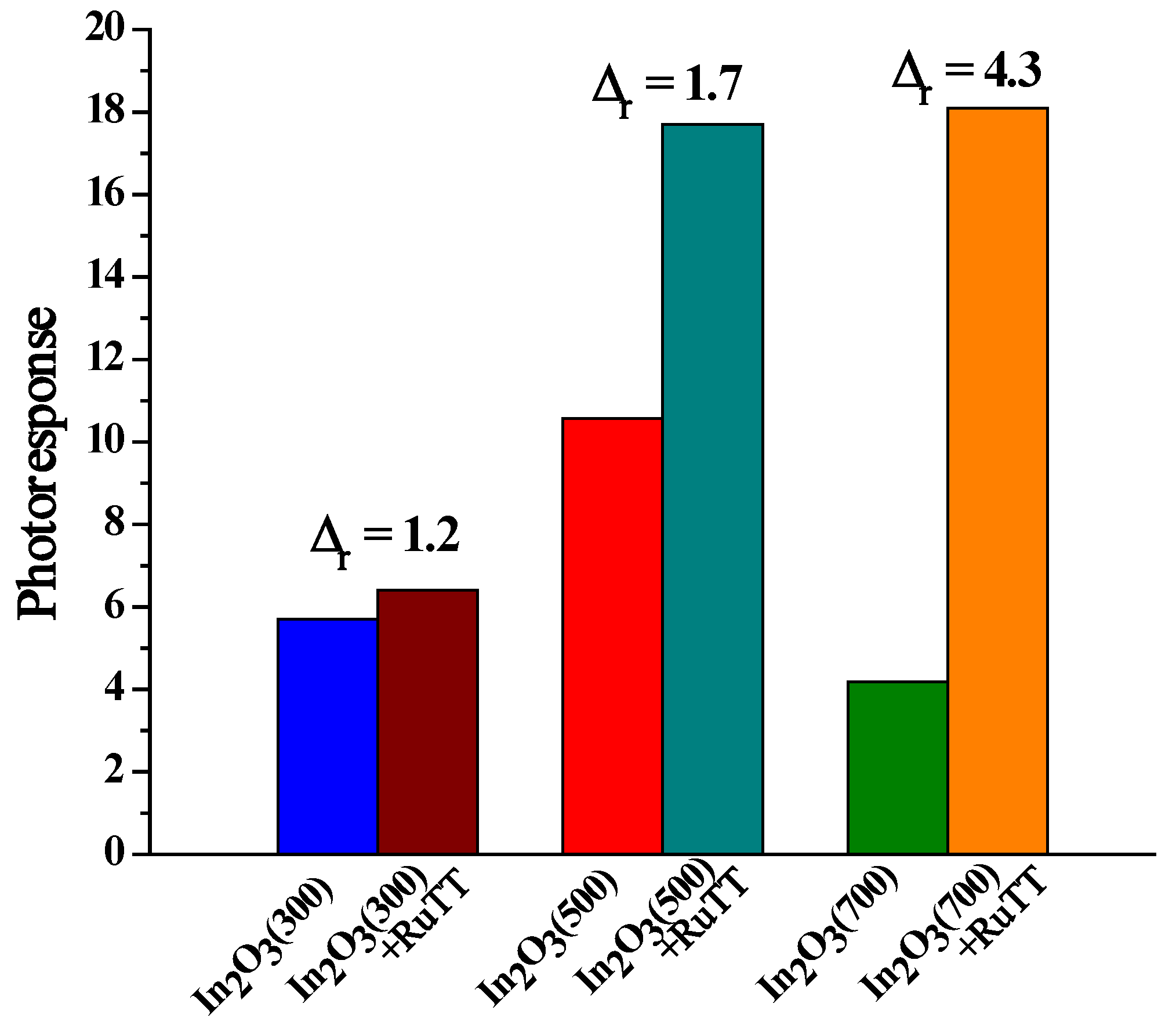
 2Cl−
2Cl−







