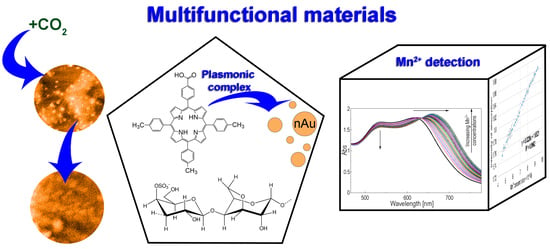
Excellent Cooperation between Carboxyl-Substituted Porphyrins, k-Carrageenan and AuNPs for Extended Application in CO2 Capture and Manganese Ion Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
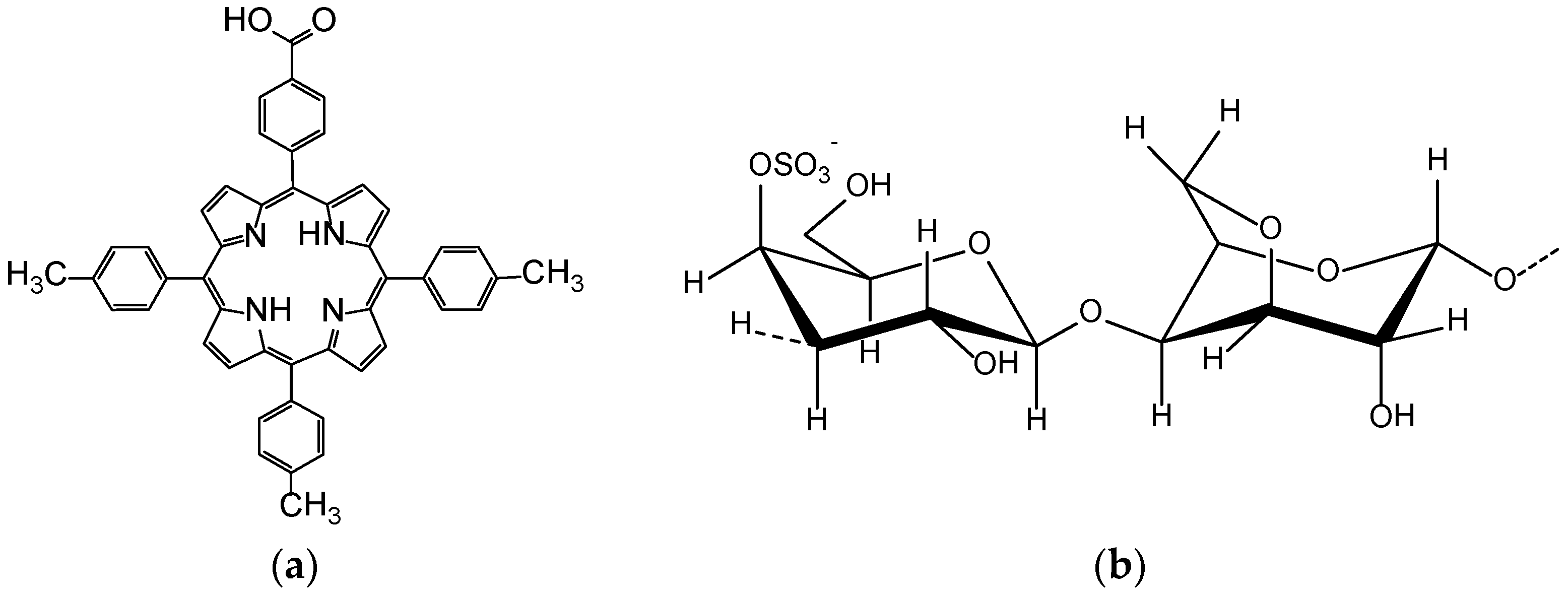
2.3. The Method for Obtaining of 5-(4-Methoxy-carbonyl-phenyl)-10,15,20-tris-(4-methyl-phenyl)-porphyrin (5-COOCH3-3MPP)
2.4. Hydrolysis of Porphyrin Ester (5-COOCH3-3MPP) to 5-(4-Carboxy-phenyl)-10,15,20-tris-(4-methyl-phenyl)-porphyrin (5-COOH-3MPP)
2.5. Physical-Chemical Characterization of the Two Obtained Porphyrins
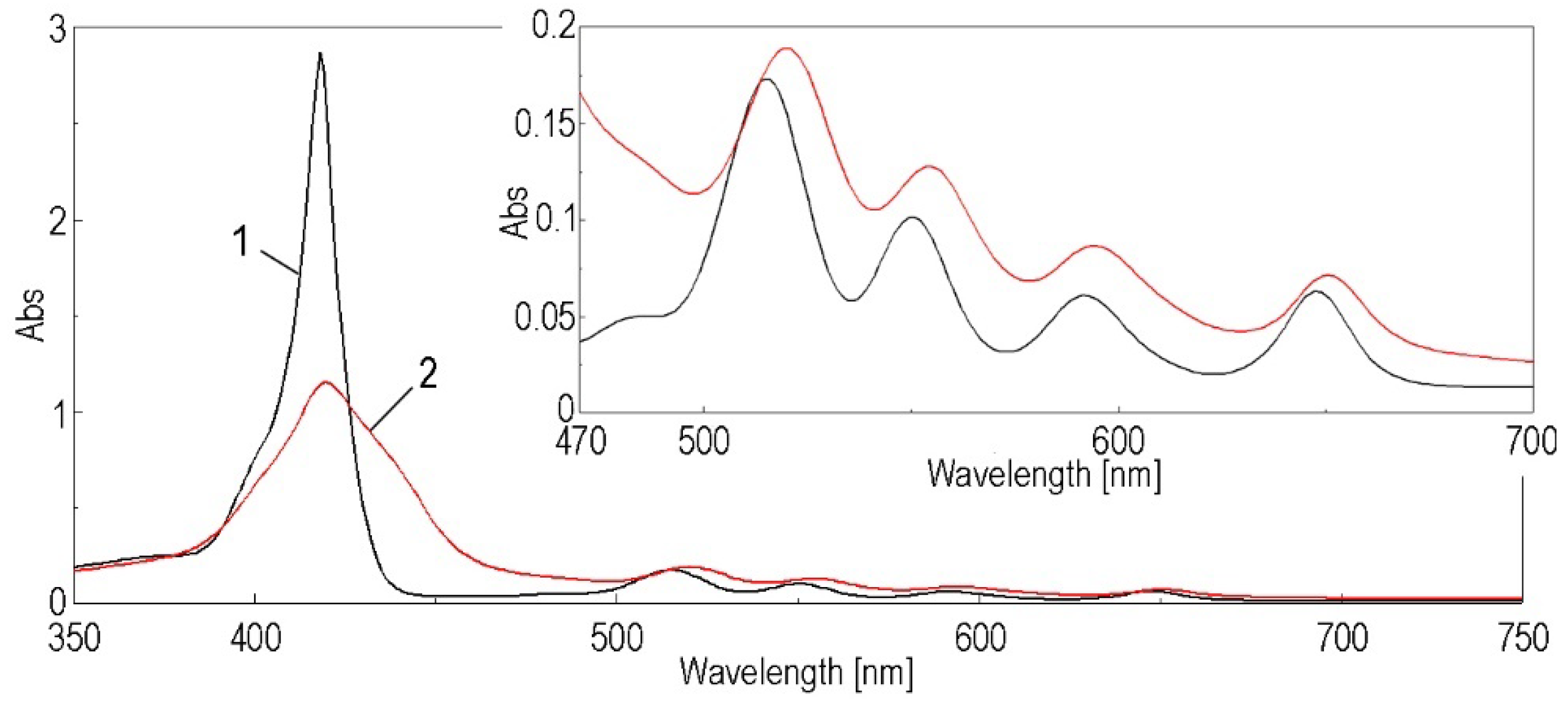
3. Results
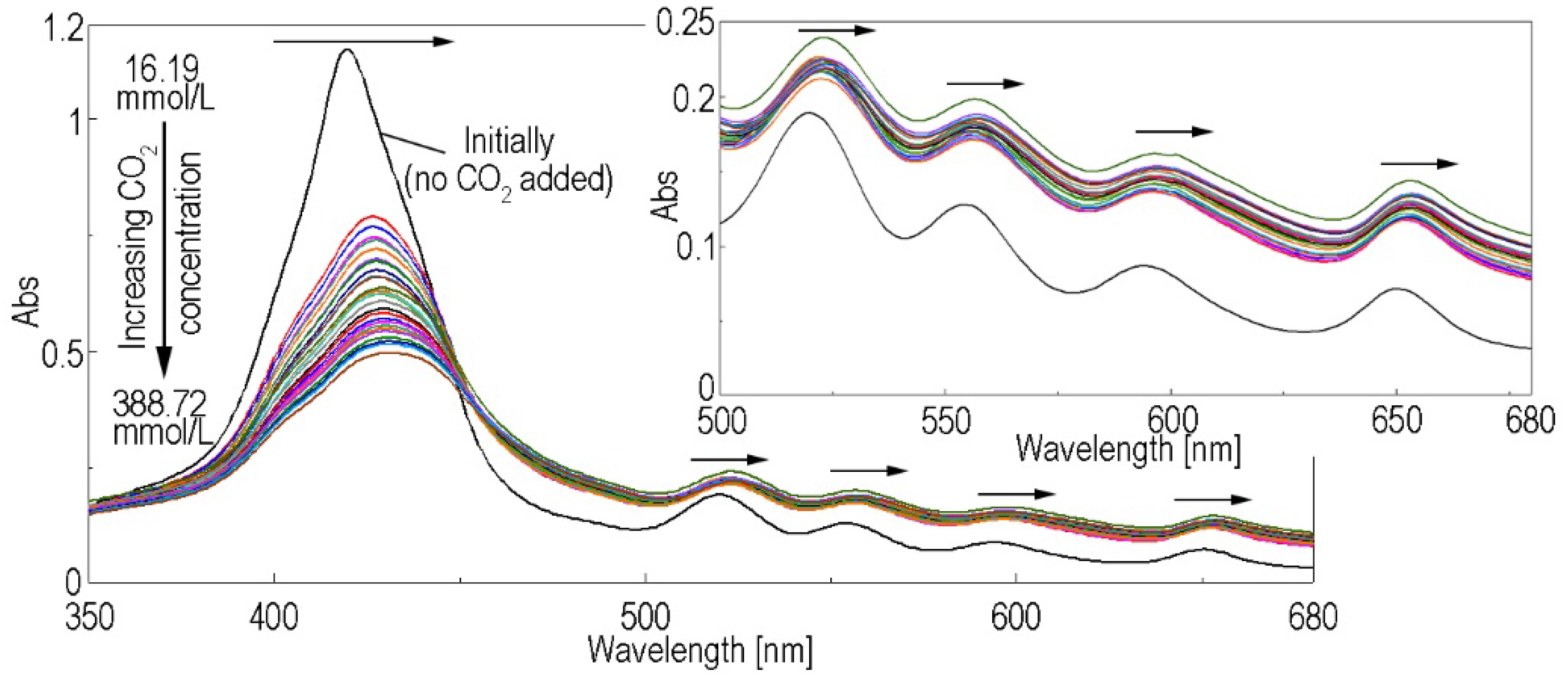
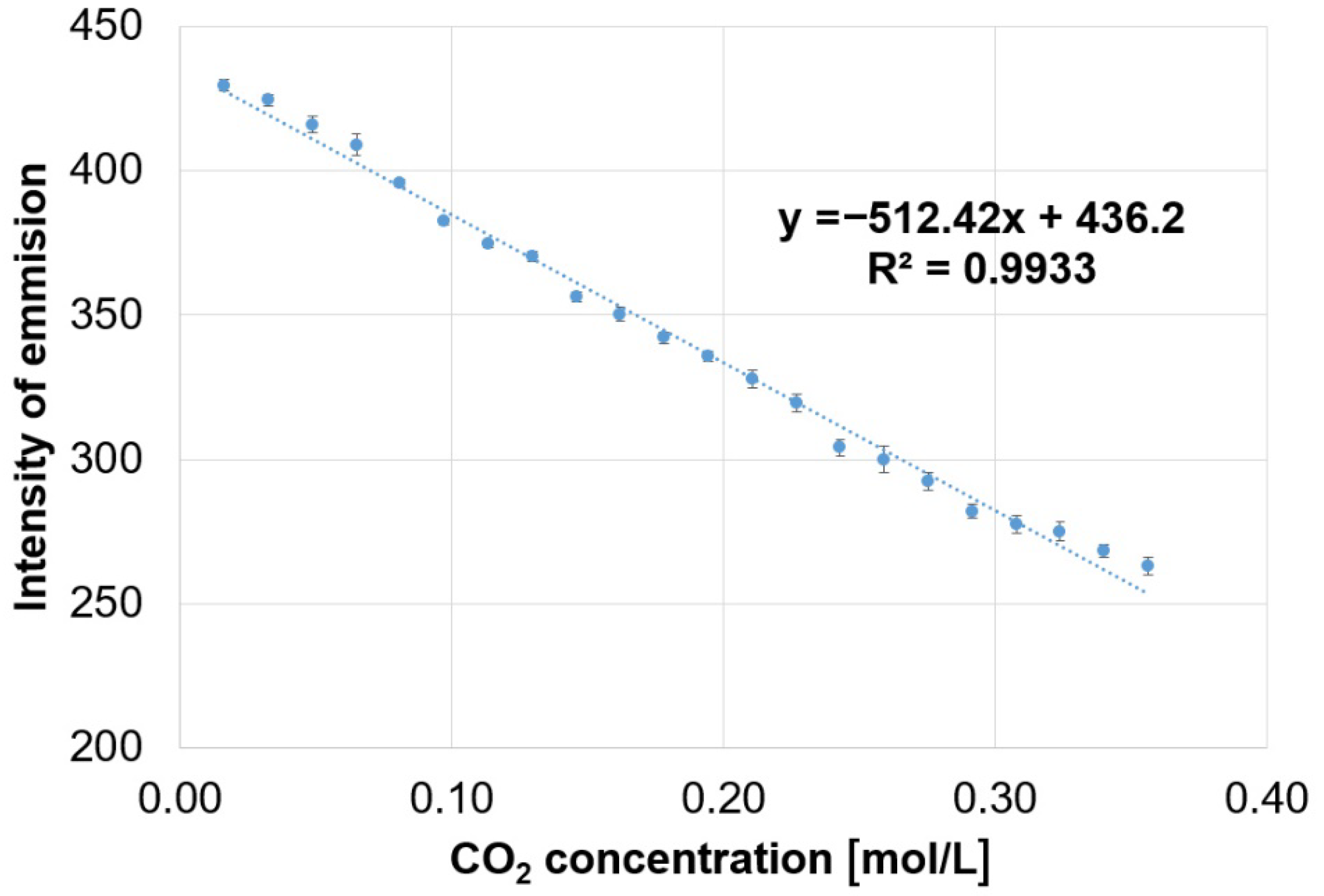
3.1. Method for Capturing CO2 Gas by the (5-COOH-3MPP)-k-Carrageenan Composite Material, in DMF/Water Mixture
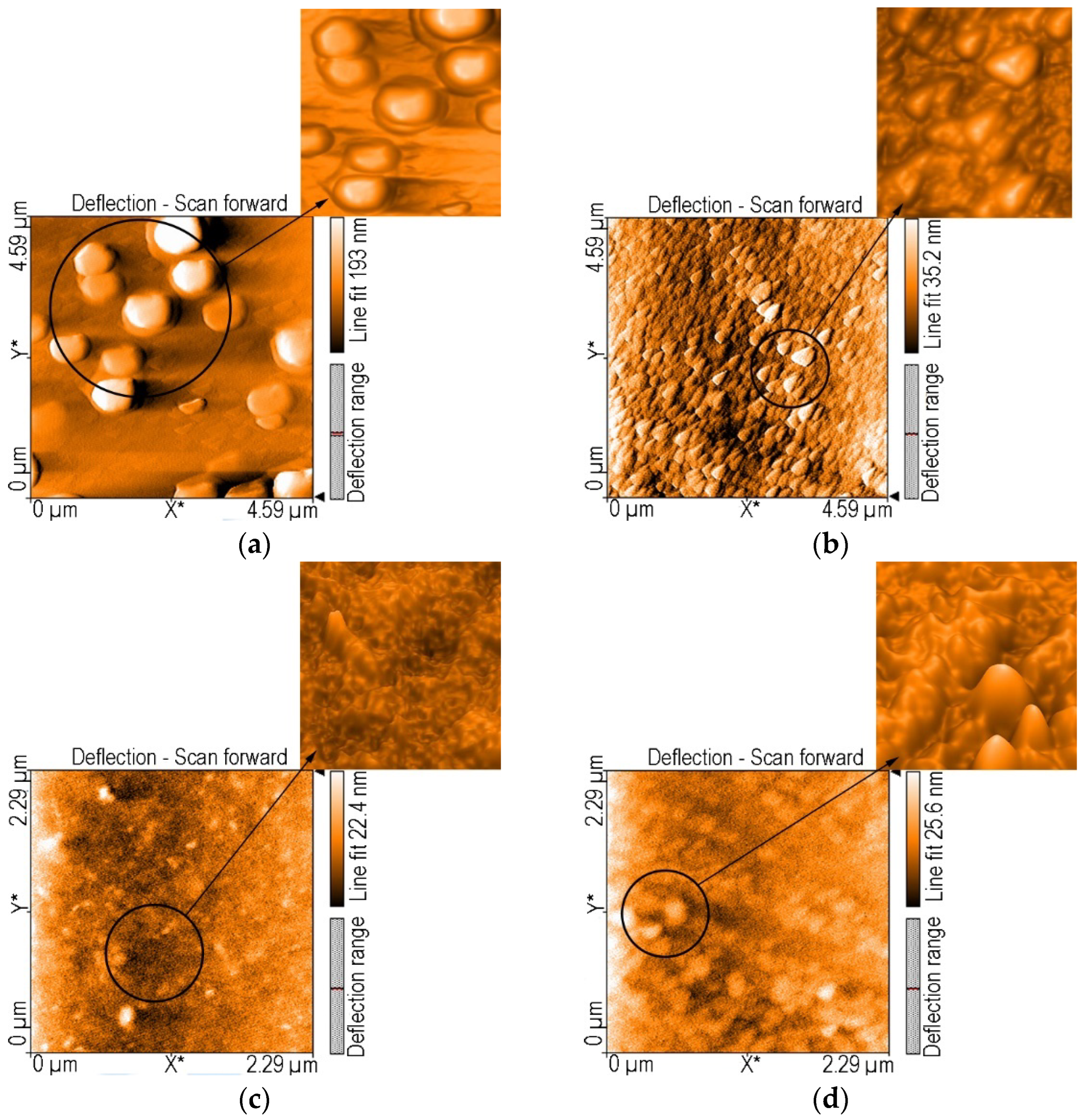
AFM Investigation of the (5-COOH-3MPP) and (5-COOCH3-3MPP) Porphyrins and of the Composite Material (5-COOH-3MPP)-k-Carrageenan, before and after treatment with CO2
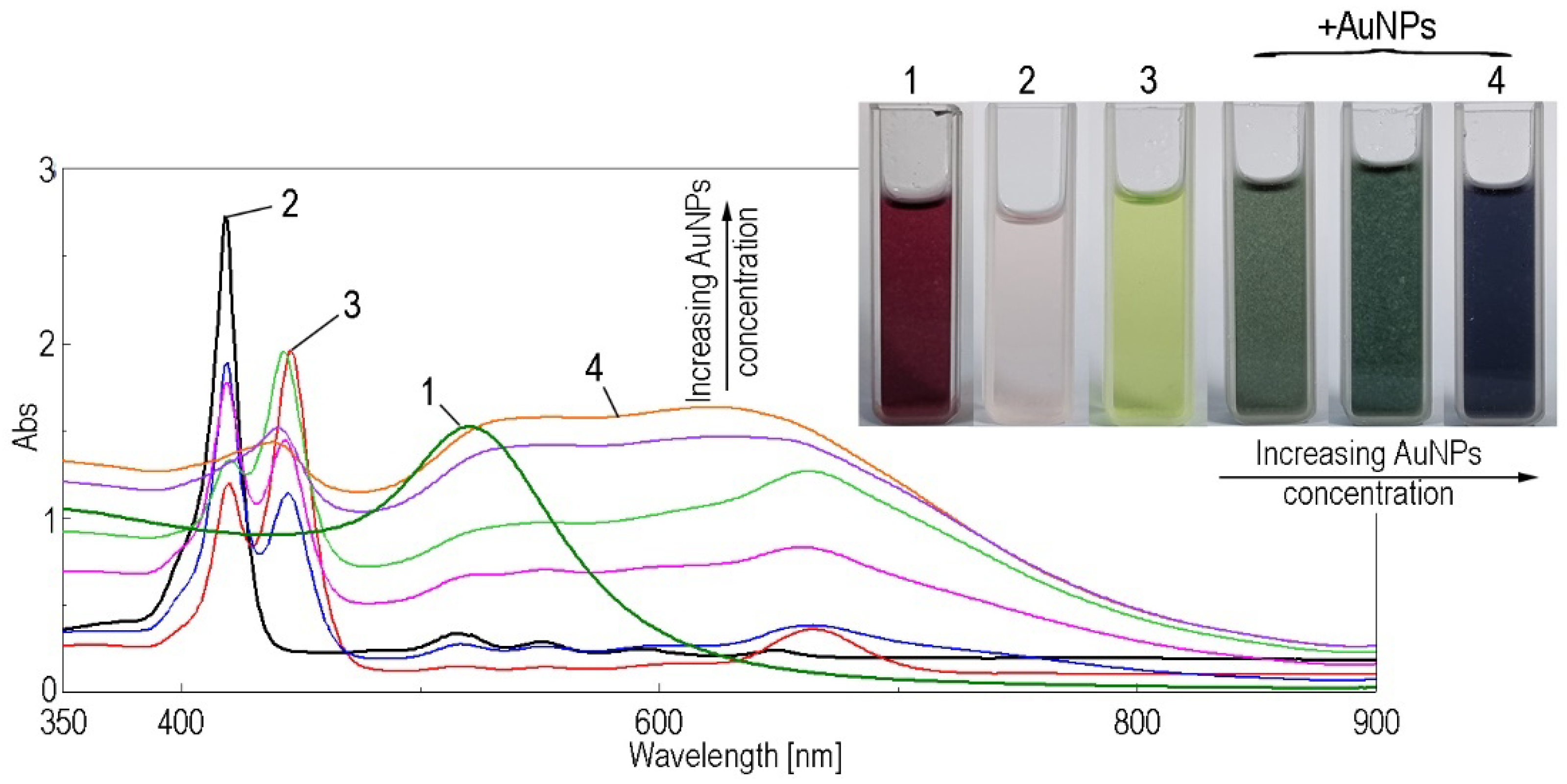
3.2. Method for Obtaining (5-COOH-3MPP)-k-Carrageenan-AuNPs Hybrid Material
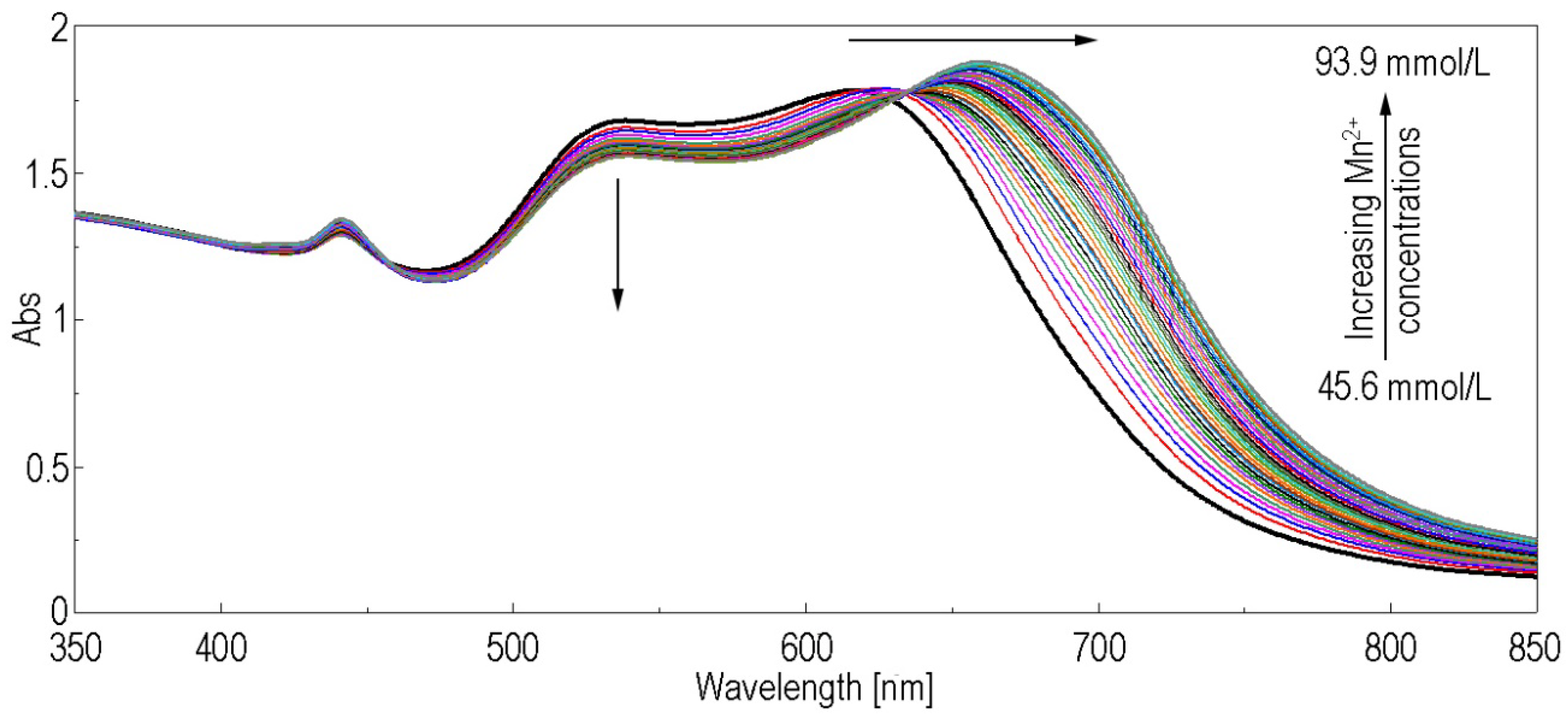
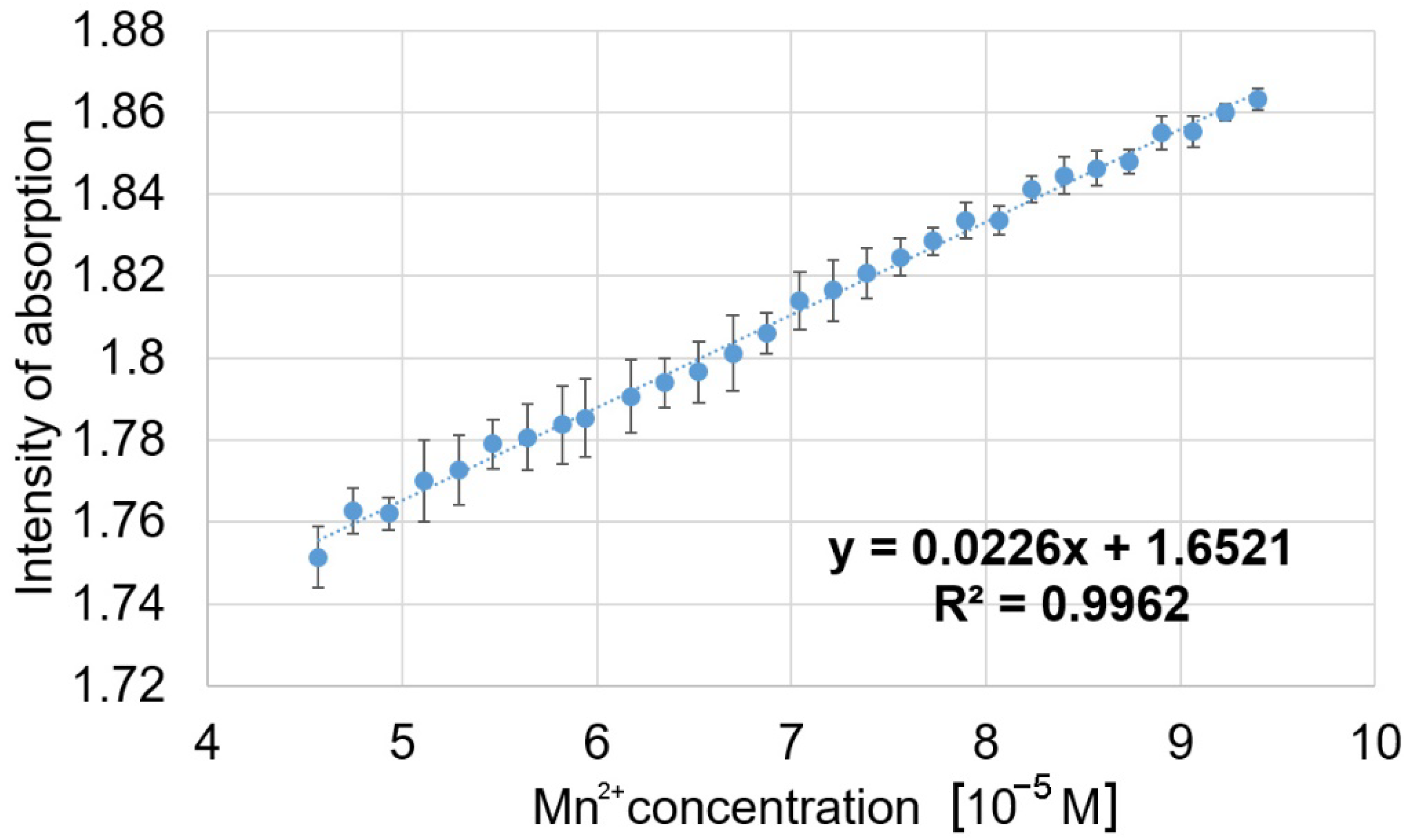
3.3. Spectrophotometric Detection of Mn2+ Ions by (5-COOH-3MPP)-k-Carrageenan-AuNPs Hybrid Material
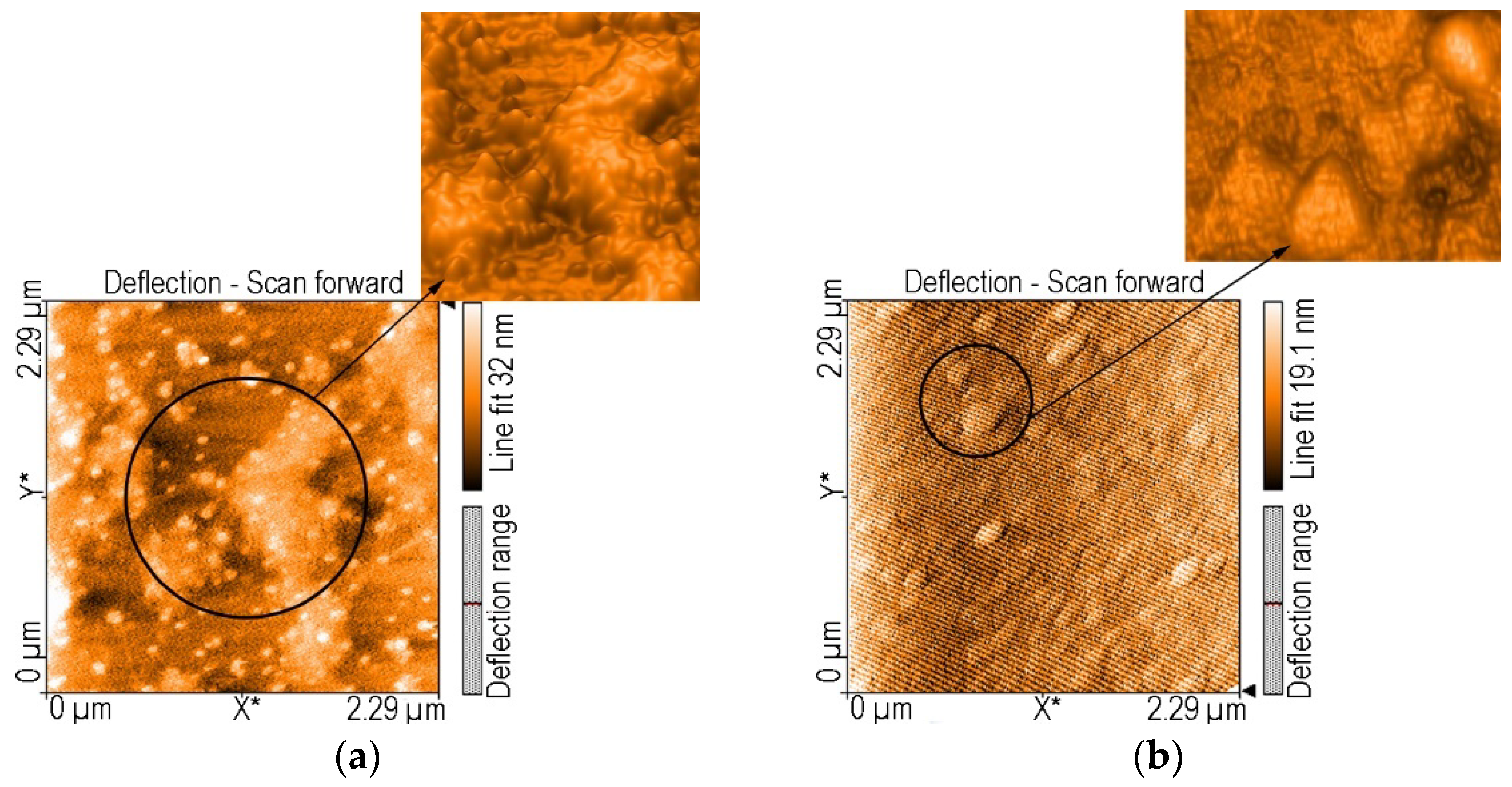
AFM Investigation of (5-COOH-3MPP)-k-Carrageenan-AuNPs Hybrid Material, before and after Mn2+ Detection
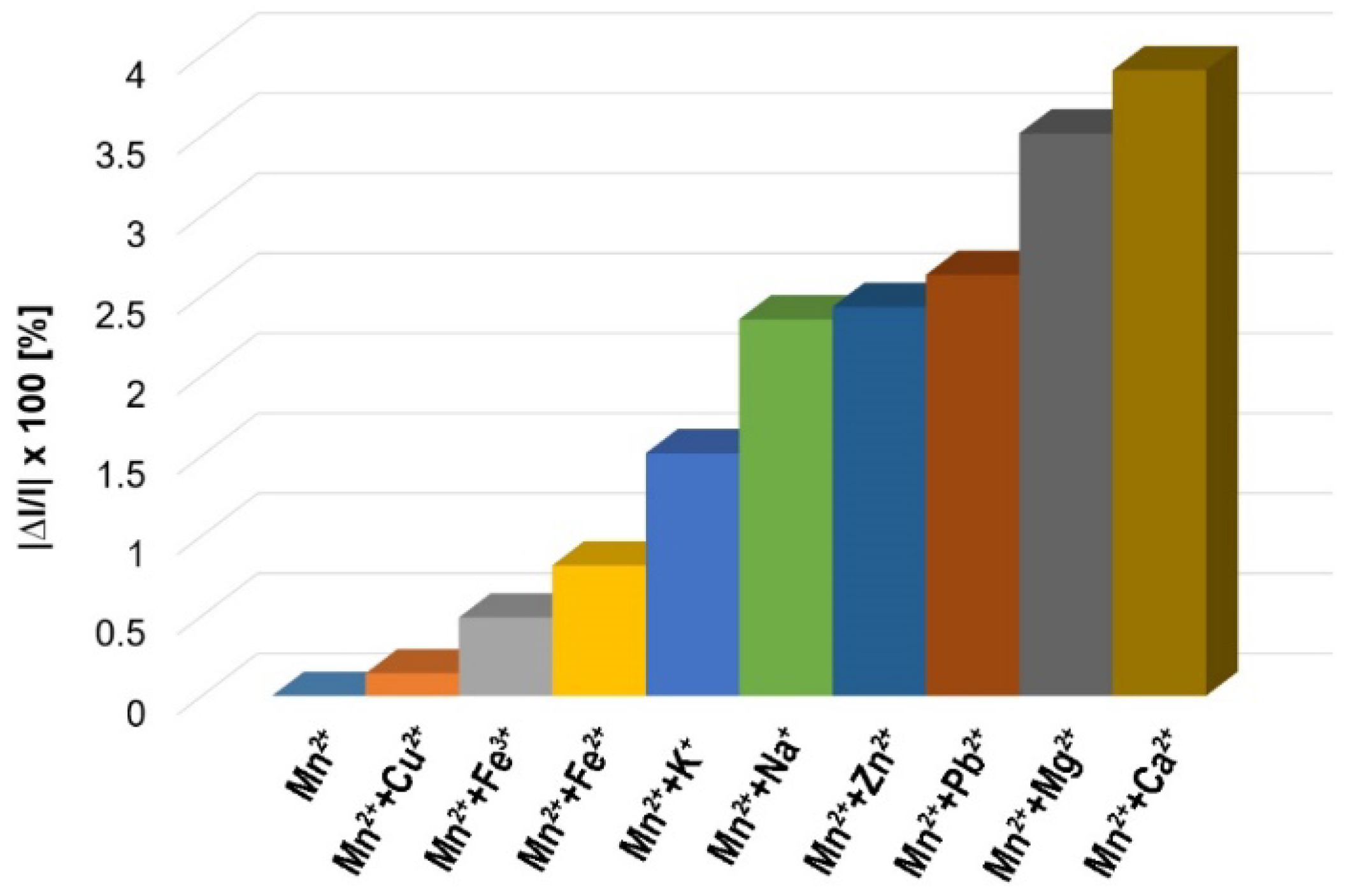
3.4. Interference Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Feng, Y.; He, H.; Liu, Y.; Weng, J.; Zhang, P.; Huang, W. Construction of hexanuclear Ce(III) metal−porphyrin frameworks through linker induce strategy for CO2 capture and conversion. Catal. Today 2021, 374, 38–43. [Google Scholar] [CrossRef]
- Chang, S.; Xie, W.; Yao, C.; Xu, G.; Zhang, S.; Xu, Y.; Ding, X. Construction of 2D porphyrin-based covalent organic framework as adsorbent for organic dyes removal and carbon dioxide adsorption. J. Solid State Chem. 2021, 304, 122577. [Google Scholar] [CrossRef]
- Chi, H.; Chen, H.; Gong, K.; Wang, X.; Zhang, Y. Protein-caged zinc porphyrin as a carbonic anhydrase mimic for carbon dioxide capture. Sci. Rep. 2020, 10, 19581. [Google Scholar] [CrossRef] [PubMed]
- Radford, S.E. GroEL: More than Just a Folding Cage. Cell 2006, 125, 831–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, J.; Zuo, K.; Li, Z.; Wang, Y.; Hu, H.; Zeng, C.; Xu, H.; Wang, B.; Gao, Y. Covalent Organic Frameworks for Simultaneous CO2 Capture and Selective Catalytic Transformation. Catalysts 2021, 11, 1133. [Google Scholar] [CrossRef]
- Kaplan, E.; Gümrükçü, S.; Gençten, M.; Şahin, Y.; Hamuryudan, E. Thiophene Functionalized Porphyrin for Electrochemical Carbon Dioxide Reduction. J. Electrochem. Soc. 2021, 168, 126512. [Google Scholar] [CrossRef]
- Mak, C.A.; Pericas, M.A.; Fagadar-Cosma, E. Functionalization of A3B-type porphyrin with Fe3O4 MNPs. Supramolecular assemblies, gas sensor and catalytic applications. Catal. Today 2018, 306, 268–275. [Google Scholar] [CrossRef]
- Anghel, D.; Lascu, A.; Epuran, C.; Fratilescu, I.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal. Int. J. Mol. Sci. 2020, 21, 4262. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.Z.; Li, X.; Xu, Y.W.; Zhang, L.; Su, C.Y. Catalytic Space Engineering of Porphyrin Metal-Organic Frameworks for Combined CO2 Capture and Conversion at a Low Concentration. ChemSusChem 2018, 11, 2340–2347. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Vlascici, D.; Fagadar-Cosma, G.; Palade, A.; Lascu, A.; Creanga, I.; Birdeanu, M.; Cristescu, R.; Cernica, I. A Sensitive A3B Porphyrin Nanomaterial for CO2 Detection. Molecules 2014, 19, 21239–21252. [Google Scholar] [CrossRef] [Green Version]
- Fagadar-Cosma, E.; Tarabukina, E.; Zakharova, N.; Birdeanu, M.; Taranu, B.; Palade, A.; Creanga, I.; Lascu, A.; Fagadar-Cosma, G. Hybrids formed between polyvinylpyrrolidone and an A3B porphyrin dye: Behavior in aqueous solutions and chemical response to CO2 presence. Polym. Int. 2016, 65, 200–209. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hipolito Júnior, E.; Gonçalves, A.G.; Noseda, M.D.; Duarte, M.E.R.; Murakami, F.S.; Ducatti, D.R.B. Semi-synthesis of N-alkyl-kappa-carrageenan derivatives and evaluation of their antibacterial activity. Carbohydr. Res. 2021, 499, 108234. [Google Scholar] [CrossRef]
- Larotonda, F.D.S.; Torres, M.D.; Gonçalves, M.P.; Sereno, A.M.; Hilliou, L. Hybrid carrageenan-based formulations for edible film preparation: Benchmarking with kappa carrageenan. J. Appl. Polym. Sci. 2015, 133, 42263. [Google Scholar] [CrossRef]
- Sepúlveda, M.R.; Dresselaers, T.; Vangheluwe, P.; Everaerts, W.; Himmelreich, U.; Mata, A.M.; Wuytack, F. Evaluation of manganese uptake and toxicity in mouse brain during continuous MnCl2 administration using osmotic pumps. Contrast Media Mol. Imaging 2012, 7, 426–434. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Ma, H.; Hursthouse, A.S. Removal of Manganese(II) from Acid Mine Wastewater: A Review of the Challenges and Opportunities with Special Emphasis on Mn-Oxidizing Bacteria and Microalgae. Water 2019, 11, 2493. [Google Scholar] [CrossRef] [Green Version]
- Du, J. Drinking Water Health Advisory for Manganese in: United States Environmental Protection Agency: Prepared by: U.S. Environmental Protection Agency Office of Water (4304T). 2004. Available online: https://www.epa.gov/sites/default/files/2014-09/documents/support_cc1_magnese_dwreport_0.pdf (accessed on 15 February 2022).
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Klemnmann, R.L.P.; Watzlar, G.R. Should the efluent limits for manganese be modified? In Mine Drainage and Surface Mine Reclamation, Volume II: Mine Reclamation, Abandoned Mine Lands and Policy Issues, Proceedings of the Annual meeting of the American Society for Surface Mining and Reclamation, Pittsburgh, PA, USA, 17 April 1988; Brown, D.S., Ed.; Department of the Interior, Bureau of Mines: Washington, DC, USA, 1988; pp. 305–310. [Google Scholar]
- Fuchida, S.; Tajima, S.; Nishimura, T.; Tokoro, C. Kinetic Modeling and Mechanisms of Manganese Removal from Alkaline Mine Water Using a Pilot Scale Column Reactor. Minerals 2022, 12, 99. [Google Scholar] [CrossRef]
- Palade, A.; Fagadar-Cosma, G.; Lascu, A.; Creanga, I.; Birdeanu, M.; Fagadar-Cosma, E. New porphyrin-based spectrophotometric sensor for Ag0 detection. Dig. J. Nanomater. Bios 2013, 8, 1013–1022. [Google Scholar]
- Anghel, D.; Birdeanu, M.; Lascu, A.; Epuran, C.; Fagadar-Cosma, E. Amino-substituted porphyrins at the border of hybrid materials generation and platinum nanoparticles detection. Studia Univ. Babes-Bolyai Chem. 2020, 2, 107–120. [Google Scholar] [CrossRef]
- Ţaranu, B.O.; Vlascici, D.; Sebarchievici, I.; Fagadar-Cosma, E. The aggregation behavior of an A3B free base porphyrin and its application as chromium(III)-selective membrane sensor. Studia Univ. Babes-Bolyai Chem. 2016, 61, 199–212. [Google Scholar]
- Vlascici, D.; Popa, I.; Chiriac, V.A.; Fagadar-Cosma, G.; Popovici, H.; Fagadar-Cosma, E. Potentiometric detection and removal of copper using porphyrins. Chem. Cent. J. 2013, 7, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlascici, D.; Fagadar-Cosma, E.; Popa, I.; Chiriac, V.; Gil-Agusti, M. A Novel Sensor for Monitoring of Iron(III) Ions Based on Porphyrins. Sensors 2012, 12, 8193–8203. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Zhu, J.; Hu, X.; Yang, Y. A Phosphorescence Quenching-Based Intelligent Dissolved Oxygen Sensor on an Optofluidic Platform. Micromachines 2021, 12, 281. [Google Scholar] [CrossRef]
- Fratilescu, I.; Dudás, Z.; Birdeanu, M.; Epuran, C.; Anghel, D.; Fringu, I.; Lascu, A.; Len, A.; Fagadar-Cosma, E. Hybrid silica materials applied for fuchsine B color removal from wastewaters. Nanomaterials 2021, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Sebarchievici, I.; Lascu, A.; Fagadar-Cosma, G.; Palade, A.; Fringu, I.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, E. Optical and electrochemical mediated detection of ascorbic acid using manganese porphyrin and its gold hybrids. Comptes Rendus Chim. 2018, 21, 327–338. [Google Scholar] [CrossRef]
- Salageanu, L.; Lascu, A.; Birdeanu, M.; Fagadar-Cosma, E. Plasmonic Material Based on Silver Colloid and Zn-Metalloporphyrin for Drug Detection of p-Aminosalicylic Acid. Dig. J. Nanomater. Biostructures 2018, 13, 653–659. [Google Scholar]
- Lascu, A.; Palade, A.; Birdeanu, M.; Fagadar-Cosma, E. Procaine detection using hybrids of cobalt-metalloporphyrin with gold and silver nanoparticles. J. Chem. Soc. Pak. 2019, 41, 43–51. [Google Scholar]
- Lascu, A.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, E. Hybrid Mn-Porphyrin-Nano Gold Nanomaterial Applied for the Spectrophotometric Detection of β-Carotene. J. Chem. 2018, 2018, 5323561. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, P.; Abraham John, S. Gold nanoparticles decorated on cobalt porphyrin-modified glassy carbon electrode for the sensitive determination of nitrite ion. J. Colloid Interface Sci. 2014, 421, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Lascu, A.; Shova, S.; Zaltariov, M.F.; Birdeanu, M.; Croitor, L.; Balan, A.; Anghel, D.; Stamatin, S. X-ray structure elucidation of a Pt-metalloporphyrin and its application for obtaining sensitive AuNPs-plasmonic hybrids capable to detect triiodide anions. Int. J. Mol. Sci. 2019, 20, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmoni, E.; Brunetti, B. About Estimating the Limit of Detection by the Signal to Noise Approach. Pharm. Anal. Acta 2015, 6, 1000355. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Hu, X.; Chen, L.; Zuo, Y.; Yang, Y.; Jiang, F.; Sun, C.; Zhao, W.; Han, X. Rapid nitrate determination with a portable lab-on-chip device based on double microstructured assisted reactors. Lab Chip 2021, 21, 1109–1117. [Google Scholar] [CrossRef]
- Bakar, M.B.; Oelgemöller, M.; Senge, M.O. Lead structures for applications in photodynamic therapy. Part 2: Synthetic studies for photo-triggered release systems of bioconjugate porphyrin photosensitizers. Tetrahedron 2009, 65, 7064–7078. [Google Scholar] [CrossRef]
- Scalise, I.; Durantini, E.N. Synthesis and photodynamic activity of metallo 5-(4-carboxyphenyl)-10,15,20-tris(4-methylphenyl) porphyrins. In Proceedings of the Sixth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-6), Internet, 1–30 September 2002; Available online: https://www.mdpi.org/ecsoc/ecsoc-6/Papers/C007/C007.htm (accessed on 15 February 2022).
- Fagadar-Cosma, E.; Vlascici, D.; Birdeanu, M.; Fagadar-Cosma, G. Novel fluorescent pH sensor based on 5-(4-carboxy-phenyl)-10,15,20-tris(phenyl)-porphyrin. Arab. J. Chem. 2019, 12, 1587–1594. [Google Scholar] [CrossRef] [Green Version]
- Max, J.J.; Chapados, C. Infrared Spectroscopy of Aqueous Carboxylic Acids: Comparison between Different Acids and Their Salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar] [CrossRef]
- Sanches, N.B.; Pedro, R.; Diniz, M.F.; Mattos ED, C.; Cassu, S.N.; Dutra, R.D.C.L. Infrared Spectroscopy Applied to Materials Used as Thermal Insulation and Coatings. J. Aerosp. Technol. Manag. 2013, 5, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M.K. Fourier Transform Infrared Spectrophotometry Studies of Chromium Trioxide-Phthalic Acid Complexes. Chem. Sci. Trans. 2016, 5, 770–774. [Google Scholar] [CrossRef] [Green Version]
- Pangajavalli, S.; Ranjithkumar, R.; Sridhar, B.; Ramaswamy, S. Hirshfeld and Vibrational Analysis of 5-Benzyl-7a-Hydroxy-1-Methyl-2,3,5,6,7,7a-Hexahydro-1H-3a, 7-Methanoindeno [2,1-d]Pyrrolo[3,2-c]Azepine-12,13(4H)-Dione. MMSE 2017. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Falagan-Lotsch, P.; Grzincic, E.M.; Murphy, C.J. One low-dose exposure of gold nanoparticles induces long-term changes in human cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13318–13323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Coradeghini, R.; Gioria, S.; García, C.P.; Nativo, P.; Franchini, F.; Gilliland, D.; Ponti, J.; Rossi, F. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol. Lett. 2013, 217, 205–216. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epuran, C.; Fratilescu, I.; Macsim, A.-M.; Lascu, A.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Excellent Cooperation between Carboxyl-Substituted Porphyrins, k-Carrageenan and AuNPs for Extended Application in CO2 Capture and Manganese Ion Detection. Chemosensors 2022, 10, 133. https://doi.org/10.3390/chemosensors10040133
Epuran C, Fratilescu I, Macsim A-M, Lascu A, Ianasi C, Birdeanu M, Fagadar-Cosma E. Excellent Cooperation between Carboxyl-Substituted Porphyrins, k-Carrageenan and AuNPs for Extended Application in CO2 Capture and Manganese Ion Detection. Chemosensors. 2022; 10(4):133. https://doi.org/10.3390/chemosensors10040133
Chicago/Turabian StyleEpuran, Camelia, Ion Fratilescu, Ana-Maria Macsim, Anca Lascu, Catalin Ianasi, Mihaela Birdeanu, and Eugenia Fagadar-Cosma. 2022. "Excellent Cooperation between Carboxyl-Substituted Porphyrins, k-Carrageenan and AuNPs for Extended Application in CO2 Capture and Manganese Ion Detection" Chemosensors 10, no. 4: 133. https://doi.org/10.3390/chemosensors10040133
APA StyleEpuran, C., Fratilescu, I., Macsim, A.-M., Lascu, A., Ianasi, C., Birdeanu, M., & Fagadar-Cosma, E. (2022). Excellent Cooperation between Carboxyl-Substituted Porphyrins, k-Carrageenan and AuNPs for Extended Application in CO2 Capture and Manganese Ion Detection. Chemosensors, 10(4), 133. https://doi.org/10.3390/chemosensors10040133