Abstract
This paper proposes a novel strategy for low humidity detection, an optical waveguide (OWG) sensor that is locally coated with polyvinylpyrrolidone (PVP) film. The humidity sensor was fabricated using a spin coating on a K+-exchanged glass optical waveguide with PVP film. Its sensing properties were investigated by injecting a humid air range of 10.6~32%RH (relative humidity) at room temperature. The surface morphology of the PVP film was characterized by an atomic force microscope (AFM). The possible humidity sensing mechanism of the proposed sensor was discussed by using absorption spectra. This study showed that the PVP-coated OWG sensor possessed high sensitivity, stability, and rapid response/recovery. Therefore, these observed results demonstrate that the low-cost OWG humidity sensor could be applied in real-time low concentration water vapor monitoring.
1. Introduction
As we know, humidity monitoring is required in many fields, such as industry, pharmaceutical industry, storage, farming, etc. Especially in semiconductor components’ production and storage process, the manufacturing area and storage environment should be low relative humidity (RH). According to IEC 60721-3-1-2018 Classification of environmental conditions—Part 3-1: Classification of groups of environmental parameters and their severities—Storage, the low RH is defined as ≤ 30 RH% [1]. Recently, optical humidity sensors, especially optical fibers [2,3,4,5], have attracted widespread interest due to their intrinsic advantages over conventional capacitive or resistive humidity sensors that are not affected by ambient temperature changes. However, optical fiber is rigid, expensive, and not easy to process. Because of this, it is crucial to develop low humidity sensors with high sensitivity, fast response, low cost, and real-time monitoring.
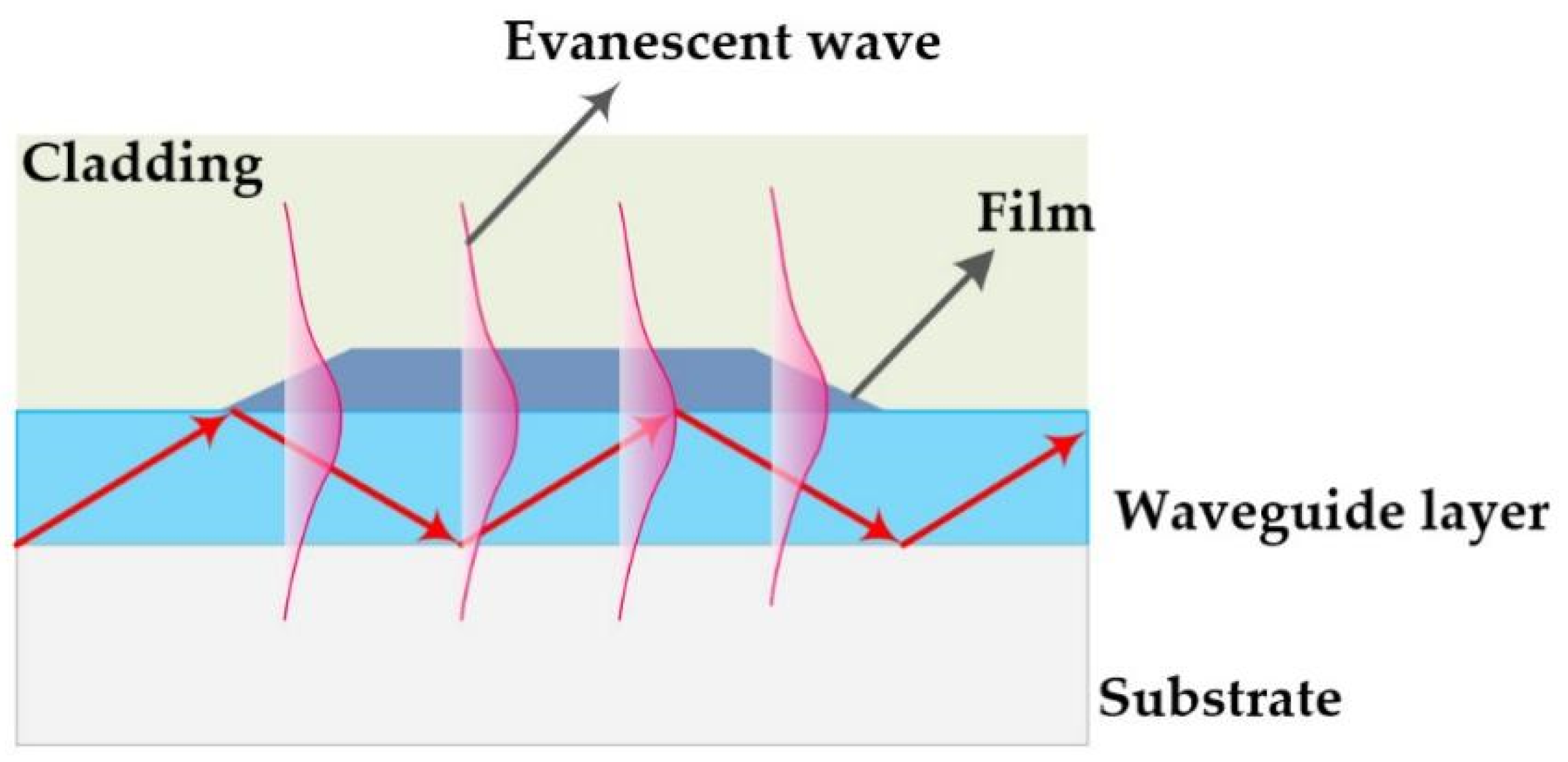
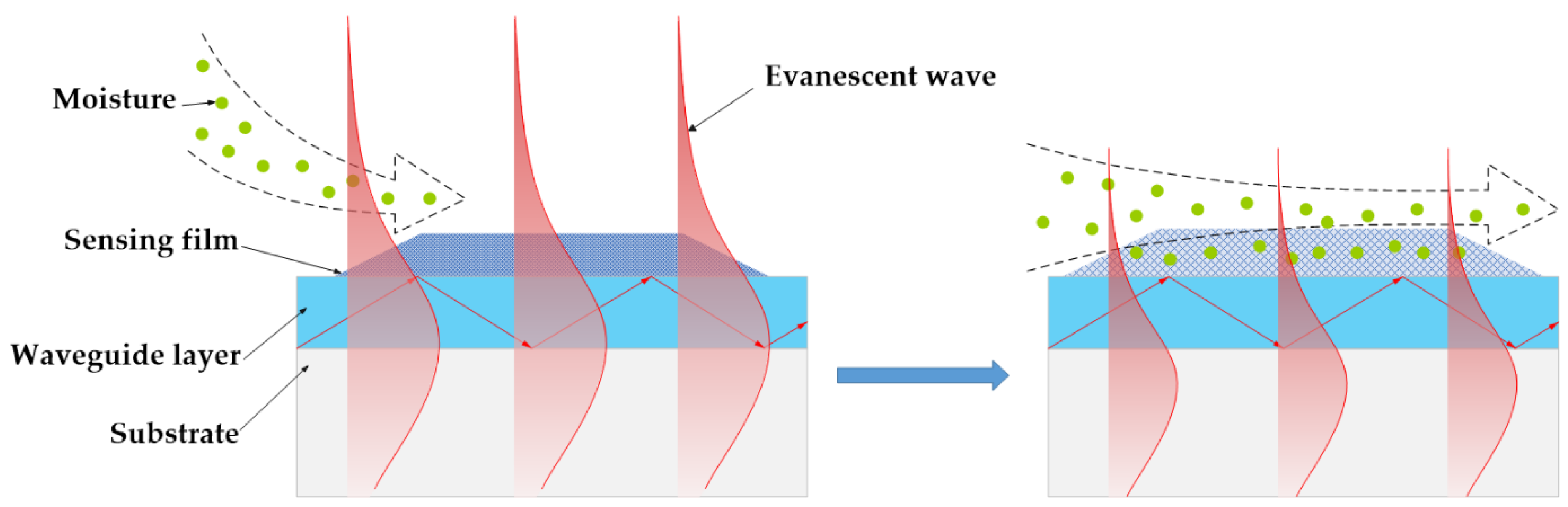
The optical waveguide (OWG) is an optical device and is usually used to develop an optical sensor [6,7,8,9]. The typical OWG sensor is composed of a single-mode planar OWG and a film that is locally overlaid on its surface. The OWG sensor is based on the evanescent wave. In short, when the light was transmitted through in the waveguide layer that possesses a higher refractive index (RI) than that of the substrate, the evanescent wave was excited by the total internal reflection (TIR) on the interface between waveguide layer and substrate [10,11]. A schematic diagram of the OWG and evanescent wave is illustrated in Scheme 1. The evanescent wave could penetrate into cladding or film coated on the surface of the waveguide layer. It is sensitive to variations of film’s optical properties, such as RI, density, absorbance, etc., which could be used as a sensitive probe. As a new optical sensor, OWG sensors possess the advantage of high sensitivity, fast response, compact system, etc., as well as the potential for easy integration [12,13]. OWG sensors have witnessed widespread application in the detecting biological molecules [14], metal ions [15], and particularly low-concentration gas (such as xylene [16,17], chlorobenzene [18], ammonia [19,20], hydrogen chloride [21], simulant of chemical weapon agents [22,23]).
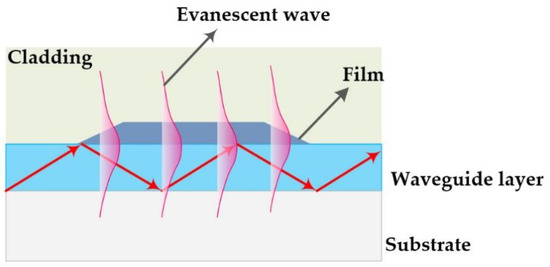
Scheme 1.
Schematic diagram of the OWG and evanescent wave.
As is generally known, certain kinds of polymer, such as hydroxyethylcellulose [24] and polyvinyl alcohol [25], show swelling or changes in RIs when exposed in a humid atmosphere. Optical humidity sensors could be easily fabricated utilizing this effect. Polyvinylpyrrolidone (PVP) possesses excellent solubility and physiological compatibility and is ordinarily used to manufacture various functional membrane materials [26,27]. Meanwhile, excellent hygroscopicity [28,29,30,31] of PVP favors improving its sensitivity to moisture when it exposes in humid vapor.
In this paper, a new OWG sensor for low humidity sensing is reported. As a water-sensitive material that causes a change in RI by attachment of water molecules, PVP was used as steam-sensitive cladding layer and coated on the surface of K+-exchanged glass OWG. The optical properties of PVP before and after interaction with water vapor were studied, and the humidity response performance was investigated. Then, a new method of PVP/K+-exchange glass OWG humidity sensor monitoring of RH with satisfactory sensitivity, real-time low humidity monitoring and simplified operation was established. The fabricated sensor possesses unique features, such as simple configuration and low cost, and these advantages have a few distinguishing features, such as electromagnetic immunity, high integration and stable sensing in real-time low humidity measuring.
2. Materials and Methods
2.1. Reagents and Instruments
Polyvinylpyrrolidone K30 (PVP) was available from Amresco (Solon, OH, USA). Diiodomethane (n = 1.74) was purchased from J&K Scientific Ltd. (Beijing, China). Other chemicals of high purity analytical grade were procured from Beijing Chemical Works (Beijing, China).
Microscope glass slides (S1111, Matsunami Glass Ind., Ltd., Osaka, Japan) with a thickness of 1 mm were adopted to prepare a single-mode planar optical waveguide. K+-exchange glass OWGs were fabricated in an MF-0610F muffle furnace (Huagangtong Technology (Beijing) Co., Ltd., Beijing, China). The thin film was coated on the OWG using a KW-4A spin coater (Institute of Microelectronics of the Chinese Academy of Sciences, Beijing, China). A SOLVER NEXT scanning probe microscope (NT-MDT Co., Moscow, Russia) was utilized to characterize the topographies. A BioMATE 3S UV-Vis spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, U.S.A.) was used to determine absorption spectra. RIs of the PVP aqueous solutions were measured by an Abbe refractometer (2WAJ, Shanghai CSOIF Co., Ltd., Shanghai, China). Humidity was monitored by a hygrothermograph (DT-625, Shenzhen Everbest Machinery Industry Co., Ltd., Shenzhen, China). Samples were introduced by a QC-1S gas sampler (Beijing Municipal Institute of Labour Protection, Beijing, China).
2.2. Preparation of the OWG Humidity Sensing Chip
Normally, the OWG sensing chip was fabricated by coating a sensitive layer onto the surface of waveguide layer. The PVP film/K+-exchanged glass OWG humidity sensing chip was fabricated in the following way.
In this paper, microscope glass slides were used as substrate and immersed into potassium nitrate melt at 400 °C for 40 min, followed by cooling and washing. Finally, K+-exchanged glass OWGs with waveguide layer with a RI of 1.52 were obtained [32].
The PVP thin-film acted as a sensing component was deposited onto the surface of K+-exchanged glass OWGs by a spin coating method. A total of 100 μL 3% PVP ethanol solution was dropped onto the OWG, and the spin coating parameters used for the spin coating of PVP thin film was 3000 rpm for 30 s. The obtained PVP film/K+-exchanged glass OWG sensing chip was then baked for 2 h at 60 °C.
2.3. Construction of OWG Humidity Sensor
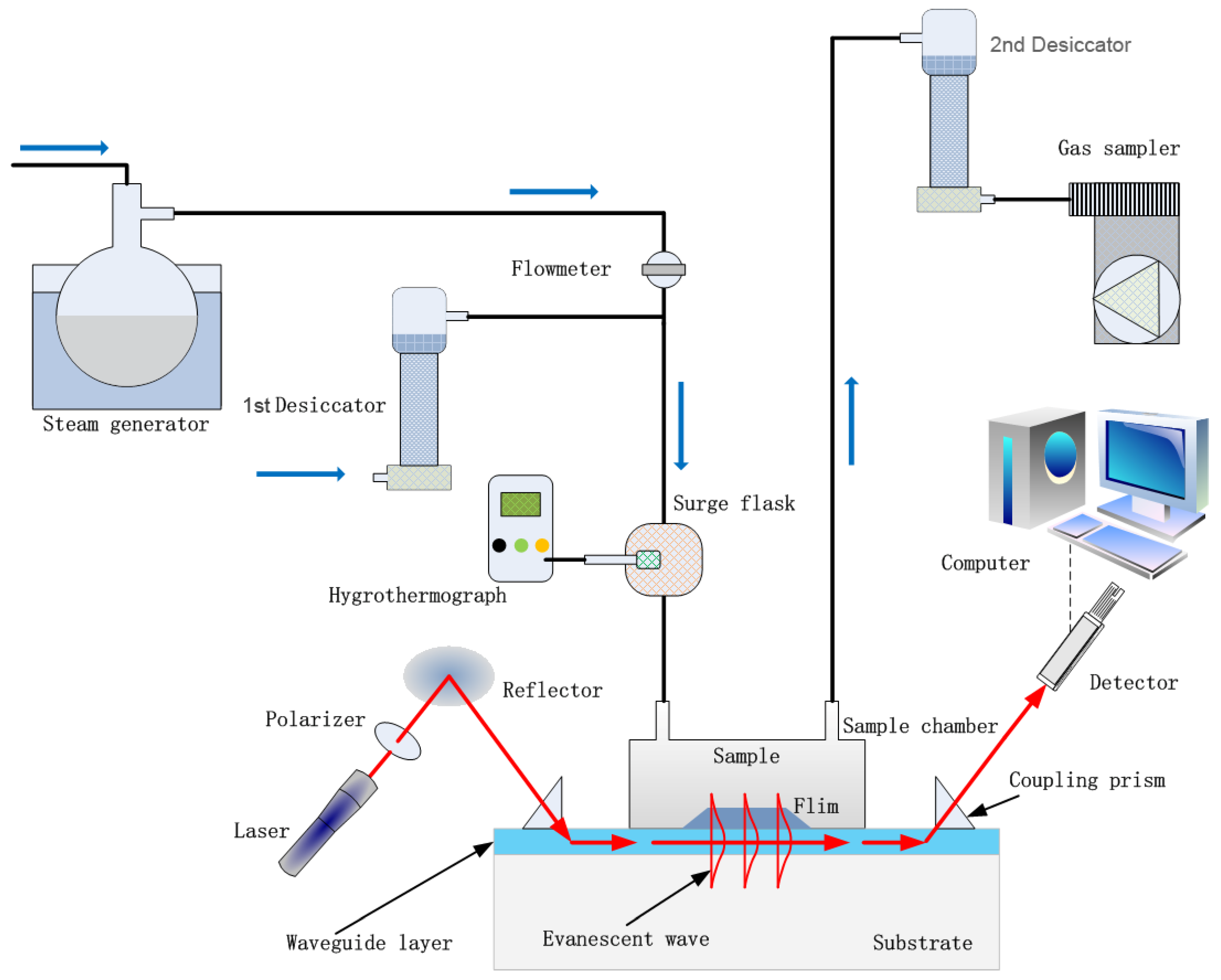
The humidity sensor based on OWG proposed in this work is illustrated in Scheme 2. The humid gas testing apparatus consists of a steam generator, desiccators, surge flask, hygrothermograph, gas sampler, light source, reflector, glass prisms, PVP film/K+-exchanged glass OWG sensing chip, sample chamber, photodetector and computer. A He-Ne laser (633 nm) beam was coupled into and out of the waveguide layer by two glass prisms (n = 1.75) placed at the ends of the sensing chip, and the evanescent wave was excited by total internal reflection. To improve the coupling efficiency, diiodomethane was introduced into the interface of glass prisms and waveguide layer as matching liquid. A sample chamber was used to cover the PVP film tightly to measure the sensing performance of the device. The photodetector received output light signals and then converted them into electric signals.
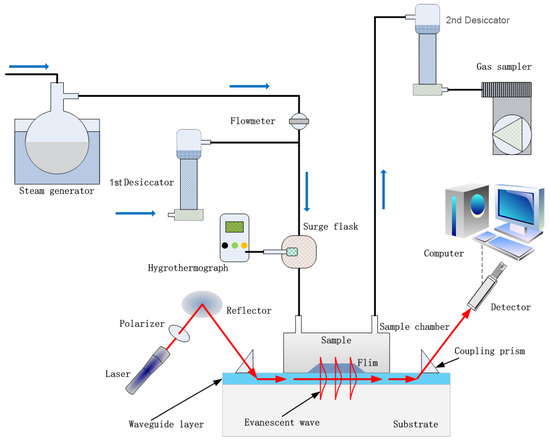
Scheme 2.
Illustration of the OWG humidity sensor detecting system.
2.4. Measurement Procedures
The steam generator consisted of a thermostat and 100 mL flask containing 50 mL water provided multiple steams of varying humidity sustainably by adjusting the heating temperature. The surge flask was used to mix the dry air from the 1st desiccator and humid vapor from the steam generator, and a hygrothermograph monitored the humidity of streams in the surge flask. The inlet and the outlet of the sample chamber were connected to the surge flask and the 2nd desiccator, respectively, and the outlet of the 2nd desiccator was connected to a gas sampler. The pumping rate of the gas sampler was set as 0.5 L/min. Then, the humid gas stream in the surge flask was pumped into the sample chamber under the driving of the gas sampler and interacted with the PVP film, and the humid gas stream was then drawn into the desiccator to dehumidify. Finally, the exhaust gas was discharged through the gas sampler. After measurement, the cut-off valve between the steam generator and surge flask was cut-off, and the sample chamber was afterward purged with dry air to allow the sensor to recover under atmospheric conditions.
3. Results
3.1. Topographical Characteristics
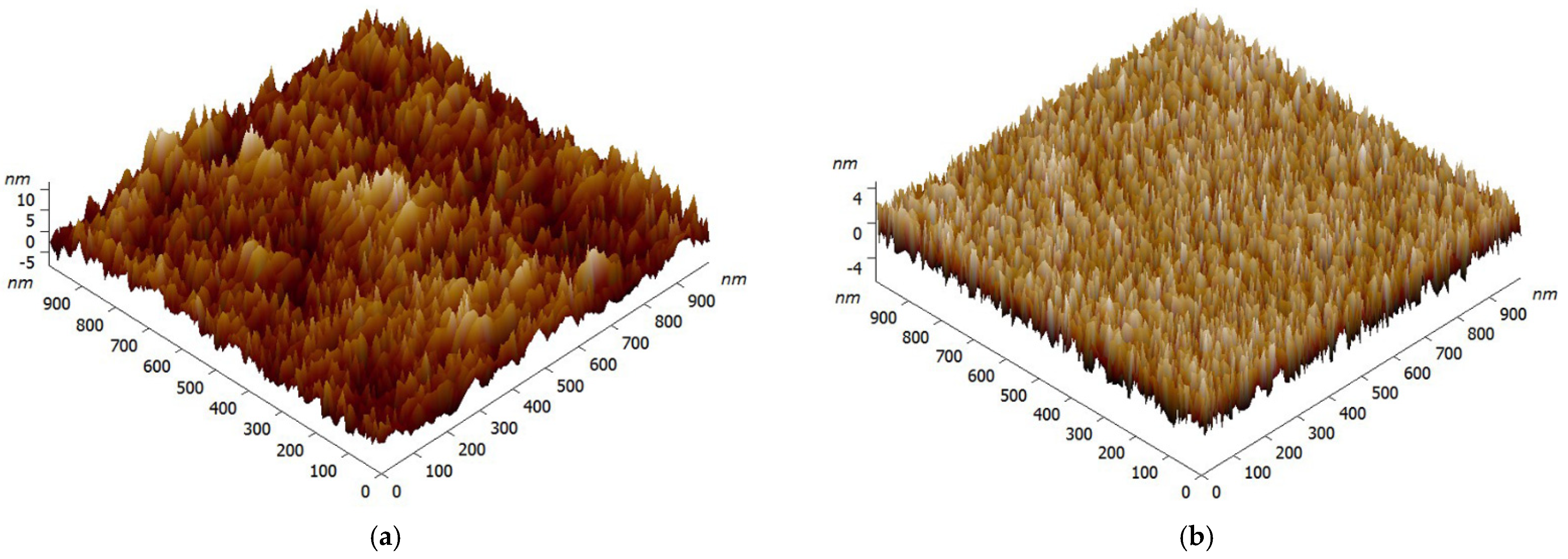
Figure 1 reveals the surface topography of PVP film, and an AFM image of the glass substrate is also displayed as a comparison. Figure 1a clearly shows the rough appearance of the original glass substrate with ruleless particle distribution, and its root-mean-square (RMS) roughness is about 2.78 nm. The characteristic AFM scan of the surface after being coated with PVP in Figure 1b shows an appearance morphologically different from that of the original glass substrate. After the PVP was coated on the glass substrate by spin coating, a dense and relatively smooth film with nearly no particles was formed. Rather than a larger surface roughness, we see a decline in RMS roughness to 1.89 nm after being coated with PVP. It is also important to note that, PVP film with a certain roughness would cause surface scattering loss of the waveguide.
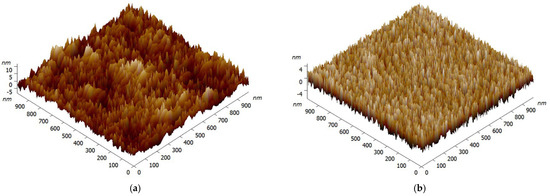
Figure 1.
AFM images (1 μm×1 μm) of the glass substrate (a) and PVP film (b).
The given AFM images indicated that an optical film with excellent topography was successfully acquired by spin coating, and the smooth morphology could reduce the loss of evanescent waves effectively.
3.2. Responses of the Sensor to Humidity
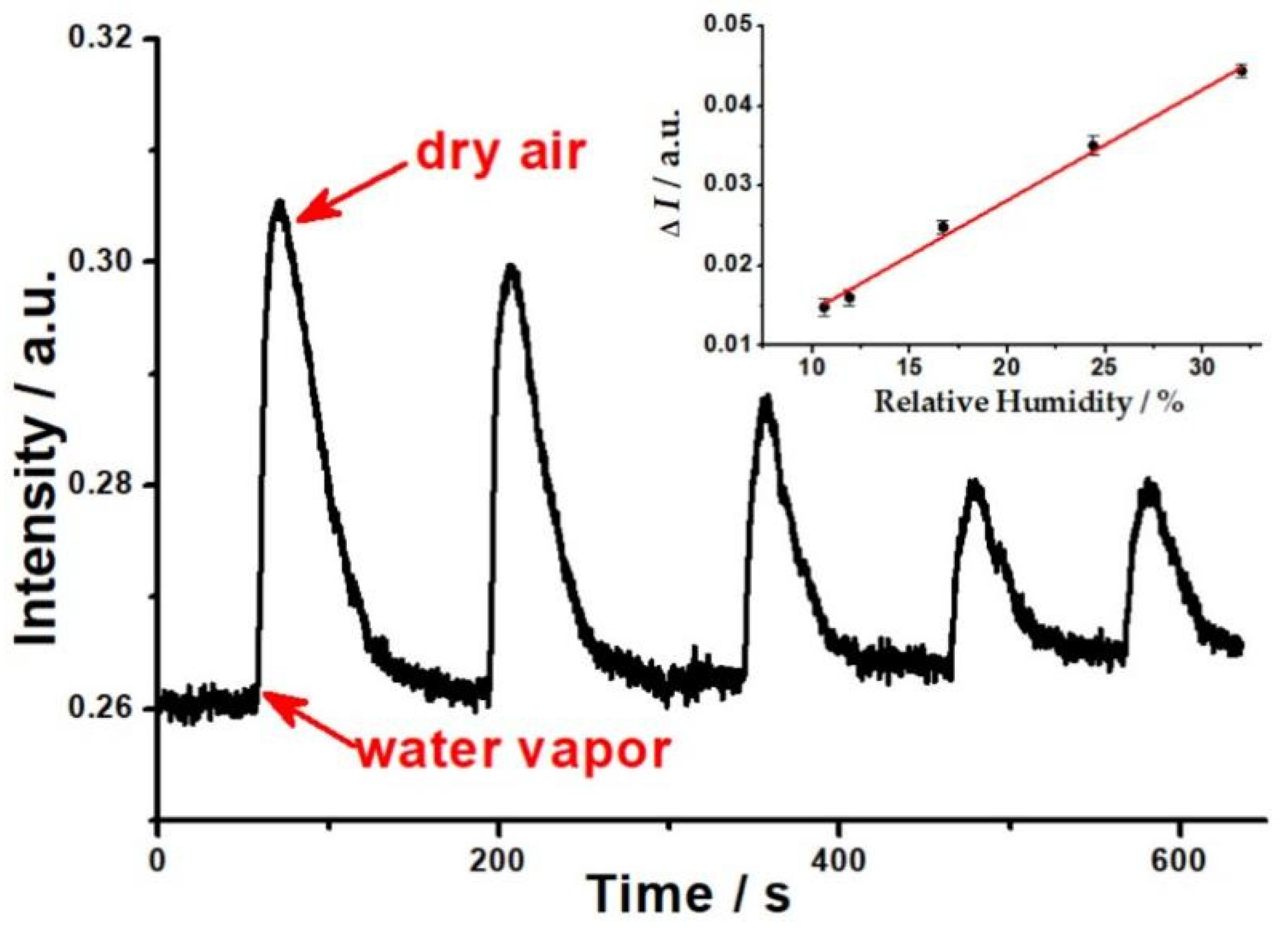
The reversible response of the OWG sensor to different humidity is shown in Figure 2. When the humid gas stream was introduced to the sample chamber, the output light intensity (I) of the OWG sensor increased rapidly with the existence of humid gas streams, and I increased with the rise of the humidity of the sample in the range of 10.6~32%RH at room temperature and decreased when the humidity reduced. Conversely, when the humid gas stream was exhausted from the sample chamber, I basically restored to its original level with dry air. Meanwhile, increasing the humidity contributed to an increase in the recovery time. It can be found that the recovery time of the OWG humidity sensor was longer than its response time, and this phenomenon may be due to more dry air being needed to squeeze out the humid vapor that was bonded on the PVP film.
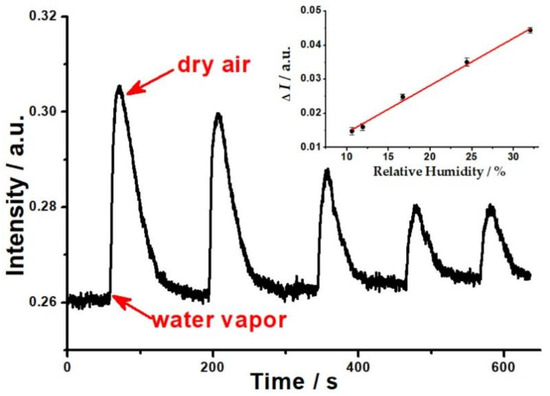
Figure 2.
The response curve of mixed humid gas at different RH (the inserted graph is plot of changes in the output light intensity of the OWG sensor against samples’ RH).
The change of output light intensity (ΔI) was measured for each of RHs, and the calibration curve is plotted in Figure 2. On the ordinate, ΔI = Ihumidity − Idry is plotted, where Idry is the original output light intensity, and Ihumidity is the maximum value of I corresponding to before and after the injection of humid gas streams into the sample chamber, respectively. A strong correlation was observed between the OWG sensor’s response and RH. As can be seen from the data, the change of the OWG sensor’s ΔI was found to be linear to RH of humid gas streams in the range of 10.6~32%RH at room temperature. The following data were obtained:
ΔI = 0.00139[humidity] + 4.74 × 10−4 (R2 = 0.99416, n = 5, p < 0.001)
3.3. Detection Principle
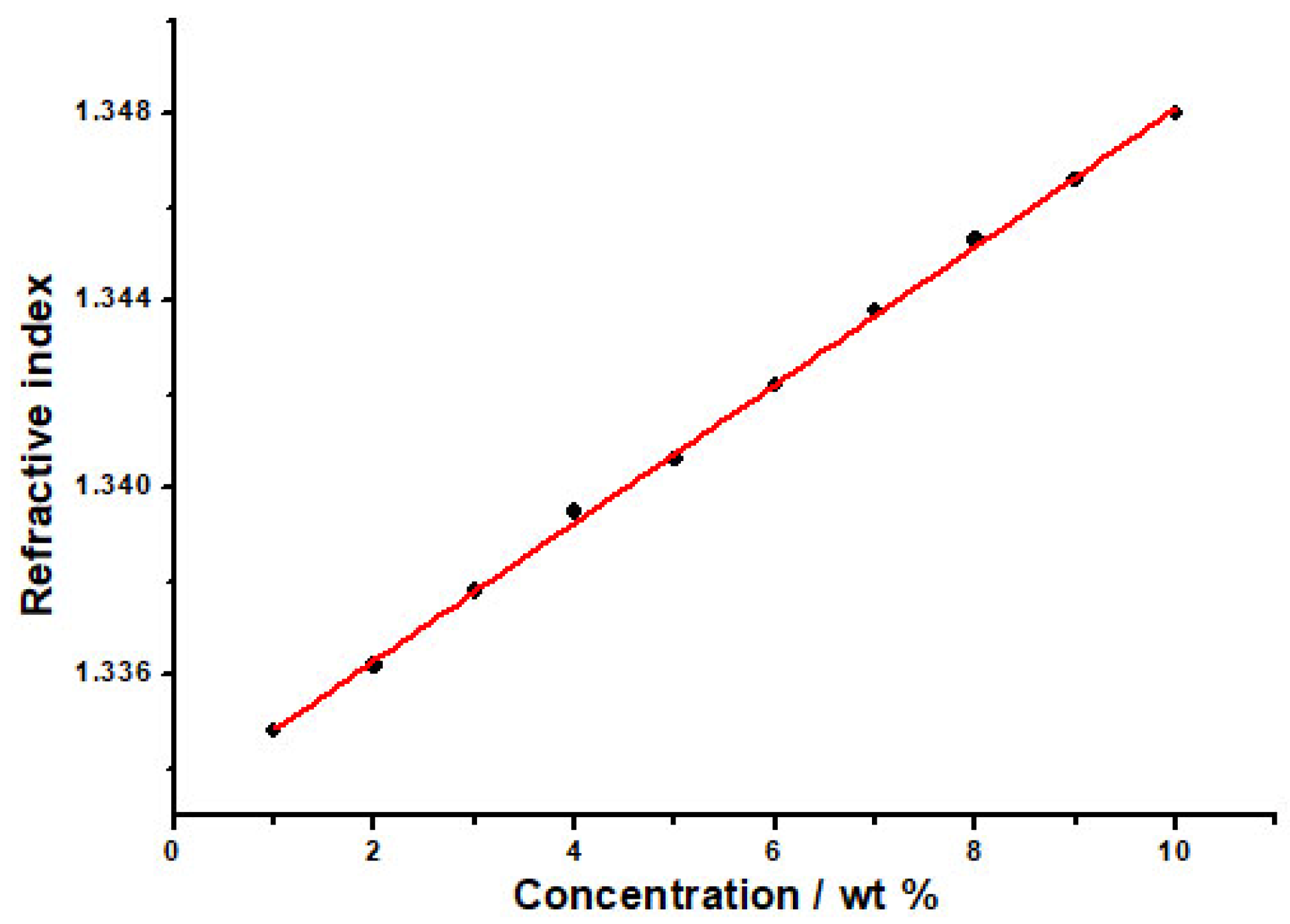
The RIs of PVP aqueous solution at different mass fractions (wt%) were determined, and the RIs were plotted versus the mass fraction of the PVP aqueous solution. As the mass fractions of PVP in the aqueous solution decreased, the RI declined (Figure 3). According to this characteristic, the PVP-sensitive film absorbed water or was dehydrated when the ambient humidity changed, and its mass fraction would change to cause the RI change. Therefore, PVP can be used as a moisture-sensitive material for the OWG humidity sensor.
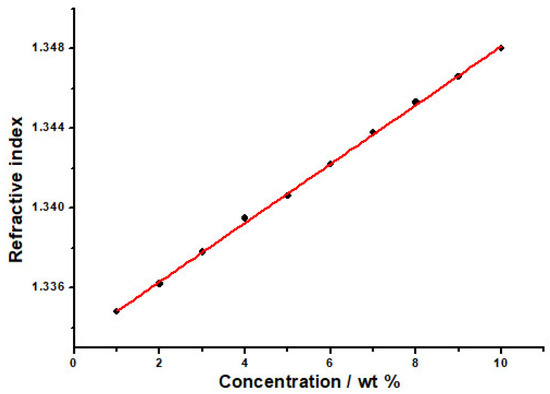
Figure 3.
The relationship between the RI of PVP aqueous solution and mass fractions (wt%).
An evanescent wave was generated as the 633 nm laser beam was TIR transmitted in the waveguide layer. When a thin film is coated on the surface of the waveguide layer, the evanescent wave could also come into the film. For the OWG humidity sensor, a minor change of PVP sensing film’s optical properties caused by exposure to moisture will affect the output optical signal.
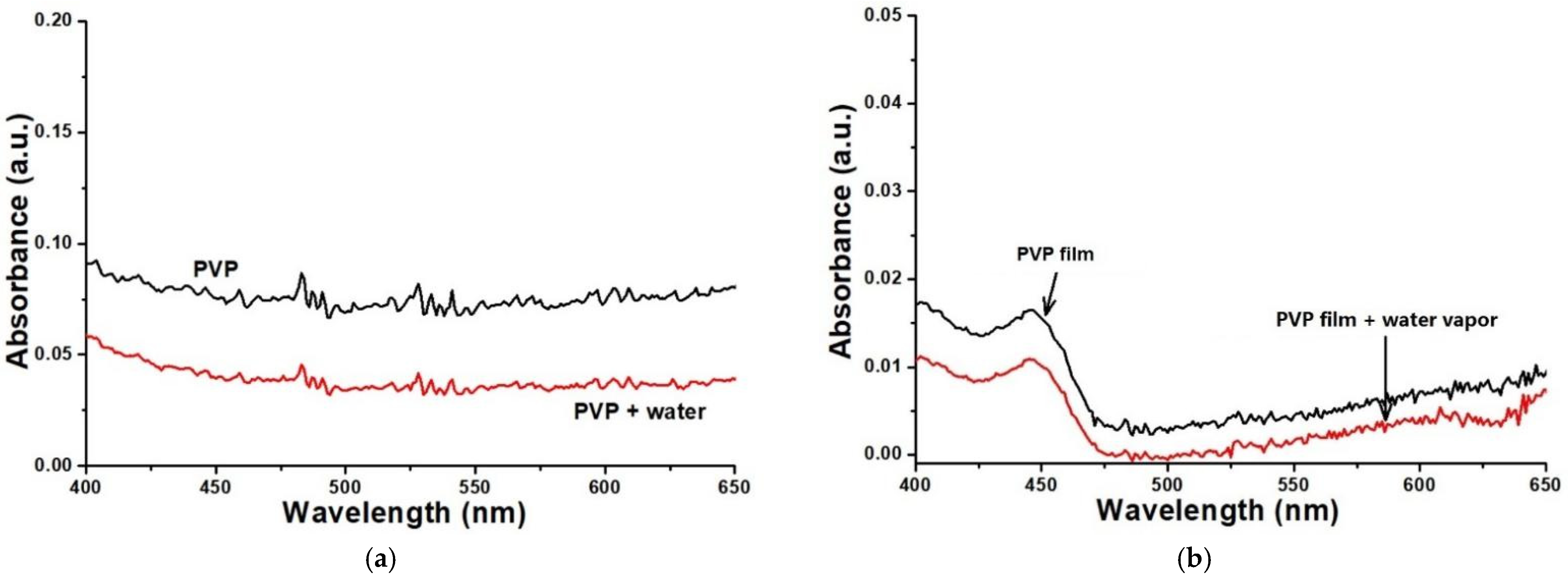
In this work, the absorption spectra of PVP solution before and after adding water were measured meanwhile the absorption spectra of PVP film coated on the surface of K+ exchange glass OWG before and after being put into water vapor were acquired. From Figure 4a, when 10% water was dropped into PVP ethanol solution, the absorbance of the solution decreased. Figure 4b shows the change of UV-vis spectra of PVP film before and after the action of specific water vapor (the slides coated with PVP film were cut into 1×1 cm and placed in a flask with a specific humidity of water vapor, then its absorption spectra were determined). It can be seen that, after the PVP film reacted with water vapor, its absorbance at 633 nm was reduced obviously, which is similar to that of the PVP in ethanol solution.
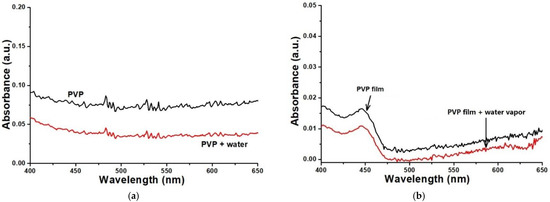
Figure 4.
Variation of PVP ethanol solution (a) and PVP film (b) absorption spectra.
The relationship between absorbance and RI of the thin film can be explained by the following equation:
where A is the absorbance of PVP film, n1 and n2 are the RI of the PVP film and substrate, respectively. According to Equation (1), a decreased RI of the PVP film can result in a decreased A of the film. Therefore, the decreased A of the PVP film observed in Figure 4b indicates that the water vapor exposure causes the n1 to reduce.
According to waveguide theory, the power fraction of the evanescent wave in the PVP film increases with rise of n1. On the other hand, an enhanced evanescent field would result in an increased surface-scattering loss of the OWG. With this reason the OWG sensor response to humidity can be easily understood: the water vapor exposure of the PVP film cause the n1 to reduce, which in turn decreased the evanescent power fraction in the film (see Scheme 3), consequently resulting in an increases in the output light intensity of the OWG sensor.
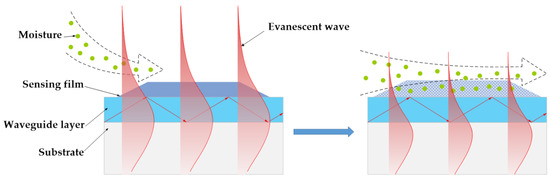
Scheme 3.
Schematic illustration of OWG sensor for water vapor monitoring.
3.4. Reproducibility of the OWG Sensor
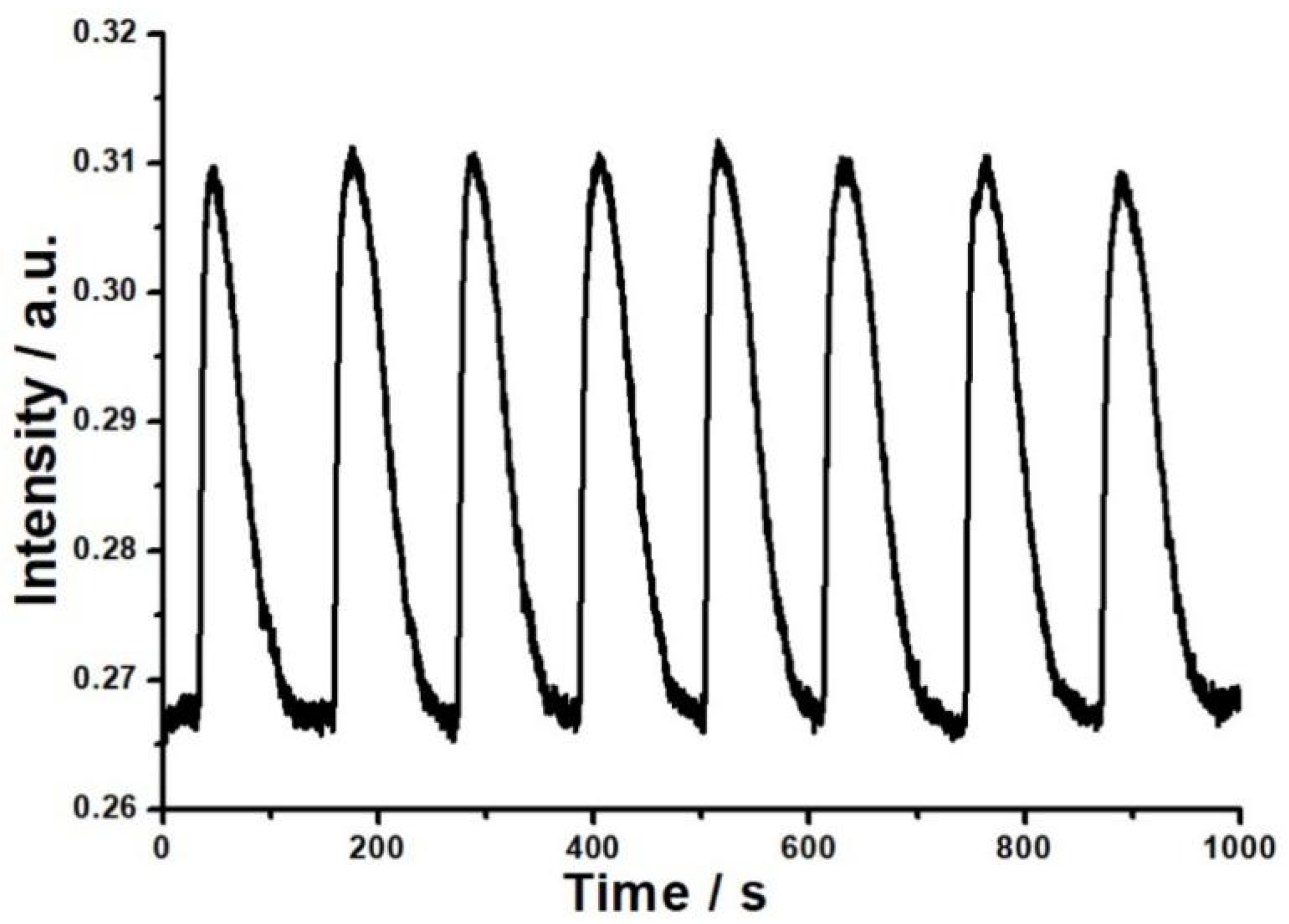
The reversibility, stability and sensitivity of the PVP film/K+ exchange glass OWG humidity sensor were investigated. In this work, dry air and humid gas stream with a humidity of 32%RH were alternately introduced into the sample chamber for eight cycles. As shown in Figure 5, when humid vapor was injected, the output light intensity increased rapidly. Afterward, dry air was injected to replace humid vapor, leading to the output light intensity falling back to the original level. More importantly, the ΔI determined in each cycle were basically the same, and each humidity measuring cycle was reversible. Meanwhile, good reversibility shows that the bond between PVP film and water vapor was physical and relatively weak. This demonstrated that the OWG sensor had a reversible and repetitive response to humid air with a fast response time and its response and recovery time were 15 s and 100 s, respectively. At this humidity, the relative standard deviation of ΔI was 1.803%.
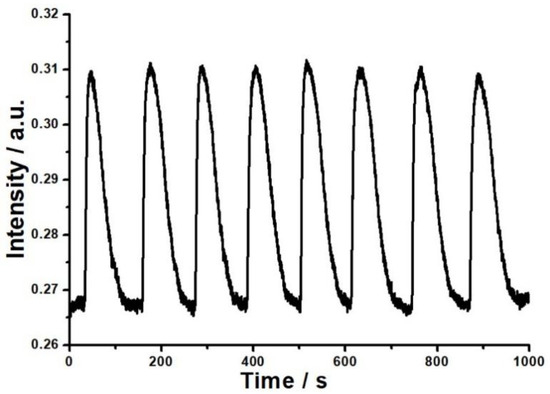
Figure 5.
The reversibility of the OWG sensor to 32%RH water vapor.
4. Discussion
To summarize, a sensitivity humidity sensor based on OWG with PVP film coating with good repeatability and stability was demonstrated. PVP was coated on the surface of the OWG’s waveguide layer as the sensitive film due to its moisture sensitivity to different RH levels. The novel OWG sensor was extremely inexpensive and yet is sensitive, particularly easy to manufacture and operate. The humidity sensing properties of the OWG sensor were investigated by exposing it to a humidity range of 10.6~32%RH at room temperature. This should be compared with humidity sensors reported in previous works [33,34], which could detect as low as 18%RH and 40%RH water vapor, and the detection limit of the OWG humidity sensor is much lower. In contrast with Ni/SiNWs, nanocomposite-based capacitive humidity sensors that use electroplating methods to prepare sensitive film [35], the OWG humidity sensor possesses a similar detection limit and a simpler preparation procedure. This study presents that the fabricated OWG humidity sensor has a reversible and repetitive response to humid air. Its response/recovery rates were quick. This OWG humidity sensor is a promising new method for monitoring low concentration water vapor. Future research is underway, and the main work is focused on the modification of moisture-sensitive film materials and the improvement of fabrication, which leads to the humidity measurement range to be expended, and its response/recovery time to be reduced. Finally, it should be noted that, by using other sensitive materials as the cladding thin film of the OWG, such a sensor could also be designed for determining chemical gas, biological molecule, temperature, etc.
Author Contributions
Conceptualization, investigation, supervision, and writing—original draft, B.D.; funding acquisition, X.M. and S.F.; formal analysis, S.L. and Z.L.; supervision, L.G. and J.X.; project administration, Z.T.; methodology, Z.-M.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation of State Key Laboratory of NBC Protection for Civilian (SKLNBC2020-03, SKLNBC2020-07).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IEC 60721-3-1-2018; Classification of Environmental Conditions—Part 3-1: Classification of Groups of Environmental Parameters and Their Severities—Storage. International Electrotechnical Commission: Geneva, Switzerland, 2018.
- Miao, Y.; Liu, B.; Zhang, H.; Li, Y.; Zhou, H.; Sun, H.; Zhang, W.; Zhao, Q. Relative humidity sensor based on tilted fiber Bragg grating with polyvinyl alcohol coating. IEEE Photonics Technol. Lett. 2009, 21, 441–443. [Google Scholar] [CrossRef]
- Woyessa, G.; Nielsen, K.; Stefani, A.; Markos, C.; Bang, O. Temperature insensitive hysteresis free highly sensitive polymer optical fiber Bragg grating humidity sensor. Opt. Express 2016, 24, 1206–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, F.U.; Morgan, S.P.; Hayes-Gill, B.R.; Harvey, D.; Kinnear, W.; Norris, A.; Evans, D.; Hrdman, J.G.; Korposh, S. Characterisation and use of a fiber optic sensor based on PAH/SiO2 film for humidity sensing in ventilator care equipment. IEEE Trans. Biomed. Eng. 2016, 63, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Dong, X.; Chan, C.C.; Ni, K.; Zhang, S.; Shum, P.P. Humidity sensor with a PVA-coated photonic crystal fiber interferometer. IEEE Sensors J. 2013, 13, 2214–2216. [Google Scholar] [CrossRef]
- Koxmak, S.; Yimamumaimaiti, T.; Abdukeremu, H.; Nizamidin, P.; Yimit, A. Detection of amines in lamb spoilage by optical waveguide sensor based on bromophenol blue-silicon composite film. Chem. Res. Chin. Univ. 2019, 35, 193–199. [Google Scholar] [CrossRef]
- Zhu, M.; Kari, N.; Yan, Y.; Yimit, A. The fabrication and gas sensing application of a fast-responding m-CP-PVP composite film/potassium ion-exchanged glass optical waveguide. Anal. Methods 2017, 9, 5494–5501. [Google Scholar] [CrossRef]
- Nizamidin, P.; Yimit, A.; Turdi, G.; Chen, Z.J.; Zhang, F.; Kutilike, B. Fabrication and characterization of photo-responsive metal–organic framework membrane for gas sensing using planar optical waveguide sensor. Anal. Chim. Acta 2021, 1158, 338385. [Google Scholar] [CrossRef]
- Liu, L.h.; Zhou, X.h.; Xu, W.q.; Song, B.d.; Shi, H.c. Highly sensitive detection of sulfadimidine in water and dairy products by means of an evanescent wave optical biosensor. RSC Adv. 2014, 4, 60227–60233. [Google Scholar] [CrossRef]
- Bradshaw, J.T.; Mendes, S.B.; Saavedra, S.S. Planar integrated optical waveguide spectroscopy. Anal. Chem. 2005, 77, 28A–36A. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Wu, J.; Wang, Q.; Xu, J.; Fang, S.; Chen, J.; Duan, M. Optical sensor for fluoride determination in tea sample based on evanescent-wave interaction and fiber-optic integration. Talanta 2017, 174, 372–379. [Google Scholar] [CrossRef]
- Adányi, N.; Majer-Baranyi, K.; Nagy, A.; Németh, G.; Szendrő, I.; Székács, A. Optical waveguide lightmode spectroscopy immunosensor for detection of carp vitellogenin. Sens. Actuators B 2013, 176, 932–939. [Google Scholar] [CrossRef]
- Xu, J.; Suarez, D.; Gottfried, D.S. Detection of avian influenza virus using an interferometric biosensor. Anal. Bioanal. Chem. 2007, 389, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Majer-Baranyi, K.; Zalán, Z.; Mörtl, M.; Juracsek, J.; Szendrő, I.; Székács, A.; Adányi, N. Optical waveguide lightmode spectroscopy technique-based immunosensor development for aflatoxin B1 determination in spice paprika samples. Food Chem. 2016, 211, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.f.; Li, J.; Qi, Z.m. Nonspecific detection of lead ions in water using a simple integrated optical polarimetric interferometer. J. Appl. Phys. 2013, 113, 213109. [Google Scholar] [CrossRef]
- Mohemaiti, M.; Keram, A.; Nezamidin, P.; Yimit, A. Preparation of zinc oxide thin film/tin-diffused optical waveguide sensor and gas-sensing detection. Acta Chim. Sin. 2011, 69, 1840–1844. [Google Scholar]
- Nizamidin, P.; Yimit, A.; Wang, J.D.; Itoh, K. Optical properties and sensing applications of lithium iron phosphate thin films. Thin Solid Films 2012, 520, 6250–6255. [Google Scholar] [CrossRef]
- Abdurahman, R.; Yimit, A.; Ablat, H.; Mahmut, M.; Wang, J.D.; Itoh, K. Optical waveguide sensor of volatile organic compounds based on PTA thin film. Anal. Chim. Acta 2010, 658, 63–67. [Google Scholar] [CrossRef]
- Nizamidin, P.; Yimit, A.; Yan, Y.; Kutilike, B.; Kari, N.; Mamtimin, G. Fast fabrication and gas-sensing characteristics of petal-like Co-MOF membrane optical waveguide. Sens. Actuators B 2021, 346, 130342. [Google Scholar] [CrossRef]
- Gao, L.; Yang, X.; Shu, Y.; Chen, X.; Wang, J. Ionic liquid-based slab optical waveguide sensor for the detection of ammonia in human breath. J. Colloid Interface Sci. 2018, 512, 819–825. [Google Scholar] [CrossRef]
- Rahman, E.; Kerim, A.; Yasin, P.; Nizamidin, P.; Abdurahman, A.; Yimit, A. MB-stearic acid composite film optical waveguide sensor for the detection of HCl gas. Chem. J. Chin. Univ. 2012, 33, 2173–2177. [Google Scholar]
- Du, B.; Tong, Z.; Mu, X.; Liu, S.; Xu, J.; Liu, Z.; Qi, Z.M.; Ding, Z. Detection of diethyl chlorophosphate using a composite optical waveguide sensor. Anal. Methods 2019, 11, 1208–1213. [Google Scholar] [CrossRef]
- Du, B.; Tong, Z.; Mu, X.; Xu, J.; Liu, S.; Liu, Z.; Cao, W.; Qi, Z.M. A potassium ion-exchanged glass optical waveguide sensor locally coated with a crystal violet-SiO2 gel film for real-time detection of organophosphorus pesticides simulant. Sensors 2019, 19, 4219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muto, S.; Suzuki, O.; Amano, T.; Morisawa, M. A plastic optical fibre sensor for real-time humidity monitoring. Meas. Sci. Technol. 2003, 14, 746–750. [Google Scholar] [CrossRef]
- Ueda, H.; Aikawa, S.; Kashima, Y.; Kikuchi, J.; Ida, Y.; Tanino, T.; Kadota, K.; Tozuka, Y. Anti-plasticizing effect of amorphous indomethacin induced by specific intermolecular interactions with PVA copolymer. J. Pharm. Sci. 2014, 103, 2829–2838. [Google Scholar] [CrossRef]
- Lv, J.; Gu, W.; Cui, X.; Dai, S.; Zhang, B.; Ji, G. Nanofiber network with adjustable nanostructure controlled by PVP content for an excellent microwave absorption. Sci. Rep. 2019, 9, 4271. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhuang, X.; Zhou, K.; Cai, C.; Hu, Z.; Zhang, J.; Zhu, Y. Amorphous polymer with C=O to improve the performance of perovskite solar cells. J. Mater. Chem. C 2017, 5, 9037–9043. [Google Scholar] [CrossRef]
- Rumondor, C.F.; Stanford, L.A.; Taylor, L.S. Effects of polymer type and storage relative humidity on the kinetics of felodipine crystallization from amorphous solid dispersions. Pharm. Res. 2009, 26, 2599–2606. [Google Scholar] [CrossRef]
- Konno, H.; Taylor, L.S. Ability of different polymers to inhibit the crystallization of amorphous felodipine in the presence of moisture. Pharm. Res. 2008, 25, 969–978. [Google Scholar] [CrossRef]
- Shamblin, S.L.; Zografi, G. Effects of absorbed water on the properties of amorphous mixtures containing sucrose. Pharm. Res. 1999, 16, 1119–1124. [Google Scholar] [CrossRef]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-vinylpyrrolidone: Synthesis, characterization and uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Matsuda, N.; Santos, J.; Itoh, K.; Takatsu, A.; Kato, K. A Study of Molecular adsorption of bromothymol blue by optical waveguide spectroscopy. Langmuir 2003, 19, 214–217. [Google Scholar] [CrossRef]
- Chung, V.P.J.; Yip, M.C.; Fang, W. Resorcinol–formaldehyde aerogels for CMOS-MEMS capacitive humidity sensor. Sens. Actuators B 2015, 214, 181–188. [Google Scholar] [CrossRef]
- Yang, M.Z.; Dai, C.L.; Wu, C.C. Sol–gel zinc oxide humidity sensors integrated with a ring oscillator circuit on-a-chip. Sensors 2014, 14, 20360–20371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, B.R.; Zhang, J.; Miao, F.J.; Li, H.L.; Wan, L.J.; Wang, Y.T. Capacitive humidity sensors based on Ni/SiNWs nanocomposites. Sens. Actuators B 2009, 136, 144–150. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).