Abstract
In this paper, the optical properties of viburnum extract flavonoids in the visible region of the spectrum were investigated and their use as a potential photosensitizer of singlet oxygen for photodynamic therapy was evaluated. The presence of long-lived excited states in the extract molecules was established by spectral methods and time-resolved spectroscopy methods and the dependences of the absorption capacity and luminescence intensity of the extract molecules on the concentrations of oxygen and ablative nanoparticles of the gold in the reverse micelles of AOT (sodium dioctyl sulfosuccinate) were established. The plasmonic enhancement of the luminescence of the extract molecules and the processes of their complexation with oxygen were also established. Furthermore, the rate constants of the processes of conversion of exciting energy in complexes were determined.
1. Introduction
It is well known that natural plant extracts have a wide range of physical and biological properties including antioxidant, anti-inflammatory, and immunomodulatory effects [1,2]. In the modern literature, extracts of many plants are considered as photosensitizers of reactive oxygen species for use against some bacteria, molds, viruses, and tumor cells [3,4,5]. Various parts of plants have been actively studied: all aboveground parts [6] and underground [7], separately stems [8,9], flowers [10,11], and leaves [12] as well as fruits [13,14,15,16] for the presence and identification of biologically active compounds capable of singlet oxygen generation under irradiation.
Viburnum opulus L. (VO) is one of the most important and actively studied medicinal and food plants [16,17,18,19,20,21,22]. It is actively cultivated, and also occurs in its natural habitat on the territory of Russia, Europe, some regions of North Africa and Northern Asia [22].
The advantage of Viburnum opulus L. use for human health is the presence of biologically active components such as vitamin C, carotenoids, iridoids, essential oils, and phenolic compounds. Due to this composition, the VO extract shows antioxidant activity, antimicrobial potential (especially against to positive bacteria), and has proven anti-inflammatory, anti-obesity, antidiabetic, osteogenic, cardio-, and cytoprotective properties [3,4,5,16]. In vivo studies have proven the applicability of Viburnum opulus L. for the treatment of urinary tract diseases, endometriosis, and some types of cancer [3,4,5,16,18,19,20,21,22]. Viburnum opulus L. biologically active substance samples are most often represented by water decoctions or infusions of fresh or dried berries as well as extracts obtained using ethanol, acetone, methanol, and other extractants by maceration. The juice fabricated by pressing fresh berries is used less often. The highest antioxidant properties have been confirmed in the ethanol extract of Viburnum opulus L. [16,18]. Flavonoids, organic and phenolic acids, mono-, di-, and triterpene compounds, vitamins, macro- and microelements of the plant have all shown biological activity [17].
In photodynamic therapy (PDT), the fight against malignant cells is carried out with the help of three main components: the photoactive compound from plants, light, and oxygen. The search for plants such as photosensitizers is constantly underway and considered as an urgent task [3]. Thus, when the electron-vibrational states of sensitizer molecules are excited by light, radiative and non-radiative transitions occur, leading to the active (singlet) states of molecular oxygen generation [23]. These states are of major significance for PDT. In addition, the delivery of active anticancer substances obtained from plant extracts into the cancer cell through a semi-permeable protective cell wall is also a separate task, the solution of which can result in emulsion synthesis (for example, micelles with AOT), based on water–alcohol extracts in a fatty acid environment. Similar experiments on the creation of two-phase solutions as a result of the formation of micelles containing an active photosensitizer (PS) are given in [24,25].
It is known that organic molecules near the metal surface and, in particular, near micro- and nanoparticles of silver and gold significantly change their characteristics [26,27]. In this case, the intensity of the organic molecules luminescence in some cases weakens, while in others, on the contrary, it increases. These changes in the properties of molecules are associated with the resonance of the electronic transition with the so-called localized (surface) plasmons in metal nanoparticles, which take part in energy transfer processes [27]. Surface plasmons enhance the luminescence of biological molecules as a result of the dipole-dipole transfer of electron energy [28]. Currently, gold nanoparticles, due to their plasmonic properties in the visible region (500–600 nm), are actively used in such areas as photothermal therapy, theranostics, immunochromatographic identification, biosensors, photocatalysis and electronics [29,30,31,32,33,34,35]. Gold NPs are also known to be applied in targeted cancer therapy [36].
Thus, the purpose of this work was the study of spectral fluorescent and time-resolved characteristics of the Viburnum opulus L. extract obtained from berries in a micellar solution of AOT and active substance identification for evaluating the potential use as a PS for PDT. To enhance the optical properties, the mechanisms of plasmon enhancement of the absorption-fluorescent characteristics [37] as a result of the addition of ablative gold nanoparticles (NPs) [38] and the mechanisms of complexation with molecular oxygen have been used in this work.
2. Materials and Methods
2.1. Sample Preparation
Fresh berries of Viburnum opulus L. weighing of 213 g were ground in a mortar, then the resulting mass was squeezed out. The resulting juice was repeatedly passed through the paper filters until a clear solution with a bright red color was obtained, from which, after settling for 24 h, no precipitate came out. The final filtration was carried out through a filter with a pore size of 200 nm. Next, 100 mL of water–ethanol solution was added to 100 mL of juice in the ratio of ethanol (Eth) and water of 3:7. The resulting solution was kept for a 48 h at room temperature. The extract solution was a light red color and after settling in two days, no precipitation appeared. Next, solutions of the extract with ablative gold nanoparticles were fabricated in a ratio of 1:1. The concentration of gold NPs varied in a (1 ÷ 10) × 10−10 M range. The volume of the extract remained constant.
Next, a microemulsion with the extract and Au NPs was prepared in oil (triglycerides of fatty acids—FA). The AOT surfactant (sodium dioctyl sulfosuccinate) was dissolved in the oil until the critical concentration of micelle formation was obtained (C = 10−3 M). Then, a water–ethanol solution with VO extract with Au NPs of 1.2 mL was added drop by drop to the oil with reverse micelles under constant stirring with a magnetic stirrer at 580 rpm for 30 min. AOT with VO extract was mixed in a ultrasonic bath (Elmascript P 30 H, Germany) at a temperature of 25 °C for 5 min (ν = 37 kHz).
2.2. Gold Nanoparticle Preparation
Gold nanoparticles (Au NPs) were prepared by the nanosecond laser ablation method of a gold plate in distilled water by means of Solar Laser System (Belarus) operating in the Q-switched mode with the following laser radiation parameters: λ = 532 nm, τ = 10 ns, ν = 15 Hz, E = 20 µJ. Each ablation session time lasted 5 min. The solution volume per one ablation session was V = 1.2 mL. After the ablation process, the solution became a pink color.
2.3. Methods of Quantitative and Qualitative Determination of Flavonoids in Viburnum opulus L. Berries
Quantitative determination of flavonoids in a VO water–ethanol extract was determined by the Leventhal titrimetric method [39] based on the ability of flavonoids to be oxidized by potassium permanganate. Prepared according to the method described above and filtered, the VO water–ethanol extract was taken with a 25 mL pipette and transferred to a conical flask with the capacity of 750 mL. Then, 500 mL of water and 25 mL of indigosulfonic acid solution were added and titrated with constant stirring with a solution of potassium permanganate (0.02 M) until it became a golden yellow color. At the same time, the control experiment was conducted in which the water–ethanol extract was carried out in which the water–ethanol extract was replaced with distilled water. The sum of flavonoids was estimated taking into account that 1 mL of potassium permanganate solution (0.02 M) corresponds to 0.0041 g of flavonoid substances in terms of tannin.
The qualitative reaction to flavonoids was carried out using FeCl (III): 2 ÷ 3 drops of 1% FeCl (III) solution were added to a 1 mL of VO extracts, resulting in a color change: an initially pink extract acquired a reddish-brown color, which indicates the presence of flavones and their derivatives in the sample [40].
2.4. Determination of Antioxidant Activity of Viburnum Extract with Gold Nanoparticles (DPPH Method)
This method is based on the ability of binding molecules of the reactive radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) with antioxidants contained in the studied samples. The test sample of the extract/extract with Au NPs (V = 20 µL) was mixed with V = 300 µL of a freshly prepared of 2,2-diphenyl-1-picrylhydrazyl (DPPH) 0.1 mM solution in ethanol. The sample was incubated for 60 min at room temperature in the dark. The decrease in optical density at 515 nm was measured by a spectrophotometry technique (CLARIOstar, BMG Labtech, Ortenberg, Germany) [41].
2.5. Experimental Measurements
The fluorescence spectra of the microemulsion with the extract were measured by means of the Fluorolog 3 optical system (Horiba). The lifetime measurements of long-lived (triplet) states of extract molecules and phosphorescence spectra were carried out by means of this unit using the pulsed Xe lamp. The solid state NanoLed with a wavelength of 405 nm and pulse duration of 200 ps was used for lifetime fluorescence measurements. The IR absorption spectra were measured by means of the Shimadzu IR spectrometer (Japan). The absorption spectra in the visible region were recorded with a Shimadzu spectrophotometer (Japan).
The size distribution of gold nanoparticles was investigated by the dynamic scattered light method with using a Photocor-Compact Z unit. Zeta-potential measurements were also recorded on this system.
3. Results and Discussion
3.1. IR-Spectroscopy Results
In the first series of the experiment, the IR spectrum of the absorption of the water–ethanol solution of the VO extract was studied. The results are shown in Figure 1.
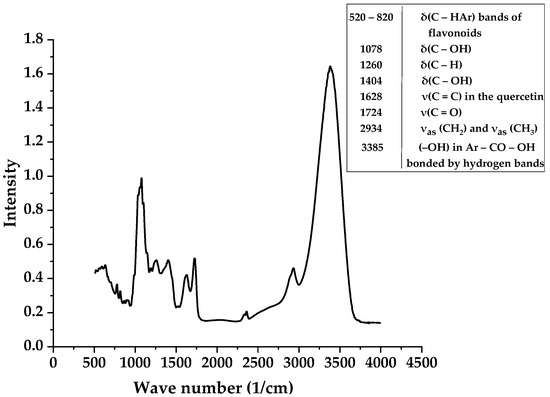
Figure 1.
The IR spectrum and interpretation of the peaks of the water–Eth Viburnum opulus L. extract in the KBr pellet.
It should be noted that the interpretation of the IR spectrum is quite difficult due to the presence of a large number of different compounds and components. Thus, the VO berries contain the polyalcohols: mannitol, sorbitol, and inositol. VO berries are also rich in pectin substances. The ripe Viburnum berries contain oxalic, malic, succinic, formic, citric, caprylic, valerian, isovaleric, and chlorogenic acids. The amino acid composition of berries is represented by free amino acids: serine, glutamic acid, and alanine predominate. The others ten amino acids are arginine, valine, aspartic acid, histidine, glycine, leucine, isoleucine, lysine, proline, and threonine. Phenolic (P-active) compounds of viburnum berries are represented by leucoanthocyanins, flavonols, catechins, anthocyanins, and phenol-carboxylic acids. A characteristic feature of groups of chemical compounds such as anthocyanins, leucoanthocyanins, and bioflavonoids is the P-vitamin activity [17]. The skin of the berries contains tannins and other coloring substances. Since this work was devoted to the study of the optical properties of the extract and the possibility of its use as a photosensitizer for PDT, compounds whose molecules contain chromophore and auxochromic bond groups (R–OH, C=O) responsible for the ability to absorb light and luminesce, were of the greatest interest in the IR spectrum. Therefore, we noted the presence of aromatic compounds as well as the presence of chromophore and auxochromic groups at v = 1404 and 1724 cm−1, which determine the color of the solution and intense absorption of light (UV and visible wavelength ranges) by the extract and luminescent features.
At the same time, based on the experimental results of the quantitative determination of flavonoids in the extract solution by the Leventhal titrimetric method, it was found that the sum of flavonoids in the extract was 0.12% in terms of tannin (the average values of the same ten samples).
Thus, based on the above results, we can say that the Viburnum opulus L. extract solution is a complex multicomponent composition in which flavonoids play the main role, providing the ability of the extract to absorb luminescence in the visible spectral region. This conclusion will be further confirmed by absorption–luminescent studies of the extract.
3.2. Absorption and Luminescence Dynamics of Flavonoids from Viburnum opulus L. Extract with Gold Nanoparticles
Figure 2 shows the absorption spectra of water–Eth extracts of VO with Au NPs at different concentrations; VO extract without Au NPs was taken as a reference.
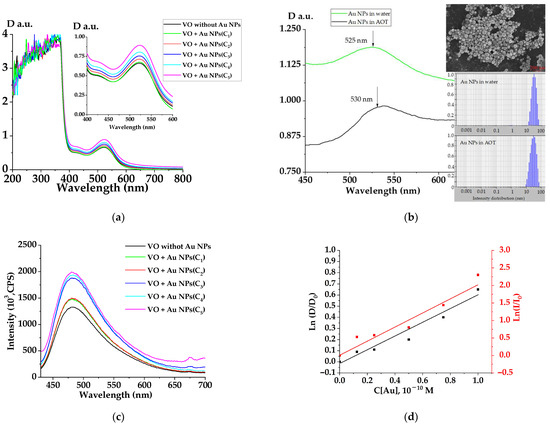
Figure 2.
Optical characteristics. (a) Absorption spectrum of water–Eth VO extract with Au NPs of various concentrations C1 = 1 × 10−10, C2 =0. 75 × 10−10, C3 = 0.5 × 10−10, C4 = 0.25 × 10−10, C5 = 0.125 × 10−10 M. (b) Absorption spectrum of Au NPs in water (green curve) and in microemulsion (black curve), inset—SEM image of ablative Au NPs and size distributions. (c) Luminescence spectrum of water–Eth extract with Au NPs. (d) The dynamics of changes in the optical density and luminescence intensity of the extract on the concentration of Au NPs in the AOT reverse micelles at a wavelength of 480 nm.
As can be seen from Figure 2, the VO extract optical density spectrum had peaks at a wavelength of 370, 420, and 525 nm. According to the literature data, these peaks correspond to quercetin (370 and 420 nm) and cyanidine (525 nm) [42,43,44]. The optical density at wavelengths of 420 and 525 nm increased with an increase in the Au NP concentration.
The optical density of a sample without Au NPs at a wavelength of 523 nm was 0.675, and for a sample with a concentration of C1, it was 0.892. Thus, the optical density gain factor was 0.75. Figure 2b shows the absorption spectrum of ablative gold NPs in a water solution and in a micellar AOT solution. The position of the maximum was located in the region of 500–550 nm and indicates the presence of colloidal gold nanoparticles in the solution after the laser ablation process. As can be seen from Figure 2b, the localization of the plasmon maximum practically did not shift after incubation of Au NPs in the microemulsion. The inset of Figure 2b shows a SEM image of a gold NP cluster. As can be seen from the figure, gold NPs had a spherical shape with the particle radius of 10 ÷ 70 nm. Size distribution of gold NPs in water and micellar solutions are also presented in Figure 2b (inset). The average hydrodynamic radius of NPs obtained from the distributions was r ≈ 30 nm and it did not depend on the environment in which the gold nanoparticles were located. Zeta-potentials for NPs were −15.65 ± 0.11 and −25.26 ± 0.34 for water and micellar solution without O2 molecules, respectively. The zeta-potential reflects the degree and nature of the interaction between gold nanoparticles and the dispersion medium. The combination of these interactions determines the magnitude of the zeta-potential, and consequently, the stability of hydrosols. The increase in the zeta-potential value can be explained by an increase in the negative charge on the surface of nanoparticles due to the adsorption of AOT anion molecules. Consequently, the electrostabilization of nanoparticles will increase in the microemulsion and Au NPs with a large surface charge will be located inside the micelles.
It can also be seen in Figure 2a,b that the VO extract and gold NPs absorption spectral ranges coincided, therefore, the NP-doped VO extract’s optical density increase was associated with spectral addition of the absorption ability of the extract molecules and NPs. It should be noted that the VO extract luminescence spectra had up to 25% increase in intensity when doped with gold NPs. The increase in the luminescence intensity of the VO extracts was caused by the plasmon mechanism [31] of energy transfer from gold NPs. An enhancement in the fluorescence of molecules located near the surface of metal nanoparticles (metal-enhanced fluorescence, MEF) is observed when the plasmon resonance wavelength coincides with the fluorophore excitation wavelength [28]. The physical essence of such a process is based on the nonradiative dipole–dipole transfer of electromagnetic energy between the nanoparticle and the unexcited and excited states of a closely spaced molecule. Gold and silver nanoparticles are the most common MEF materials. The MEF effect depends on the size and shape of the nanoparticles, the distance between the donor and acceptor, the overlap integral of the plasmon resonance, and absorption spectra of the fluorophore. In Figure 2a,b, it is shown that the absorption spectra of the extract molecules and gold nanoparticles overlapped in the region of 500–600 nm, therefore, the probability of energy transfer by the dipole–dipole mechanism during resonant photoexcitation from the gold nanoparticles to the extract molecule was rather high.
Furthermore, it was of interest to consider the dynamics of the absorption–luminescent characteristics of the VO extract with gold NPs in a microemulsion (AOT in FA) and to establish the possibility of plasmon energy transfer between gold NPs and luminescent molecules of the VO extract (microemulsion). The results are shown in Figure 2d. The optical density and luminescence intensity of the VO extract increased in the presence of Au NPs. Let us consider the mechanism of plasmon enhancement in our system. Thus, when metal nanoparticles are photoexcited, a part of the electromagnetic wave is absorbed. This energy is lost by converting it into thermal energy. The scattered wave, together with the incident wave, increases the local field at the locations of the molecules surrounding the particle. This can lead to an additional increase in the intensity of the scattered light and luminescence.
An increase in the probability of quantum transitions of an atom or molecule located inside the resonator or near it (gold nanoparticle) leads to an increase in the number of electronic transitions in the molecule, and consequently to an increase in the absorption and emission of electromagnetic radiation by it. A high intensity field is created in the resonating particle and in the near-field part of space. This means that molecules entering this field will either be strongly perturbed (during light scattering) or excited (in the case of luminescence). As a result, the intensity of the scattered or emitted light will increase. Thus, in this paper, it was proven that the dependence of the optical density and intensity of luminescence at the same angle slope of the lines (Figure 2d) on Au NP concentration inside the AOT micelles is due to the NP plasmon amplification effect.
Since the maximal enhancement of the VO extracts’ luminescence was observed at the highest NP concentration (Figure 2c), further kinetic studies were carried out with this concentration (C = C5). In the next section, we present the study of saturated water–alcohol VO extracts doped with gold NPs at a C5 = 10−10 M concentration in AOT micelles.
3.3. Optical Properties and Time-Resolved Spectroscopy of Oxygen-Saturated Microemulsions with VO Extract and Gold Nanoparticles
In this part, the photonics of oxygen-saturated VO water–Eth extracts doped with Au NPs in the AOT microemulsion were investigated. For this purpose, additional oxygen saturation of the microemulsion with an extract and NPs (C = 10−10 M) was carried out, followed by the registration of absorption, luminescence, and kinetic decay curves at wavelengths of 530 nm (fast luminescence) and 680 nm (phosphorescence—T state deactivation). The oxygen saturation process was performed by decomposition of hydrogen peroxide when heated on a burner in the presence of a catalyst (platinum plate). The process occurred with a constant stirring of the microemulsion by using a magnetic stirrer at 520 rpm (Supplemental Materials). During complete decomposition of hydrogen peroxide contained in 10 g of 3% peroxide solution (V = 10 mL), O2 molecules in a volume of 105 mL at 20 °C was released, enough to completely fill the space above the microemulsion. In the initial state (before oxygen saturation), the partial pressure of oxygen was = 1.013 × 105 · = 0.213 × 105 ( = 0.21).
By applying Henry’s law, the following system of equations can be written:
where and are the solubility of oxygen in the microemulsion (initial and after additional oxygen saturation, respectively). Substituting the and values, we obtained / = 4.7. Thus, the O2 concentration in the microemulsion increased 4.7 times at the partial pressure of oxygen equal to atmospheric pressure. Assuming that (mic) = t· (oil), where t is the coefficient of proportionality; (oil) denotes the oxygen solubility in oil; and (mic) is the oxygen solubility in micelle, the last expression can be written as follows:
Thus, the oxygen concentration in the micelle also increased 4.7 times and was C1 = 2 × 10−4 M at oxygen partial pressure equal to atmospheric pressure. Adjusting the partial pressure of O2 in the space above the microemulsion, different concentrations of oxygen in micelles were created. The oxygen pressure in the space above the emulsion was regulated by the reaction time (τ) and the oxygen concentration. C[O2] grew linearly with an increase in pressure: at τ1 = 5 min—p1 = 0.25 atm (C1[O2] = 2 × 10−4 M); at τ2 = 7 min—p2 = 0.65 atm (C2[O2] = 3.5 × 10−4 M); at τ1 = 12 min—p3 = 1 atm (C3[O2] = 8.5 × 10−4 M). The process of oxygen oversaturation of the microemulsion led to the destruction of micelles and the precipitation of a pink sediment. This was due to oxidative processes, leading to the destruction of double bonds in the triglycerides of fatty acids (oil).
The optical density, luminescence intensity peak, and lifetime of VO extracts doped with gold NPs at a wavelength of 480 nm (excited at 400 nm)) at various oxygen concentrations are shown in Figure 3.
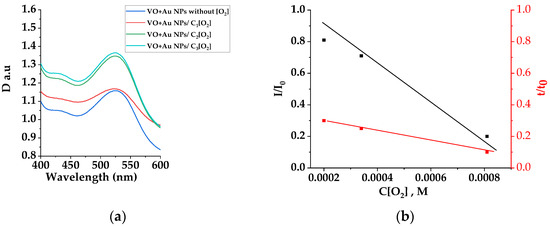
Figure 3.
Absorption spectra of microemulsion with Au NPs with oxygen C3[O2]—black curve, VO microemulsion with Au nanoparticles (C = 10−10 M) without and with oxygen of different concentrations (C1 = 2 × 10−4 M, C2 = 3.5 × 10−4 M, C3 = 8.5 × 10−4 M) (a). The dependences of changes in luminescence intensity and luminescence lifetimes on oxygen concentrations at λ = 480 nm under photoexcitation with λ = 400 nm (b).
As can be seen from Figure 3a, an increase in the optical density of the extract with nanoparticles was observed with the oxygen concentration growing in micelles. Thus, at the maximum oxygen concentration, the gain factor was 1.23.
From a comparison of Figure 2a and Figure 3a, it can be seen that the recorded spectral increase in the VO extract’s optical absorption density at a wavelength of 520 nm coincided with the optical density of the gold NPs’ plasmon absorption. It is important to note that a feature of the VO extract’s absorption density changed with an increase in the oxygen concentration in the mixture at a constant concentration of NPs, which is represented by the absence of shape changes and the absorption bands peaks’ spectral shift. The former means that the change in the optical absorption density in the visible region of the spectrum is exclusively due to the change concentrations of gold NPs. Thus, the total absorption spectra of VO with gold NPs (Figure 2a and Figure 3) proves the fact that the complexes of VO + Au NPs are stable and have the corresponding absorption and luminescence properties only at certain oxygen concentrations.
At the same time, as a result of oxygen saturation of the microemulsion, there is a change in the quenching efficiency of fast luminescence (Figure 3b, red curve), according to Stern–Vollmer theory [23]:
The obtained Stern–Vollmer quenching constants determined from the I/I0 vs. C[O2] plot slope appeared to be Kdyn = 0.96 × 103 M−1 and 0.18 × 103 M−1 for the VO extracts’ luminescence spectral and kinetic characteristics, respectively.
The experimental fast luminescence decay curves are shown in Figure 4a. Thus, the lifetime of luminescence in the absence of oxygen molecules in the microemulsion was 2.47 ns, in the presence of oxygen, the lifetime decreased in the range of 0.64 ÷ 0.38 ns. Based on these data, the bimolecular quenching constant rate was kq ~ 3.8 × 1010 M−1s−1. The order of magnitude of the quenching constant rate may indicate a dynamic quenching mechanism [23], in which the quencher (oxygen) creates a complex VO + O2 with a luminescent extract molecule in an excited state as a result of a sufficiently fast diffusion interaction with a speed of ~1010 M−1s−1. This short-lived complex inside the micelle breaks down rapidly, and all the absorbed energy of the electronic excitation of this complex, as a result of intercombination conversion, passes to long-lived (triplet) states in the extract molecule and to singlet-excited states (1Δg) in the oxygen molecule [45], which is rather important, since it is the singlet states of oxygen (1Δg) that participate in photodynamic processes.
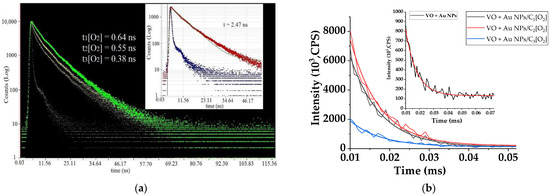
Figure 4.
(a) Kinetic decay curves of the luminescence from the VO extract microemulsion with Au NPs (C = 10−10 M) at a wavelength of 420 nm under photoexcitation with wavelength of 400 nm at different oxygen concentrations (C1, C2, C3), inset—without oxygen. (b) Kinetics decay of extract molecule triplet states with Au NPs with oxygen of different concentrations (C1, C2, C3) registered at a wavelength of 680 nm with photoexcitation of 530 nm (triplet states), inset—without oxygen. C1 = 2 × 10−4 M, C2 = 3.5 × 10−4 M, C3 = 8.5 × 10−4 M.
At the same time, the linearity of the graph I/I0 vs. C[O2] (Figure 3b, black curve) in the specified range of oxygen concentrations indicates the interaction of only one type of luminescent molecule in the extract with oxygen molecules.
It is also important to note that the processes of plasmon enhancement and quenching of luminescence with oxygen discussed above occurred in a micelle, therefore the radius of action of these mechanisms is limited by the size of the micelle itself. The process of extinguishing singlet states of extract molecules with oxygen occurs in the presence of gold nanoparticles, the average radius of which is ~40 nm. Therefore, there is only one gold nanoparticle located in the micelle. Taking into account the lifetime of luminescence and the diffusion constant rate of oxygen molecules according to the Einstein–Smolukhovsky equation [46]: cm2/s, where D denotes the diffusion coefficient of the water–ethanol extract molecules; t = 0.38 × 10−9 s is the lifetime fluorescence, it is possible to estimate the size of the micelle. Thus, the diameter of the micelle was x∼125 nm. At the same time, according to the size distribution function of gold nanoparticles (Figure 2b, insert), there were NPs with a radius of 10 ÷ 70 nm in the hydrosole including a sufficiently large quantity of NPs with a radius of 15–20 nm. As is well known, small particles are susceptible to aggregation and cluster formation. Consequently, in one micelle, the clusters consisting of smaller nanoparticles could also be located in addition to one particle with r ≈ 30 nm. Therefore, when a micelle with the extract and nanoparticles is photoexcited, there is a total increase in the generation of plasmon energy from clusters, which makes an additional contribution to the process of luminescence enhancement.
It should be noticed that the high value of the decay luminescence constant rate (kq~3.8 × 1010 M−1s−1) in the VO + O2 complexes may be due to two quenching types: oxygen quenching and the possible appearance of radical reactions that will also quench the molecular luminescence as well as accelerate the decay of VO with Au NPs under the influence of oxygen molecules.
Consequently, it is possible to say that the process of luminescence plasmon enhancement presented in the first part of the experimental work (Figure 2) can be partially decreased by the oxygen molecules as a result of dipole–dipole interactions and the exchange interactions of radical reactions.
However, in order to establish this, it is necessary to study the extract molecules’ luminescence kinetics in the presence of the quenching dynamic of fast luminescence with singlet oxygen generation processes. Moreover, the possibility of the extract molecules’ complexation with oxygen molecules in long-lived states resulting in singlet oxygen state formation should be investigated when identifying the presence of long-lived (triplet) states in luminescent extract molecules (quercetine and cyanidine).
Figure 4b shows the decay kinetics of triplet states in extract molecules during the changing oxygen concentration (C1 = 2 × 10−4 M, C2 = 3.5 × 10−4 M, C3 = 8.5 × 10−4 M) process in the presence at a constant concentration of gold NPs, C = 10−10 M (or 2 × 1013 L−1).
The decay curves were approximated by an exponential model:
where A, B1 are the kinetic parameters.
It was found that the extract molecule had triplet states at a wavelength of 680 nm with a lifetime of 3.5 microseconds, which increased up to ~8 μs in the presence of Au NPs (inset Figure 4a). The mechanism of this increase was due to the fact that the lifetime in the excited state of atoms and molecules significantly depends on the radius of the nanoparticles and the distance to their surface [47,48]. As the emitting atoms and molecules approach the nanoparticle, the probability of transition from the excited state, starting from distances of the order of the size of this particle (rAu ≈ 30 nm), increases. However, this increase occurs not only due to an increase in the rate of radiation transitions, but also due to the non-radiative relaxation of energy. As a result of non-radiative energy transfer by the dipole–dipole mechanism, energy is accumulated at the long-lived (triplet) level of the luminescent molecule.
Thus, the triplet states of VO molecules can create complexes with oxygen in the excited (triplet) state (VO3 + 3O2)1,3,5 as a result of quenching singlet states by the dynamic quenching mechanism (see the previous section).
As a result of oxygen saturation of the microemulsion, a decrease in VO extract phosphorescence lifetime was observed under photoexcitation with λ = 530 nm. Thus, the duration of long-lived (triplet) states decreased down to 7 µs, 6 µs, and 2 µs at the oxygen concentrations C1[O2], C2[O2], and C3[O2], respectively. Thus, the quenching of the VO extracts’ molecules long-lived states with the presence of NPs and molecular oxygen was observed. The corresponding quenching rate constants of triplet states (kqT) could be determined according to the following equation:
where and ; и are the lifetimes of the VO molecules’ triplet states in the presence of oxygen and without oxygen, respectively. By substituting obtained lifetime values to the previous equation, we obtained the value = 0.8 × 108 s−1 (for C1[O2]). This value corresponded to the complexation constant of the collision complex (VO3 + 3O2) inside the reverse micelles in the presence of plasmon resonance energy transfer. With an O2 concentration increase up to a C3 = 8.5 × 10−4 M value, the quenching constant rate of triplet states in (VO3 + 3O2) complex was = 4.5 × 108 M−1·s−1. Depending on the efficiency of the electrostatic interaction between the T-excited state of the VO molecule and the O2 taking into account the formula: , the diffusion oxygen constant rate in micelles was = 7.2 × 108 M−1·s−1 and = 40.5 × 108 M−1·s−1 for C1[O2] and C3[O2], respectively. Thus, the VO extracts’ molecule triplet state quenching rate constant grows with the increase in oxygen molecules and determines the rate of the accumulation of excited states 1Δg in the oxygen molecule.
Thus, based on the results presented in this and the previous sections, it can be concluded that the VO extracts’ molecules (quercetin and cyanidine) are capable of forming photosensitive complexes with oxygen molecules. The excitation energy absorbed by these complexes (530 nm, 400 nm) can transfer through excited levels in the O2 molecule, providing the emission of 1Δg-state phosphorescence, which is necessary for PDT. Additional studies on the antioxidant activity of the extract are considered to be the confirmation that flavonoids of the VO extract are active for photochemical reactions with oxygen. Based on the photometric method representing the ability of binding molecules of the reactive radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) with antioxidants, it was found that the optical density of the studied sample of VO extract at a wavelength of 515 nm was 2.06 in terms of equivalent (Trolox). It was also found that the presence of gold NPs of two different concentrations in the test solution led to an increase in the value up to 2.22 at CAu = 0.5 × 10−10 M and to 2.3 at CAu = 1 × 10−10 M. Thus, as an antioxidant, the flavonoids of the VO extract, being a result of chemical reactions, are able to neutralize free radicals (such as superoxide anions (O2•−), hydrogen peroxides (H2O2), hydroxyl radicals (OH•)) produced by oxidative stress in cells. At the same time, under the influence of light of certain wavelengths of the visible range, the optical properties of VO flavonoids can be enhanced as a result of the plasmonic interaction of Au NPs, and the reactivity to complexation with O2 molecules increases (Figure 3). Thus, the complexation constant of a triplet-excited complex (VO3 + 3O2) can reach a value of = 4.5 × 108 M−1·s−1 in the presence of plasmon energy of gold NPs due to an increase in the duration of deactivation of triplet-excited extract molecules as a result of plasmon resonance (530 nm) photoexcitation.
4. Conclusions
As a result of the spectral-kinetic study of water–Eth extracts of reverse micelles with Viburnum opulus L. and gold nanoparticles (r ≈ 40 nm) at various concentrations of molecular oxygen, plasmon amplification of fluorescence and phosphorescence of VO was studied for the first time. It was established that VO is a biologically active substance with effective absorption and luminescent properties in the visible range due to the presence of flavonoids. It was found that the extract molecules had triplet states at a wavelength of 680 nm with a duration of 3.5 microseconds, which can be increased up to ~8 μs in the presence of Au NPs. The VO molecules’ triplet states can create complexes with oxygen in the excited (triplet) state (VO3 + 3O2). It has been experimentally proven that the processes of dynamic quenching of singlet-excited states of the extract molecules and the processes of plasmon generation under stationary and pulsed excitation with a wavelength of 400 nm were observed during the additional complexation process with oxygen molecules in micelles. The constant rates of fluorescence decay of the extract with and without gold NPs were determined at different oxygen concentrations. The singlet oxygen generation constant rate of = 7.2 × 108 M−1·s−1 was also determined at a constant concentration of VO with gold NPs.
Thus, for the first time, an optical method was comprehensively developed for the study of berries and extracts of Viburnum opulus L. in order to synthesize an effective singlet oxygen generator in solution for its potential use in photodynamic processes with biological and medical objects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors10040130/s1, Scheme S1: The installation for oxygen generation and additional saturation of the microemulsion (VO) with O2: 1—an oxygen generator (a glass tube with a H2O2 solution and Pt plate); 2—a microemulsion with dissolved oxygen; 3—an air atmosphere with different partial pressure of O2; 4—an AOT micelle containing an water–Eth extract of a Viburnum opulus L.; Figure S1. IR spectra: microemulsion without Au NPs (a); microemulsion with Au NPs (b); microemulsion with oxygen molecules (c). Figure S2. Absorption spectra: Au NPs in water solution; Au NPs in microemulsion; Au NPs in microemulsion with oxygen molecules. Figure S3. Size distribution of gold nanoparticles in water solution (a); in reverse micellar solution without oxygen molecules (b); and in reverse micellar solution with oxygen molecules (c). Figure S4. Absorption spectra (a) and luminescence spectra (b) of microemulsions with the VO extract and gold nanoparticles of various concentrations. Au nanoparticle concentrations: C1 = 1 × 10−10, C2 = 0.75 × 10−10, C3 = 0.5 × 10−10, C4 = 0.25 × 10−10, C5 = 0.125 × 10−10 M. Excitation wavelength was 400 nm. Table S1. The results of size distribution and zeta-potential measurements. Table S2: The results of antioxidant activity obtained by the amperometric method. Table S3: The results of antioxidant activity obtained by the FRAP method. Table S4: The results of antioxidant activity obtained by the DPPH method. Table S5: The results of antioxidant activity obtained by the ABTS method.
Author Contributions
Conceptualization, V.B. and E.Z.; Methodology, V.S.; Software, A.K.; Validation, I.S., V.B.; Formal analysis, V.S. and E.Z.; Investigation, D.A.; Resources, L.S. and A.Z.; Data curation, A.T.; Writing—original draft preparation, V.B.; Writing—review and editing, A.T.; Visualization, I.L. and A.K.; Supervision, V.B.; Project administration, I.S.; Funding acquisition, I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the framework of the project supported by the Ministry of Science and Higher Education of the Russian Federation No. FZWM-2020-0003.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zakłos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus L. Fruit Phenolic Compounds as Cytoprotective Agents Able to Decrease Free Fatty Acids and Glucose Uptake by Caco-2 Cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Dienaitė, L.; Pukalskienė, M.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Valorization of European Cranberry Bush (Viburnum opulus L.) Berry Pomace Extracts Isolated with Pressurized Ethanol and Water by Assessing Their Phytochemical Composition, Antioxidant, and Antiproliferative Activities. Foods 2020, 9, 1413. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Amin Doustvandi, M.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic Therapy for Cancer: Role of Natural Products. Photodiagn. Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Bruschi, M.L.; da Silva, J.B.; Rosseto, H.C. Photodynamic Therapy of Psoriasis Using Photosensitizers of Vegetable Origin. Curr. Pharm. Des. 2019, 25, 2279–2291. [Google Scholar] [CrossRef]
- Monjo, A.; Pringle, E.; Thornbury, M.; Duguay, B.; Monro, S.; Hetu, M.; Knight, D.; Cameron, C.; McFarland, S.; McCormick, C. Photodynamic Inactivation of Herpes Simplex Viruses. Viruses 2018, 10, 532. [Google Scholar] [CrossRef] [Green Version]
- Arciniegas, A.; Gómez-Vidales, V.; Pérez-Castorena, A.; Nieto-Camacho, A.; Villaseñor, J.L.; Romo de Vivar, A. Recognition of Antioxidants and Photosensitizers in Dyssodia Pinnata by EPR Spectroscopy. Phytochem. Anal. 2020, 31, 252–261. [Google Scholar] [CrossRef]
- Shi, S.; Cho, H.; Sun, Q.; He, Y.; Ma, G.; Kim, Y.; Kim, B.; Kim, O. Acanthopanacis Cortex Extract: A Novel Photosensitizer for Head and Neck Squamous Cell Carcinoma Therapy. Photodiagn. Photodyn. Ther. 2019, 26, 142–149. [Google Scholar] [CrossRef]
- Alam, S.T.; Hwang, H.; Son, J.D.; Nguyen, U.T.T.; Park, J.-S.; Kwon, H.C.; Kwon, J.; Kang, K. Natural Photosensitizers from Tripterygium Wilfordii and Their Antimicrobial Photodynamic Therapeutic Effects in a Caenorhabditis Elegans Model. J. Photochem. Photobiol. B Biol. 2021, 218, 112184. [Google Scholar] [CrossRef]
- Warowicka, A.; Popenda, Ł.; Bartkowiak, G.; Musidlak, O.; Litowczenko-Cybulska, J.; Kuźma, D.; Nawrot, R.; Jurga, S.; Goździcka-Józefiak, A. Protoberberine Compounds Extracted from Chelidonium Majus L. as Novel Natural Photosensitizers for Cancer Therapy. Phytomedicine 2019, 64, 152919. [Google Scholar] [CrossRef]
- Giacone, L.; Cordisco, E.; Garrido, M.C.; Petenatti, E.; Sortino, M. Photodynamic Activity of Tagetes Minuta Extracts against Superficial Fungal Infections. Med. Mycol. 2020, 58, 797–809. [Google Scholar] [CrossRef]
- Postigo, A.; Schiavi, P.C.; Funes, M.; Sortino, M. Mechanistic Studies of Candida Albicans Photodynamic Inactivation with Porophyllum Obscurum Hexanic Extract and Its Isolated Thiophenic Compounds. Photodiagn. Photodyn. Ther. 2019, 26, 420–429. [Google Scholar] [CrossRef]
- da Silva Souza Campanholi, K.; Jaski, J.M.; da Silva Junior, R.C.; Zanqui, A.B.; Lazarin-Bidóia, D.; da Silva, C.M.; da Silva, E.A.; Hioka, N.; Nakamura, C.V.; Cardozo-Filho, L.; et al. Photodamage on Staphylococcus Aureus by Natural Extract from Tetragonia Tetragonoides (Pall.) Kuntze: Clean Method of Extraction, Characterization and Photophysical Studies. J. Photochem. Photobiol. B Biol. 2020, 203, 111763. [Google Scholar] [CrossRef]
- Obi, K.; Frolova, L.; Fuierer, P. Preparation and Performance of Prickly Pear (Opuntia phaeacantha) and Mulberry (Morus rubra) Dye-Sensitized Solar Cells. Sol. Energy 2020, 208, 312–320. [Google Scholar] [CrossRef]
- Purushothamreddy, N.; Dileep, R.K.; Veerappan, G.; Kovendhan, M.; Joseph, D.P. Prickly Pear Fruit Extract as Photosensitizer for Dye-Sensitized Solar Cell. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117686. [Google Scholar] [CrossRef]
- Sampaio, D.M.; Babu, R.S.; Costa, H.R.M.; de Barros, A.L.F. Investigation of Nanostructured TiO2 Thin Film Coatings for DSSCs Application Using Natural Dye Extracted from Jabuticaba Fruit as Photosensitizers. Ionics 2019, 25, 2893–2902. [Google Scholar] [CrossRef]
- Paşayeva, L.; Kararenk, A.C.; Fatullayev, H. Screening of Different Fruit Extracts from Viburnum opulus L. as Inhibitors of Key Enzymes Linked to Type 2 Diabetes and Antioxidants: A Comparative Evaluation. J. Food Meas. Charact. 2021, 15, 4403–4410. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Z.; Zhang, X.; Shao, J.; Zhao, C. Recent Advance on Chemistry and Bioactivities of Secondary Metabolites from Viburnum Plants: An Update. Chem. Biodivers. 2021, 18, e2100404. [Google Scholar] [CrossRef]
- Pietrzyk, N.; Zakłos-Szyda, M.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus L. Fruit Phenolic Compounds Protect against FFA-Induced Steatosis of HepG2 Cells via AMPK Pathway. J. Funct. Foods 2021, 80, 104437. [Google Scholar] [CrossRef]
- Wu, X.; Xie, J.; Qiu, L.; Zou, L.; Huang, Y.; Xie, Y.; Xu, H.; He, S.; Zhang, Q. The Anti-Inflammatory and Analgesic Activities of the Ethyl Acetate Extract of Viburnum Taitoense Hayata. J. Ethnopharmacol. 2021, 269, 113742. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Chen, J.-J.; Li, D.-Q.; Zhang, Y.-; Wang, X.-B.; Yao, G.-D.; Song, S.-J. Network Pharmacology Uncovers Anti-Cancer Activity of Vibsane-Type Diterpenes from Viburnum Odoratissimum. Nat. Prod. Res. 2021, 35, 637–640. [Google Scholar] [CrossRef]
- Bozali, K.; Guler, E.M.; Gulgec, A.S.; Kocyigit, A. Cytotoxic, Genotoxic and Apoptotic Effects of Viburnum opulus L. on Colon Cancer Cells: An in Vitro Study. Turkish J. Biochem. 2020, 45, 803–810. [Google Scholar] [CrossRef]
- Kajszczak, D.; Zakłos-Szyda, M.; Podsędek, A. Viburnum opulus L.—A Review of Phytochemistry and Biological Effects. Nutrients 2020, 12, 3398. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Zhen, S.; Yi, X.; Zhao, Z.; Lou, X.; Xia, F.; Tang, B.Z. Drug Delivery Micelles with Efficient Near-Infrared Photosensitizer for Combined Image-Guided Photodynamic Therapy and Chemotherapy of Drug-Resistant Cancer. Biomaterials 2019, 218, 119330. [Google Scholar] [CrossRef]
- Kustov, A.V.; Krestyaninov, M.A.; Kruchin, S.O.; Shukhto, O.V.; Kustova, T.V.; Belykh, D.V.; Khudyaeva, I.S.; Koifman, M.O.; Razgovorov, P.B.; Berezin, D.B. Interaction of Cationic Chlorin Photosensitizers with Non-Ionic Surfactant Tween 80. Mendeleev Commun. 2021, 31, 65–67. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Ray, K.; Chowdhury, M.; Szmacinski, H.; Fu, Y.; Zhang, J.; Nowaczyk, K. Plasmon-controlled fluorescence: A new paradigm in fluorescence spectroscopy. R. Soc. Chem. 2008, 133, 1308–1346. [Google Scholar] [CrossRef] [Green Version]
- Sarid, D.; Challener, W. Modern Introduction to Surface Plasmons: Theory, Mathematica Modeling, and Applcations, 1st ed.; Sarid, D., Ed.; Cambridge University Press: Cambridge, UK, 2010; ISBN-13: 978-0521767170. [Google Scholar]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: New York, NY, USA, 2007; ISBN 978-0-387-33150-8. [Google Scholar]
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-Friendly Synthesis and Biomedical Applications of Gold Nanoparticles: A Review. Microchem. J. 2020, 152, 104296. [Google Scholar] [CrossRef]
- Darabdhara, G.; Das, M.R.; Singh, S.P.; Rengan, A.K.; Szunerits, S.; Boukherroub, R. Ag and Au Nanoparticles/Reduced Graphene Oxide Composite Materials: Synthesis and Application in Diagnostics and Therapeutics. Adv. Colloid Interface Sci. 2019, 271, 101991. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in Sustainable Agriculture: An Emerging Opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Sudhani, H.P.K.; Bajpai, V.K.; Chen, L.; Shukla, S.; Mukherjee, A. Plant Extract Mediated Silver Nanoparticles and Their Applications as Antimicrobials and in Sustainable Food Packaging: A State-of-the-Art Review. Trends Food Sci. Technol. 2021, 112, 651–666. [Google Scholar] [CrossRef]
- Khandanlou, R.; Murthy, V.; Wang, H. Gold Nanoparticle-Assisted Enhancement in Bioactive Properties of Australian Native Plant Extracts, Tasmannia Lanceolata and Backhousia Citriodora. Mater. Sci. Eng. C 2020, 112, 110922. [Google Scholar] [CrossRef]
- Omolaja, A.A.; Pearce, B.; Omoruyi, S.I.; Badmus, J.A.; Ismail, E.; Marnewick, J.; Botha, S.; Benjeddou, M.; Ekpo, O.E.; Hussein, A.A. The Potential of Chalcone-Capped Gold Nanoparticles for the Management of Diabetes Mellitus. Surf. Interfaces 2021, 25, 101251. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Al Jomaa, A.; Kamoun, M. Gold Nanoparticles: Synthesis Properties and Applications. J. King Saud Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Dan, S.; Upadhyay, S.K.; Girdhar, M.; Mandal, M. Sakshi Oral Carcinoma and Therapeutic Approaches of Nanotechnology: From Fundamental Concepts, Incidence, Molecular Mechanism to Emerging Treatment Techniques. Biointerface Res. Appl. Chem. 2022, 12, 3900–3937. [Google Scholar] [CrossRef]
- Huang, X.; Hu, X.; Song, S.; Mao, D.; Lee, J.; Koh, K.; Zhu, Z.; Chen, H. Triple-Enhanced Surface Plasmon Resonance Spectroscopy Based on Cell Membrane and Folic Acid Functionalized Gold Nanoparticles for Dual-Selective Circulating Tumor Cell Sensing. Sens. Actuators B Chem. 2020, 305, 127543. [Google Scholar] [CrossRef]
- Uwada, T.; Wang, S.-F.; Liu, T.-H.; Masuhara, H. Preparation and Micropatterning of Gold Nanoparticles by Femtosecond Laser-Induced Optical Breakdown. J. Photochem. Photobiol. A Chem. 2017, 346, 177–186. [Google Scholar] [CrossRef]
- Atanassova, M.; Christova-Bagdassarian, V. Determination of Tannins Content by Titrimetric Method for Comparison of Different Plant Species. J. Chem. Technol. Metall. 2009, 44, 413–415. [Google Scholar]
- Bagchi, T.B.; Chattopadhyay, K.; Sivashankari, M.; Roy, S.; Kumar, A.; Biswas, T.; Pal, S. Effect of Different Processing Technologies on Phenolic Acids, Flavonoids and Other Antioxidants Content in Pigmented Rice. J. Cereal Sci. 2021, 100, 103263. [Google Scholar] [CrossRef]
- Ben Ahmed, Z.; Hefied, F.; Yousfi, M.; Demeyer, K.; Vander Heyden, Y. Study of the Antioxidant Activity of Pistacia Atlantica Desf. Gall Extracts and Evaluation of the Responsible Compounds. Biochem. Syst. Ecol. 2022, 100, 104358. [Google Scholar] [CrossRef]
- Souichi, N.; Keishi, O.; Kazuo, M. Kinetic Study of the Quenching Reaction of Singlet Oxygen by Flavonoids in Ethanol Solution. J. Phys. Chem. B. 2005, 109, 4234–4240. [Google Scholar] [CrossRef]
- Amjad, M.S.; Talaat, A.A.; Md Mizanur, R.; Yousef, M.H. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT—Food Sci. Technol. 2021, 150, 111932. [Google Scholar] [CrossRef]
- Deineka, V.I.; Kulchenko, Y.Y.; Sidorov, A.N.; Blinova, I.P.; Varushkina, S.M.; Deineka, L.A.; Anh, T.N.V. Determination of Catharanthus flower anthocyanins. Anal. Control 2019, 23, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Minaev, B.F. Electronic Mechanisms of Activation of Molecular Oxygen. Russ. Chem. Rev. 2007, 76, 1059–1083. [Google Scholar] [CrossRef]
- Entelis, S.G.; Tiger, R.P. Kinetics of Reactions in the Liquid Phase. Quantitative Account of the Influence of the Medium; Chemistry: Moscow, Russia, 1973. [Google Scholar]
- Esteban, R.; Laroche, M.; Greffet, J.-J. Modifying the linear and nonlinear optical susceptibilities of coupled quantum dot-metallic nanosphere systems with the Purcell effect. J. Appl. Phys. 2009, 105, 033107. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and Quenching of Single-Molecule Fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).