A Novel Truncated DNAzyme Modified Paper Analytical Device for Point-of-Care Test of Copper Ions in Natural Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
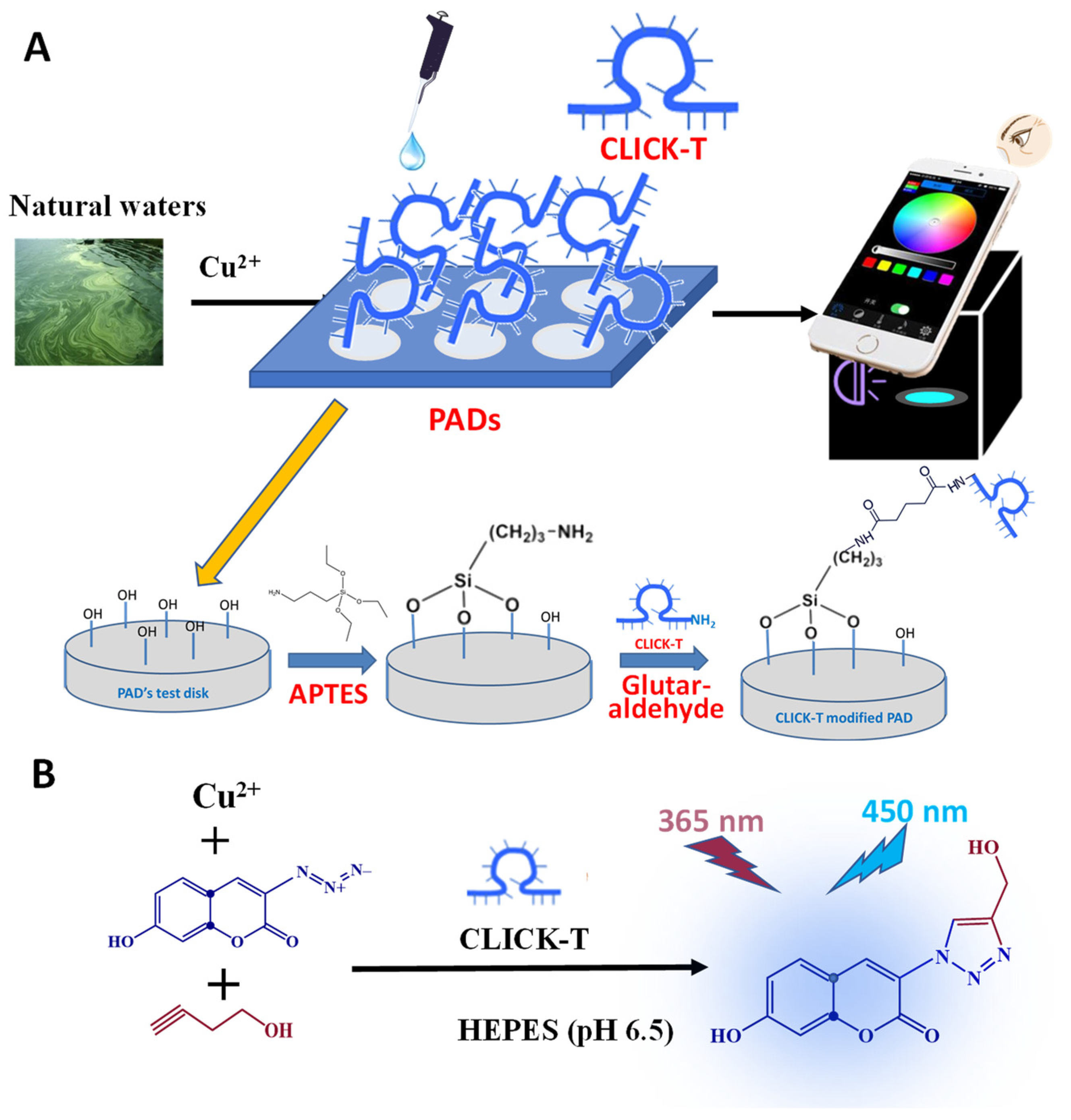
2.2. The Fabrication of PAD Arrays
2.3. Copper Ions Detection in Natural Waters by the PADs
2.4. Cyclic Voltammetry (CV)
3. Results
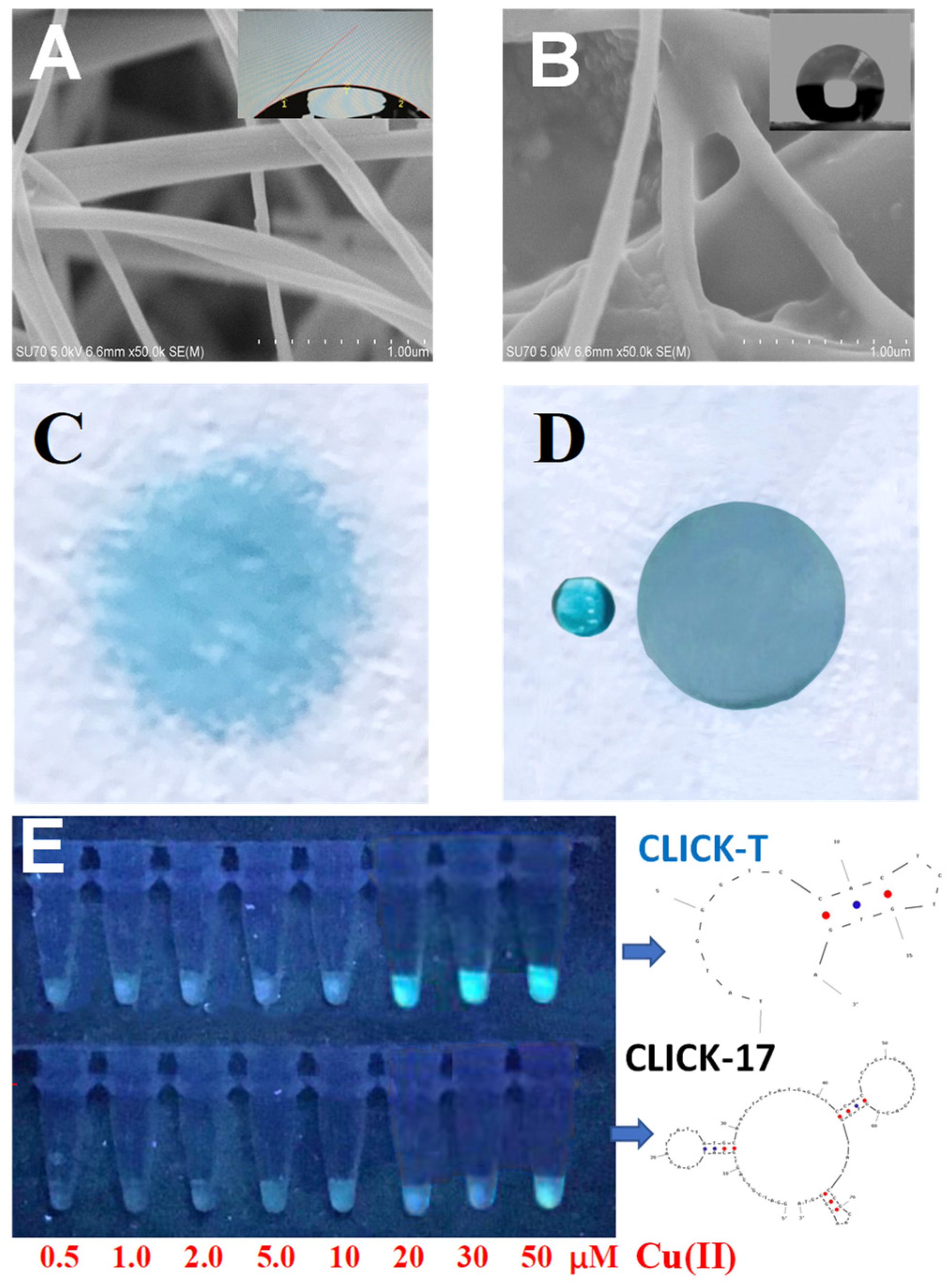
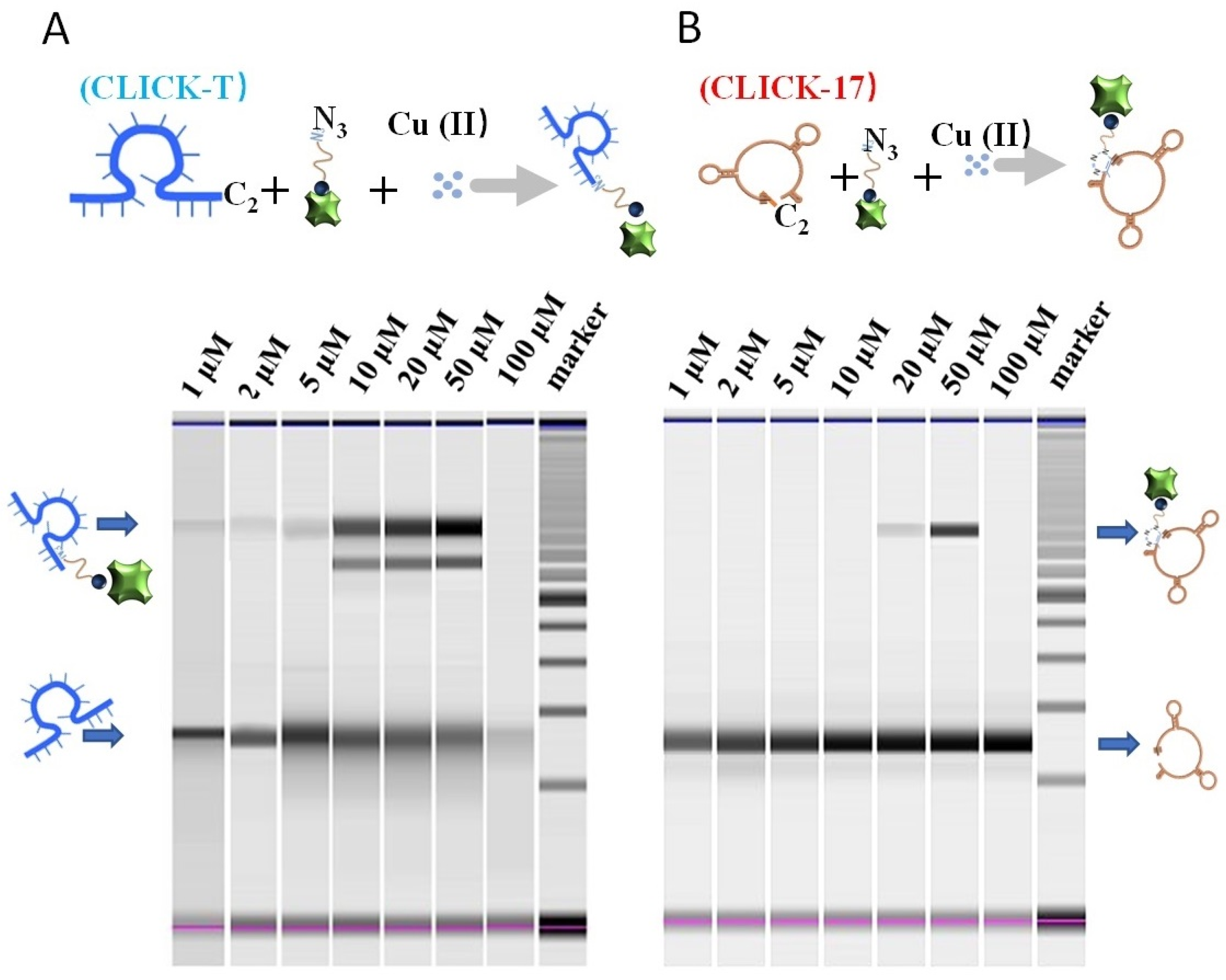
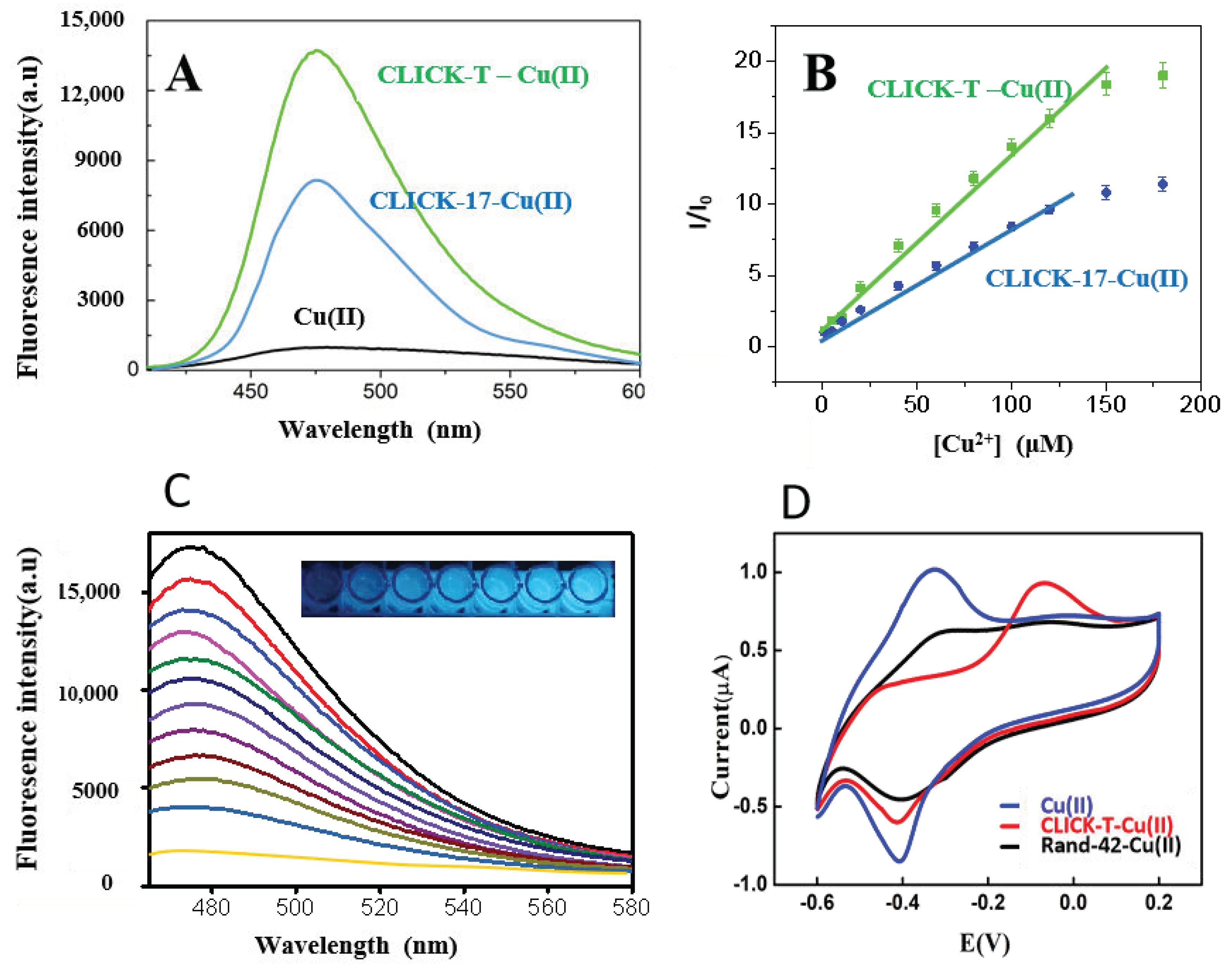
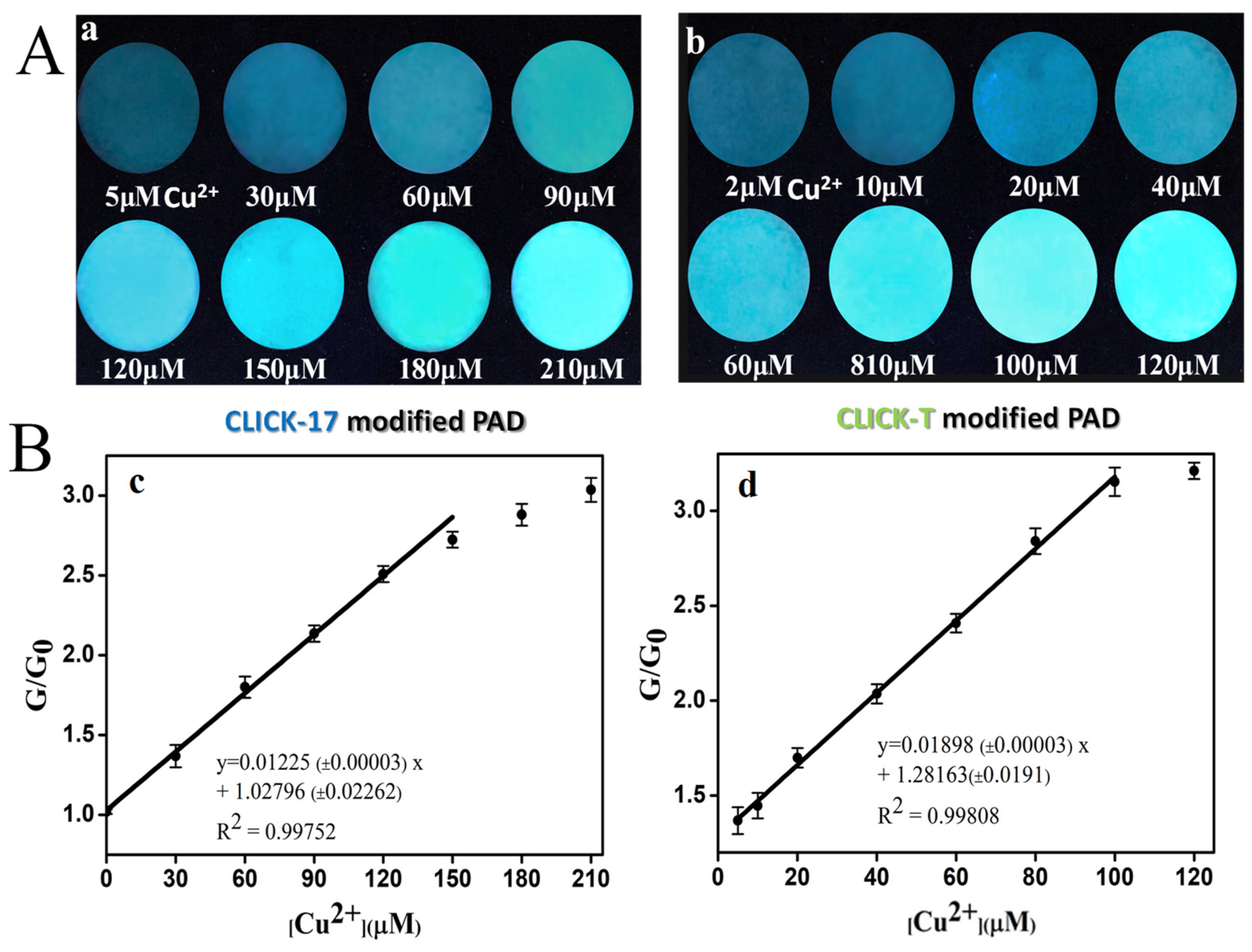
3.1. The Feasibility of Copper Detection by the CLICK-T Modified PADs
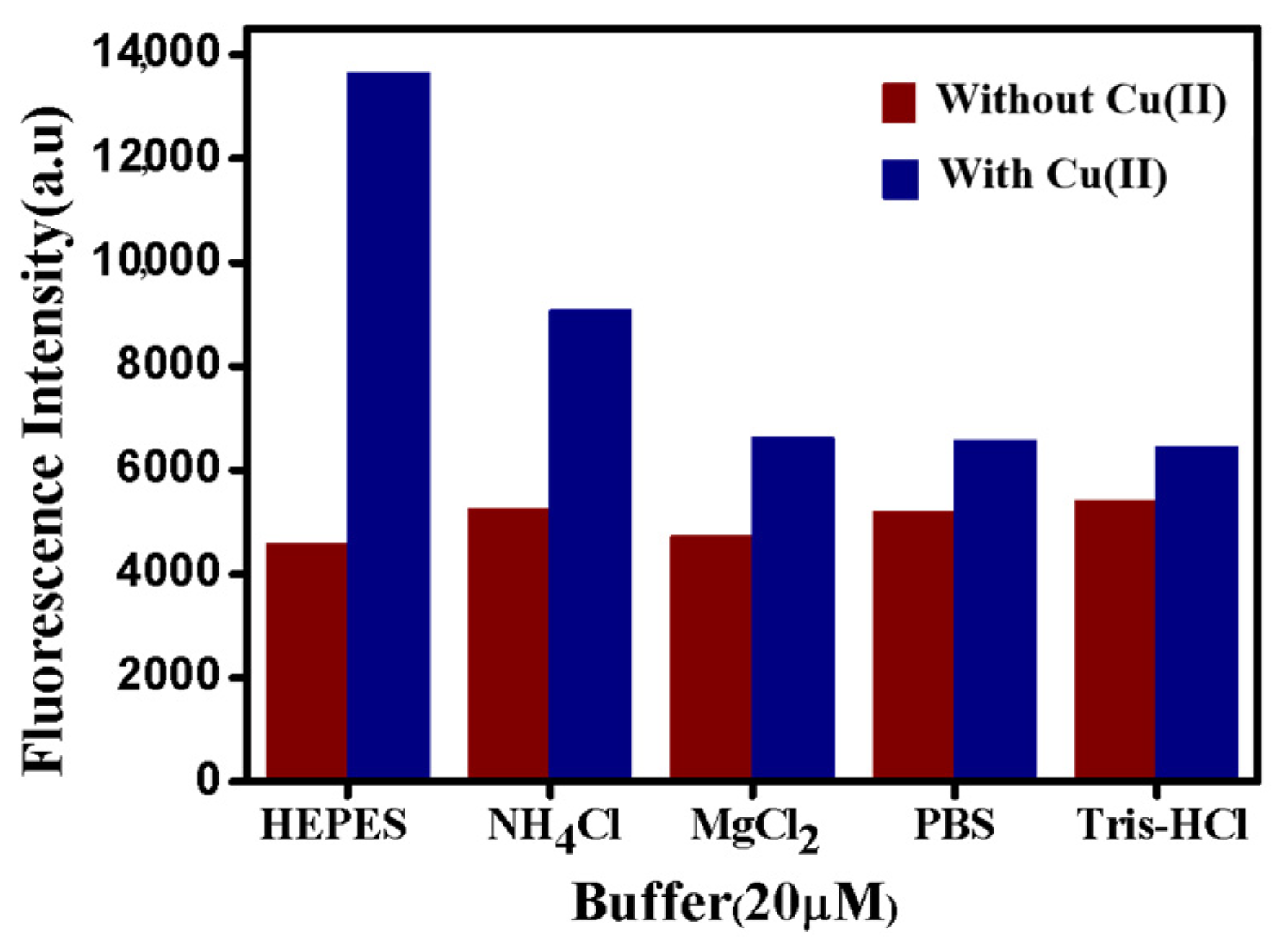
3.2. The Detection Effects and Mechanism of Cu(II) on CLICK-T Modified PADs
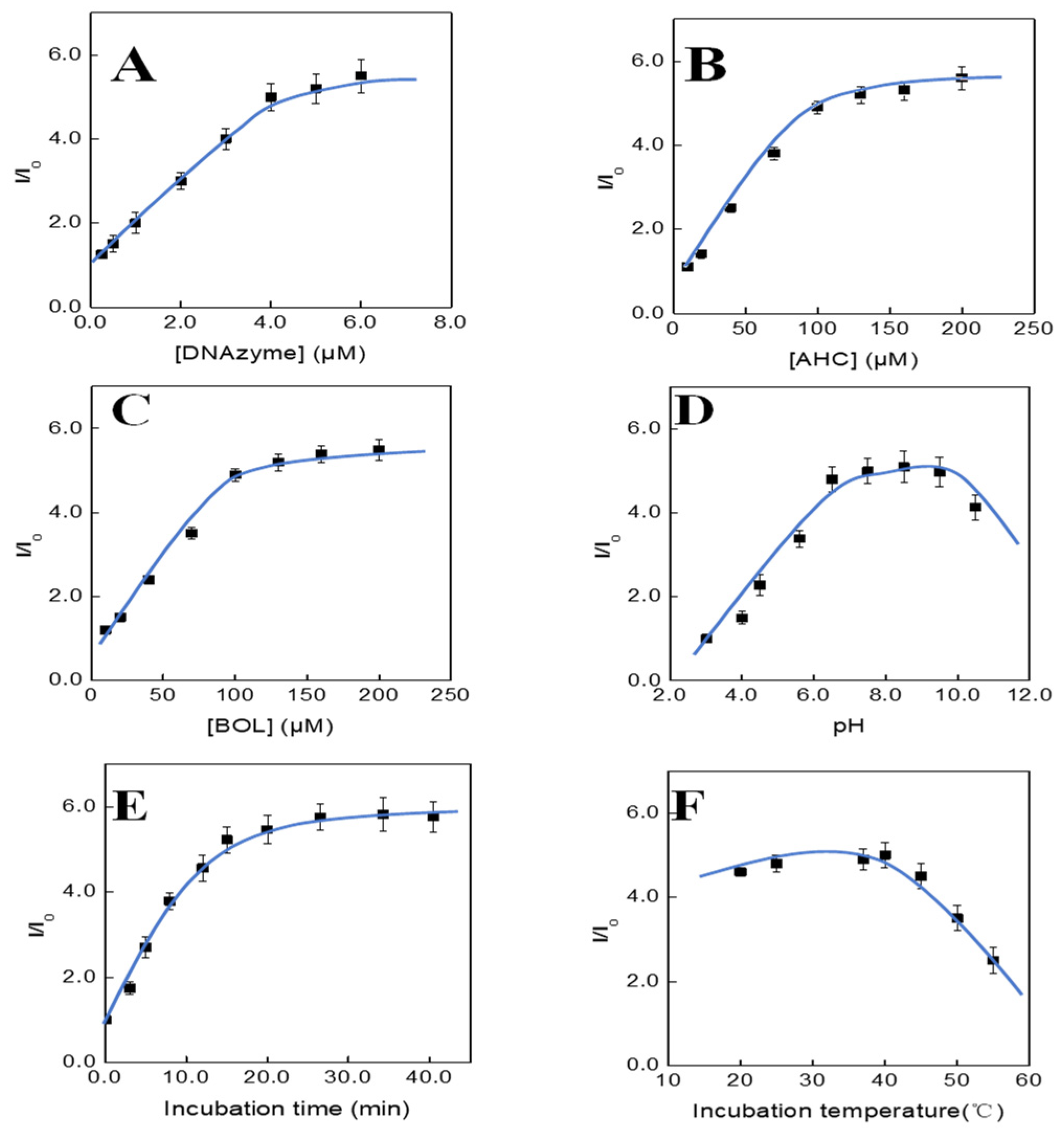
3.3. Optimization of the Assay
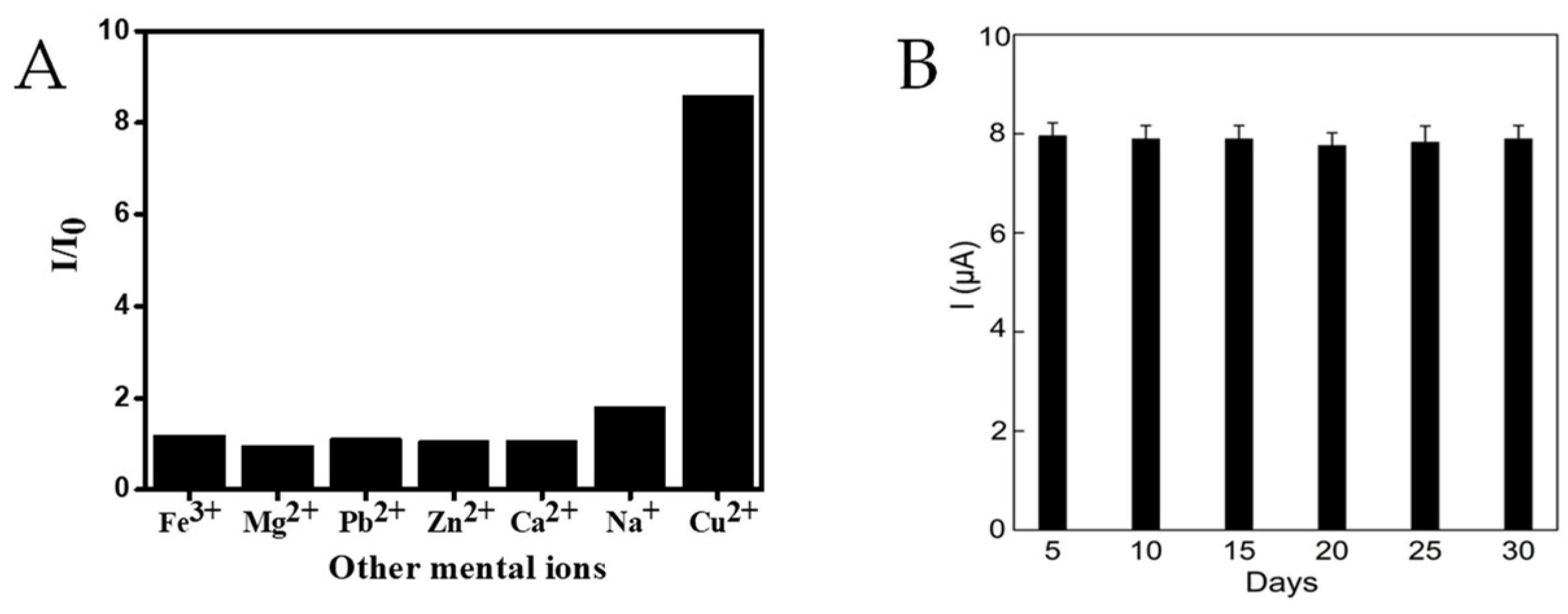
3.4. Reproducibility, Selectivity and Stability of the Assay
3.5. Detection of Copper Ions in Real Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Li, T.; Fang, D.; Li, X.; Fang, L.; Wang, X.; Mao, C.; Wang, F.; Wan, M. Micromotor for removal/detection of blood copper ion. Microchem. J. 2020, 158, 105125. [Google Scholar] [CrossRef]
- Dugandžić, V.; Kupfer, S.; Jahn, M.; Henkel, T.; Weber, K.; Cialla-May, D.; Popp, J. A SERS-based molecular sensor for selective detection and quantification of copper(II) ions. Sens. Actuators B Chem. 2018, 279, 230–237. [Google Scholar] [CrossRef]
- Ge, C.; Luo, Q.; Wang, D.; Zhao, S.; Liang, X.; Yu, L.; Xing, X.; Zeng, L. Colorimetric detection of copper(II) ion using click chemistry and hemin/G-quadruplex horseradish peroxidase-mimicking DNAzyme. Anal. Chem. 2014, 86, 6387–6392. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Feng, J.; Tang, L.; Yu, C.; Mo, G.; Deng, B. A highly efficient introduction system for single cell- ICP-MS and its application to detection of copper in single human red blood cells. Talanta 2020, 206, 120174. [Google Scholar] [CrossRef] [PubMed]
- Baghban, N.; Yilmaz, E.; Soylak, M. Nanodiamond/MoS2 nanorod composite as a novel sorbent for fast and effective vortex-assisted micro solid phase extraction of lead(II) and copper(II) for their flame atomic absorption spectrometric detection. J. Mol. Liq. 2017, 234, 260–267. [Google Scholar] [CrossRef]
- Chedia, B.A.H.; Meriem, B.; Houcine, B.; Nicole, J. Copper(II) Electrochemical Sensor Based on Aluminon as Chelating Ionophore. IEEE Sens. J. 2019, 19, 8605–8611. [Google Scholar]
- Gan, N.; Liu, K.; Qi, L.; Zhang, G.; Guo, Y.; Dipankar, S.; Yu, H.Z. DNAzyme-Catalyzed Click Chemistry for Facilitated Immobilization of Redox Functionalities on Self-Assembled Monolayers. J. Phys. Chem. C 2020, 124, 19083–19090. [Google Scholar] [CrossRef]
- Situ, B.; Zhao, J.; Lv, W.; Liu, J.; Li, H.; Li, B.; Chai, Z.; Cao, N.; Zheng, L. Naked-eye detection of copper(II) ions by a “clickable” fluorescent sensor. Sens. Actuators B Chem. 2017, 240, 560–565. [Google Scholar] [CrossRef]
- Tarnowska, M.; Krawczyk, T. Click chemistry as a tool in biosensing systems for sensitive copper detection. Biosens. Bioelectron. 2020, 169, 112614. [Google Scholar] [CrossRef] [PubMed]
- Jennah, O.; Beniazza, R.; Lozach, C.; Jardel, D.; Molton, F.; Duboc, C.; Buffeteau, T.; Kadib, A.E.; Lastécouères, D.; Lahcini, M.; et al. Photoredox Catalysis at Copper(II) on Chitosan: Application to Photolatent CuAAC. Adv. Synth. Catal. 2018, 360, 4615–4624. [Google Scholar] [CrossRef]
- Zheng, W.; Li, H.; Chen, W.; Zhang, J.; Wang, N.; Guo, X.; Jiang, X. Rapid Detection of Copper in Biological Systems Using Click Chemistry. Small 2018, 14, 1703857. [Google Scholar] [CrossRef]
- Hua, C.; Zhang, W.H.; De Almeida, S.R.; Ciampi, S.; Gloria, D.; Liu, G. A novel route to copper(II) detection using ‘click’ chemistry-induced aggregation of gold nanoparticles. Analyst 2012, 137, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Evgeniy, M.; Israel, A.; Yonatan, H.; Medrano, S.L.; Arezoo, D.; Jörg, B.; Rafael, G.; Gianaurelio, C.; Mattan, H.; Shlomo, Y. Copper Induced Conformational Changes of Tripeptide Monolayer Based Impedimetric Biosensor. Sci. Rep. 2017, 7, 9498. [Google Scholar]
- Zhang, L.; Kwok, H.; Li, X.; Yu, H.Z. Superhydrophobic substrates from off-the-shelf laboratory filter paper: Simplified preparation, patterning, and assay application. ACS Appl. Mater. Interfaces 2017, 9, 39728–39735. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Kumar, L.P.; Zhong, Y.; Dipankar, S. CLICK-T, a DNA enzyme that harnesses ultra-low concentrations of either Cu+ or Cu2+ to catalyze the azide-alkyne ‘click’ reaction in water. Nucleic Acids Res. 2020, 48, 7356–7370. [Google Scholar] [CrossRef] [PubMed]
- Baharfar, M.; Rahbar, M.; Tajik, M.; Liu, G. Engineering strategies for enhancing the performance of electrochemical paper-based analytical devices. Biosens. Bioelectron. 2020, 167, 112506. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Suzuki, K.; Citterio, D. Text-Displaying Colorimetric Paper-Based Analytical Device. ACS Sens. 2017, 2, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, K.S.; Yu, H.-Z. Superhydrophobic Glass Microfiber Filter as Background-Free Substrate for Quantitative Fluorometric Assays. ACS Appl. Mater. Interfaces 2020, 12, 7665–7672. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Alminderej, F.; Ali, I.A. Ultrasensitive Optical Chemosensor for Cu(II) Detection. Int. J. Anal. Chem. 2019, 2019, 7381046. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Yang, Y.; Chen, X.; Hu, X.; Zhang, L.; Liu, L.; Zhao, Y.; Wu, H. Visual detection of copper(II) ions based on an anionic polythiophene derivative using click chemistry. Anal. Chem. 2013, 85, 5650–5653. [Google Scholar] [CrossRef]
- Sanji, T.; Nakamura, M.; Tanaka, M. Fluorescence ‘turn-on’ detection of Cu2+ ions with aggregation-induced emission-active tetraphenylethene based on click chemistry. Tetrahedron Lett. 2011, 52, 3283–3286. [Google Scholar] [CrossRef]
- Shen, Q.; Tang, S.; Li, W.; Zhou, N.; Liu, Z.; Huang, Y.; Yao, S. A novel DNA-templated click chemistry strategy for fluorescent detection of copper(II) ions. Chem. Commun. (Camb. Engl.) 2012, 48, 281–283. [Google Scholar] [CrossRef]
- Qiu, S.; Li, X.; Xiong, W.; Xie, L.; Guo, L.; Lin, Z.; Qiu, B.; Chen, G. A novel fluorescent sensor for mutational p53 DNA sequence detection based on click chemistry. Biosens. Bioelectron. 2013, 41, 403–408. [Google Scholar] [CrossRef]
- Copper Assay Kit; Cat. No. KA1615; Abnova: California, CA, USA, 2019.
- Sun, Y.; Duan, N.; Ma, P.; Liang, Y.; Zhu, X.; Wang, Z. Colorimetric aptasensor based on truncated aptamer and trivalent DNAzyme for Vibrio parahemolyticus determination. J. Agric. Food Chem. 2019, 67, 2313–2320. [Google Scholar] [CrossRef]
| Detection Method | Reaction Time | Linear Range & Detection Limit | Reference |
|---|---|---|---|
| Fluorometric detection in solution using fluorescence spectrometer | 1 h | 0–20 µM, 1.0 µM | [21] |
| Fluorometric detection in solution using fluorescence spectrometer | 2 h | 0.5–10 µM, 0.29 µM | [22] |
| Colorimetric detection in solution using a UV/Vis spectrophotometer | 1 h | 1.8–200 µM, 1.8 µM | [12] |
| Color changes monitored by the naked eye | 40 min | 3 µM (visual detection), 0.8 µM (fluorescence spectrometer) | [20] |
| Fluorometric detection in solution using a fluorescence spectrometer | 30 min | 5.0–50 µM, 2 µM | [22] |
| Colorimetric detection in solution using a UV/vis spectrophotometer | 40 min | 0.5–50 µM, 0.3 µM | [23] |
| Copper and Iron Test Strip Kit (2994) | 4.7–47 μM | [24] | |
| The fluorescence PADs | 20 min | 0.3–150 µM, 0.1 µM | This work |
| Sample | Spiked (µM) | Measured (µM) | Recovery of the Assay (%) | ICP-AES (µM) |
|---|---|---|---|---|
| Fenghua river | 0 | 0.18 ± 0.03 | — | 0.21 ± 0.04 |
| 0.5 | 0.67 ± 0.03 | 94.4 ± 4.5 | 0.78 ± 0.03 | |
| Yuyao river-1 | 0 | ND | — | 0.07 ± 0.02 |
| 0.5 | 0.48 ± 0.02 | 96.0 ± 4.2 | 0.58 ± 0.04 | |
| Yong river-2 | 0 | 2.2 ± 0.07 | — | 1.91 ± 0.08 |
| 2.0 | 3.96 ± 0.05 | 89.0 ± 1.3 | 4.01 ± 0.05 | |
| Ningbo university | 0 | 1.32 ± 0.05 | — | 1.40 ± 0.10 |
| 2.0 | 3.21 ± 0.06 | 91.6 ± 1.9 | 3.41 ± 0.09 | |
| Baixi reservoir | 0 | ND | — | 0.05 ± 0.02 |
| 0.5 | 0.51 ± 0.03 | 102 ± 5.8 | 0.60 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wang, M.; Hong, H.; Lin, J.; Gan, N.; Bi, W. A Novel Truncated DNAzyme Modified Paper Analytical Device for Point-of-Care Test of Copper Ions in Natural Waters. Chemosensors 2022, 10, 72. https://doi.org/10.3390/chemosensors10020072
Wu J, Wang M, Hong H, Lin J, Gan N, Bi W. A Novel Truncated DNAzyme Modified Paper Analytical Device for Point-of-Care Test of Copper Ions in Natural Waters. Chemosensors. 2022; 10(2):72. https://doi.org/10.3390/chemosensors10020072
Chicago/Turabian StyleWu, Jiayi, Ming Wang, Huanhuan Hong, Jianyuan Lin, Ning Gan, and Wenchao Bi. 2022. "A Novel Truncated DNAzyme Modified Paper Analytical Device for Point-of-Care Test of Copper Ions in Natural Waters" Chemosensors 10, no. 2: 72. https://doi.org/10.3390/chemosensors10020072
APA StyleWu, J., Wang, M., Hong, H., Lin, J., Gan, N., & Bi, W. (2022). A Novel Truncated DNAzyme Modified Paper Analytical Device for Point-of-Care Test of Copper Ions in Natural Waters. Chemosensors, 10(2), 72. https://doi.org/10.3390/chemosensors10020072







