Abstract
Plastic particles smaller than 5 mm accumulate in aqueous, terrestrial, and atmospheric environments and their discovery has been a serious concern when it comes to eco-toxicology and human health risk assessment. In the following review, the potential of mass spectrometry (MS) for the detection of microplastic (MP) pollutants has been elaborately reviewed. The use of various mass spectrometric techniques ranging from gas chromatography–mass spectrometry (GC-MS), liquid chromatographic mass spectrometric (LC-MS) to matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), including their variants, have been reviewed. The lapses in the detection system have been addressed and future recommendations proposed. The challenges facing microplastics and their detection have been discussed and future directions, including mitigation methods, have been presented.
1. Introduction
Plastics, synthetic polymers, and their associates are synthesized from petrochemical-based raw materials, by covalent linking of their monomers. Plastics have become a part of our lives, owing to their outstanding and versatile properties, which include lightweight, flexibility, high stability, durability and inexpensive large-scale production. The ease in its procurement enables its widespread use, leading to its release into the environment. At present, it is more of a bane than a boon to the environment. Plastics are ringing alarm bells, posing a high-level threat to the environment and to the entire biodiversity as such. Plastics are now well-reputed for their poor biodegradability and this non-degradable material has massively piled up, becoming a serious concern [1]. A predominant portion of the annual global plastic production, which approximately accounts for 58% of wastes, pollutes the environment, as landfill dumps. Only 18% and 24% of plastic wastes are recycled and incinerated, respectively [2]. The land-fill plastic wastes undergo a series of degradations over a period of time via natural processes such as physical and chemical reactions (abiotic) and biological processes through microbial (biotic) enzyme-mediated reactions. Through these processes, the complex plastic polymers are reduced to micro- and nano-sized particles, which still tend to retain their toxicity or sometimes become even more toxic compared to their bulk counterparts. Microparticles are small sized particles that are less than 5 mm in size and when the sizes are less than 100 nm, they are categorized as nanoplastic particles. Their sizes favor their high environmental persistence, as well as easy transportation into freshwater and marine environments through atmospheric deposition, surface run-off, sewer overflows and industrial effluents as degraded plastic waste [3,4,5]. Figure 1 depicts the various sources of microplastics in the environment.

Figure 1.
Sources of microplastics.
Due to their smaller size and large surface area, micro- and nanoplastic particles adsorb a wide variety of contaminants such as heavy metals and toxicants from chemical and pharmaceutical industries; besides these, flame retardants and other plasticizers further aggravate its toxic impacts. Although the effect of micro-/nanoplastics was studied with many animal models [6], there are not many studies concerning the effect of such particles on human beings. However, the micro-/nanoplastic effects have been studied on human cell cultures. Kik et al. (2021) [7] studied the effect of polystyrene nanoparticles on human peripheral blood mononuclear cells that exhibited increased ROS levels, lipid and protein oxidation, and decreased PBMCs viability. Yet, another study by Wu et al. (2019) [8] described the effect of nano- and microplastic particles on Caco-2 (human epithelial colorectal adenocarcinoma cell line) and they observed that MPs did greatly impact cell viability; however, the particles disrupted the mitochondrial membrane and inhibited the ATP-binding cassette (ABC) transporter activity of the plasma membrane. Furthermore, in a recent study, plastic particles (~20 µm and 25–200 µm) were tested against human dermal fibroblasts, peripheral blood mononuclear cells (PBMCs), HMC-1 (human mast cell line 1), and RBL-2H3 (human basophilic leukemia cell lines). These results pointed out to marginal ROS induction and cytotoxicity at high dosages [9]; the particles were observed to trigger an elevated production of histamine in HMC-1 and RBL-2H3. In the case of PBMCs, a low induction of proinflammatory cytokines IL-6 and TNF-α was evident.
Microplastic detection is a complicated process, since what gets detected in the beginning of the degradation process, to what gets detected as degraded products differs largely. Understanding the stages in the degradation pathway is crucial, since that is what can help detect microplastics or their degraded products in a particular environment. This will provide necessary information on the degree of macro-/micro-/nanoplastic toxicity. This will also be helpful for devising comprehensive strategies for the degradation of primary and secondary products resulting from plastic degradation, enabling the detection and identification of primary and secondary products resulting from the biotic and abiotic degradation of plastics. The simplest way for qualitatively assessing plastic/microplastic degradation is through microscopic observation. PET biodegradation was observed under light and scanning electron microscope, and in some instances, AFM was used to access the surface morphological changes [10,11]. Further, the degradation process was also measured through measuring changes in their mass [12]. The extent of plastic degradation was also measured as the wettability of the plastic surfaces through contact angle measurement. Measuring the polymeric strength of the plastics undergoing degradation by dynamic mechanical analysis (DMA) and thermal analysis are also in practice. CO2 evolution is the gold-standard method used to measure the microbial biodegradation of plastic polymers. The degree of polymers released as a result of the degradation reaction has been studied using methods such as FT IR and NMR. Figure 2 gives an overview of the different analytical methods reported for use as microplastic detectors.
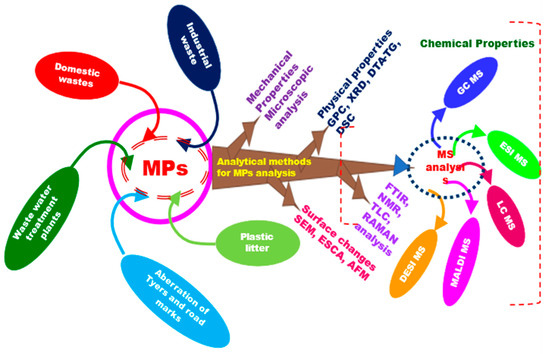
Figure 2.
Overview of analytical methods that contribute to MPs detection in the environment.
Mass spectrometry is a highly sophisticated technology used for molecular detection, identification of the structure and chemical properties of a material, and for its quantification. MS techniques have revolutionized chemistry, biochemistry, pharmacy, medicine, and many related fields of science. MS is also employed for the direct identification of plant and human diseases [13,14]. In addition, it is used as a rapid and simple tool in environmental, forensic, and drug quality control applications. MS has also successfully provided new chemical and physical insights into research concerning extra-terrestrial planetary bodies in the solar system [15].
The risk of microplastics to our natural environment, and to public health, needs to be measured in order to manage it appropriately. This requires quantification, optimization, and standardization, using highly reproducible techniques. Standardized methodologies for non-destructive collection, handling, separation, sample preparation, and the positive identification of microplastics are needed. ASTM International has published standards for collection (D8332) and preparation (D8333) of MPS. Some of the leading technologies currently available for identification of MPs include focal plane array detection, thermogravimetric analysis–pyrolysis–gas chromatography–mass spectrometry (Py–GC–MS), and infrared (IR) and Raman spectroscopy. While spectroscopic techniques measure plastic particle count, the properties of smaller plastic particles are more easily traced with mass-based concentrations. Mass-based concentrations are much more conducive, especially since spectroscopy remains time-consuming and costly, and requires technical expertise along with sophisticated laboratory equipment. Although skill is also needed for MS-based techniques, its ease in sample preparation, speed of analysis, and identification are advantageous, yielding practical information on polymer types and mass per volume.
In the following review, we survey the contribution of mass spectrometry as an analytical tool to detect microplastics in terrestrial, aquatic and environmental samples. The milestones reached and the miles to go are discussed. The gaps in the application area are pointed out and the challenges withholding mass spectrometry from maximum utilization towards microplastic detection/sensing in the environment are presented.
2. Application of Mass Spectrometry for the Detection of Microplastics
Our search for “mass spectrometry” on PubMed (an archive of citations from life science journals) yielded over 404,708 total hits, with over 28,809 articles published in the year 2021 alone. This shows the growing significance of MS (Figure 3). For developing soft desorption ionization methods for mass spectrometric analysis of biological macromolecules, John Fenn and Koichi Tanaka received the 2002 Nobel Prize in Chemistry. This was the start of the golden era of mass spectrometry in biological research as well as numerous other non-biological applications [16].
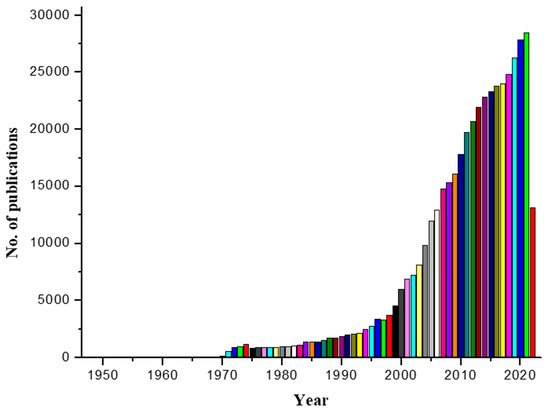
Figure 3.
PubMed search on the term, “mass spectrometry”.
2.1. Detection of MPs in Marine and Freshwater Organisms
MS is one of the rapid and reliable analytical tools that provides enhanced accuracy when it comes to the characterization of MPs, revealing their polymer composition, additives, and their associated organic toxic substances. Among all the MS techniques, gas chromatographic mass spectrometric (GC-MS) analysis of MPs was found to dominate. Peters et al. [16] detected the MPs extracted from the stomach of 1381 marine fish from the coast of Texas Gulf, using pyrolysis GC-MS (pyr-GC/MS) with Electron impact (EI+ 268, 70 eV) ionization as the analytical sensor.
Trophical transfer of MPs and the toxicants that are adsorbed were studied in model PMMA system with sorbent pollutant benzo(k)fluoranthene (BkF) using two freshwater invertebrates such as Daphnia magna and Chironomus riparius larvae (which are used as zebrafish feed) [17]. The GC-MS study revealed that the MPs and BkF were detected in lower quantities in trophical transfer than by direct exposure. The gradient centrifugation method was used to extract BkF and was then subjected to GC-MS for quantification (of BkF). The GC-MS instrumentation conditions employed for the detection of PMMA-associated sorbent BkF was as follows: electron impact (EI) SIM ionization at 250 and 252 scanning at source and quadrupole held at 200 °C.
In addition, the impact of polystyrene and polymethyl methacrylate MPs that were adsorbed on organophosphate insecticide chlorpyrifos (CPF) and on PAH (BkF) was assessed using zebrafish models [18]. GC-EI-MS was used to study the contaminant sorption and leaching of MPs in cryosections of test animals. The study revealed that the MPs that had no pollutants adsorbed on them exhibited no toxicity. In the freshwater crustacean Daphnia magna model system, Schrank et al. [19] studied their morphological and behavioral changes following the effect of flexible polyvinylchloride (PVC) MPs, with and without the plasticizer diisononylphthalate (DiNP). In this study, GC-MS was used to monitor PVC MPs and their plasticizers using butyl benzyl phthalate as an internal standard.
Andreas et al., 2021 [20] used GC-MS as a sensor to detect MPs from the digestive tract of Skipjack Tuna from the Southern Coast of Java, Indonesia. They had detected polybrominated diphenyl ethers (PBDEs) using GC-MS after processing the gastrointestinal tract with alkaline destruction and further filtration. For the extraction of PBDEs, the minced meat of Skip Jack tuna was solvent extracted and the samples were analyzed using GC-MS using two methods. An initial temperature of 150 °C with a 1 min hold time was used with a subsequent temperature increase to 330 °C at a rate of 17 °C/min in one method and 14 °C/min in another method. The mass spectrometer was operated with EI for ionization at 230 °C as the source temperature and 150 °C as the quadrupole temperature.
The effect of ingested polystyrene microspheres (10 μm in diameter) in Daphnia magna grown with 245,000 particles and the plastic content were measured using Py-GC-MS at 600 °C [21]. Liu et al. (Liu et al., 2021) described a method for the detection, quantification, and identification of MPs in Mytilus edulis, a marine mussel, using a sophisticated method that combines thermal gravimetric analysis, FT IR spectroscopy and GC-MS (TGA-FTIR-GC-MS). The MPs were extracted from the mussels using KOH, HNO3:H2O2, and HCLO4:H2O2 and the extracts were subjected to density separation and filtration; in addition, the extracted MPs such as polyethylene (PE), polystyrene (PS), polypropylene, (PP) and polyvinyl chloride (PVC) were analyzed using TGA-FTIR-GC/MS. The samples were heated for pyrolysis from 30 to 650 °C with a ramp rate of 15 °C/min from 30 to 300 °C and 30 °C/min from 300 to 650 °C. The resultant gaseous phase molecules were detected using GC-MS after passing through FTIR at 270 °C. The polymers were quantified using the calibration curves made with different concentrations of PE, PS, PP, and PVC.
The toxic effects of polyhydroxybutyrate resin (PHB), polylactic acid cups (PLA), and a polylactic acid/polyhydroxyalkanoate 3D printing filament (PLA/PHA), together with a synthetic polyvinyl chloride (PVC) toy on sea urchin larvae were studied. The results proved that PVC toy was the most toxic material, likely due to the added plasticizers; gas chromatography–mass spectrometry analysis (GC-MS) revealed the presence of a wide range of additives, suggestive of their role in the observed toxic effects on the larvae [22].
The effect of MP pollution on marine sponge tissues was determined by Saliu et al. [23]. The authors extracted MPs (<25 μm) from the tissues of Haliclona (Haplosclerida) and characterized them chemically using IR and GC-MS. The samples were pyrolyzed at 600 °C for 0.2 min. EI ionization at 70 eV was carried out and the ions (m/z) were detected in positive mode. The quadrupole analyzer and ion source were set at 150 °C and 230 °C, respectively. The study identified PP, PET, HDPE, and LDPE using solvent dichloromethane and PS using methanol.
Lo Brutto et al. [24] have detected the presence of plasticizers and toxic derivatives of microplastic contamination in amphipods such as Talitrus saltator, Parhyale plumicornis, Parhyale aquilina, Speziorchestia stephenseni, and Orchestia montagui using GC-MS. The results showed that DEP and DiBP represented the most abundant compounds in the selected amphipods. Four PAE (phthalic acid esters), namely, DEP, DBP, DiBP, and DEHP and two NPPs (non-phthalate plasticizers), namely, DEHA and DEHT, were detected using GC-MS. The samples were prepared by homogenizing the amphipod tissue and extracting the PAEs and NPPs by differential centrifugation and liquid phase extraction in ethanol and subjecting the extract to GC-MS for further analysis. To understand the evolution of MP pollution in the environment over the past decades, Halbach et al. [25] chose the Baltic Sea blue mussels as test specimens. They recorded the gradual increase in seven common polymer clusters in mussel tissue using GC-MS.
2.2. Detection of MPs in Various Water Sources
2.2.1. In Seawater and Artificial Sea Water
MPs abundance in the black sea, which is the major source of Mediterranean Sea MP pollution, was studied [26]. In order to identify the polymer content of the MPs (fibers, foils, fragments, and spherules), GC-MS was employed after the removal of organic matter associated with particles, followed by further pyrolysis (600 °C) and GC-MS analysis. Chromatographic separation was performed using the following temperature program: hold up at 40 °C for 2 min, increase at 20 °C min−1 to 320 °C and hold up for 13 min. The authors could detect particles with polymer compositions PP, PE, PS, PAN, PA, PAR, PES, and unknown.
Wu et al. [27] studied the aging process of common food pack materials (PP-based meal box and tea cups), under UV exposure in a simulated marine environment using artificial sea water for 12 days with and without addition of antioxidants (Irgafos 168), using GC-MS. After separating the sample using GC with 1mL/min flow, the samples were ionized by EI (70eV) at 280 °C and 230 °C, respectively, and the initial temperature was set at 80 °C (2 min), ramped at the rate of 15 °C/min at 300 °C, and maintained for 7 min. GC-MS revealed that the antioxidant additive Irgafos 168 (tris (2,4-di-tert-butylphenyl) phosphite) added to food packaging material inhibits the photodegradation of PP-based meal box and tea cups in the marine environment.
Another study was conducted to accelerate the photodegradation of MPs and to elucidate the toxic molecular leachates that are liberated upon the photodegradation process from LDPE, HDPE, PP, and PS in artificial seawater medium using GC-MS [28]. The GC-MS analysis was carried out with EI ionization at 70 eV, with 230 °C as the ion and source temperature and 150 °C as the quadrupole temperature. The authors identified more than 60 different compounds in which benzoic acid and phenol derivatives were the most abundant molecules from PP. The system was tested on real-world plastic particles that undergo environmental degradation. The results proved that dicarboxylic acids and oxidized species were abundant.
Gas chromatography–mass spectrometry (GC-MS) was used to analyze a total of 14 phthalate esters (PAEs) in order to better understand their distribution and occurrence in the Tropical Western Pacific Ocean (TWPO) [29]. MPs in environmental samples at trace levels may be identified and quantified using thermochemolysis and Curie-point pyrolysis–gas chromatography–mass spectrometry [30,31].
Pyrolysis-GC-TOF-MS was used to analyze MPs with pyrolyzate chemicals in marine water samples from beachfront locations in Cape Town, South Africa. The findings revealed that, of the 16 polymers that were found in the research region, polythene (PE) dominated in six of the seven locations with 87.5%, followed by polyethylene terephthalate (PET) and polyvinyl chloride (PVC) in five (71.4%) and four (57.1%), respectively. Polystyrene (PS), polyamide 12 (PA-12), polyacrylic acid (PAA), and ethyl vinyl acetate (EVA) copolymers were the additional constituent MPs that were found by pyrolyzates [32].
2.2.2. Detection of MPs in Freshwater
Yet, another study by Li et al. [33] used an isolation method for micro- and nanoplastics and validated using spherical PS and PMMA particles and also extended its extraction from real-world drinking water and river water samples. The abundance of MPs in the freshwater lake, Western Lake Superior, and their composition were evaluated by FTIR and Pyr GC/MS. The polymer content was verified by Pyr-GC-MS at 70 eV EI and the source and quadrupole temperature was set at 150 °C and 230 °C, respectively. The samples were identified as PVC, PP, PE, PET, CPE, PS, PDMS, and didecyl phthalate resin based on their polymer content [34].
2.2.3. Detection of MPs in Wastewater
Recently the diversity of MPs in a wastewater treatment plant that discharges MPs into a river system was assessed using FTIR and Pyr-GC/MS methods [35], with 70 eV ionization energy and 230 °C and 150 °C as EI-Source temperature and quadrupole temperature, respectively. The homogeneous presence of different polymers such as polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), polyamide (PA), polyethylene terephthalate (PET), polycarbonate (PC), MDI polyurethane (MDI PU), and their decomposition products were detected using GC-MS all through the year, among which polyolefins polymers were found to be dominant among the samples tested.
Funck et al. [36] developed an MP extraction system that consists of a pressure pump-assisted steel-based cascadic filtration system with a filter size of about 100 µm, 50 µm, and 10 µm, followed by MP analysis in Pyr GC-MS. They introduced a platinum filament for the efficient pyrolysis process that enabled the operation pyrolysis temperature between 550 °C and 1300 °C within 8 min. Their system was successfully validated for polystyrene (PS) and polyethylene (PE) MPs. Size, particle number, shape and color of MPs and a plastic additive, Di 2-ethyl hexyl phthalate (DEHP), were detected and identified from a wastewater treatment plant using GC-MS as one of the highlight tools [36]. According to their study, WWTP do not remove MPs sufficiently and they could observe the presence of MPs (2.419 × 107 particles/day) in the effluents even after wastewater treatment. Recently, Ibrahim et al., (2021) studied the distribution of MPs in the water and surface sediments of Setiu Wetland in South China Sea using ATR- FTIR and Pyr GC-MS [37]. Their results revealed that surface water and dry sediments contain 0.36 items/L and 5.97 items/g particles of MPs, respectively. Although Takdastan et al. [38] and Ibrahim et al. [37] have used GC-MS as one of the analytical tools to characterize the MPs, they have not elaborated their experimental methodologies. A new custom-made portable Pyr-MS was developed by Zhang et al. [39], where a compact pyrolyzer decomposes MPs and subsequent analysis in the portable MS, avoiding complex sample extraction steps. The method was validated for MPs such as PE, PP, PS, and PMMA.
2.3. Detection of MPs in Sediments
Using PS, PPE, PET, and PE models in a headspace (HS) with needle trap microextraction GC-MS, toxic volatile organic compounds (VOCs) arising from MPs/plastic debris during the course of the weathering process were determined (NTME GC–MS). In headspace vials with 25 g of MPs that had been heated to 60 °C, the VOCs were collected and then transferred to an NT device. The samples were desorbed for 20 s at 300 °C before being subjected to a 70 eV ionization GC-MS analysis. The investigation identified VOCs including aromatics, carbonyls, lactones, esters, acids, alcohols, and ethers. However, acrolein, benzene, propanal, methyl vinyl ketone, and methyl propenyl ketone VOCs were found to be released in actual samples of beach soil [40].
Gomiero et al. [41] detected MP pollution in wet sediment samples of an urban fjord in southwest Norway. The study involved the extraction of MPs from the sediment samples and further analysis by thermal desorption pyrolysis GC-MS for the identification of the polymer composition of each MP particles. The pyrolysis was carried out at 590 °C and separated in GC followed by an MS-enabled analysis of the polymer composition using EI at 70 ev [42]. The GC-MS analysis revealed MPs of PP, PE, PET, PVC, PS, and PA origin.
A technique described by Dey et al. [43] involves pyrolyzing tiny plastic particles (0.35 to 7.0 mg) at 700 °C before GC-MS analysis. By comparing the generated pyrograms of each sample to the accepted reference pyrogram, this technique could identify MPs (i.e., PE, PP, PVC, PS, PA, and PET) in sediments. This technique is time-consuming and damaging, just like any other GC-MS. Additionally, it takes a lot of time because standards must be compared with each pyrogram.
Two Portuguese beach samples were analyzed for MP pollution. The MPs were associated with organic pollutants, and the plastic type was identified through GC-MS and FTIR. The MPs were mostly polyethylene and polypropylene polymers [44], however, the GC-MS methodology was not described in detail.
Pyr GC-MS was used to detect the polymer type of the MP particles and their organic plastic additives from MPs isolated from German East Frisian Islands [45]. They isolated the MPs from the coastal sediment samples by density separation. Then, the separated particles were heated to 350 °C for thermal desorption. The temperature program was run at a ramp rate of 10 °C/min for 10 min, ranging from 40 to 350 °C. The transfer line’s temperature was 350 °C. Following the completion of TD, the CIS was heated to 280 °C at a rate of 12 °C min1 and held for 3 min. TD was followed by the registration of the first chromatogram following pyrolysis at 700 °C for 60 s. Polyethylene, polypropylene, polystyrene, polyamide, chlorinated polypropylene E, and chlorosulfonated polyethylene were identified as the MPs. The polymers contained benzaldehyde, 2,4-di-tert-butylphenol, dibutyl phthalate, diethyl phthalate, diisobutyl phthalate, and dimethyl phthalate.
2.4. Detection of MPs in Other Environmental Samples
Pyr-GC-MS is the industrial standard for analyzing polymers. The examination of complex sample combinations, such as environmental samples, cannot be carried out using this method due to limitations in the sampling amounts (0.5 mg). In order to identify microplastics in environmental samples, Erik et al. created a new thermoanalytical approach. The entire thermal degradation of a sample of 20 mg, which ensures the homogeneity of the sample, was carried out. Thermodynamic desorption gas chromatography–mass spectrometry was used to examine the individual breakdown products of the various polymers adsorbed on a solid-phase adsorber. After that, MPs were checked in genuine environmental samples taken from terrestrial (a biogas plant) and aquatic (three separate rivers) systems. The main plastics found in the biogas plant were polypropylene (PP), polyethylene (PE), and polystyrene (PS), whereas PE and PS were found in the waterways [46].
High- and low-density polyethylene, polystyrene (PS), polypropylene (PP), and polyethylene terephthalate were all ground to sizes between 857 and 509 m for the reference MP–polymer micropowders used in Biale et al. [47]. The reference MPs were purposefully aged artificially in a sun box in order to characterize the aged (photo-oxidized) MPs and their degraded fractions and understand their degradation mechanisms. The MPs were found and identified using a multi-technique approach combining evolved gas analysis-mass spectrometry (EGA-MS), pyrolysis–gas chromatography–mass spectrometry (Py-GC-MS), and size exclusion chromatography (SEC). The analytical tests showed that benzoic acid and 1,4-benzenedicarboxylic acid were the most prevalent low molecular weight photo-degradation products of PS. The most resistant to ageing was PET.
According to reports, the most significant terrestrial sources of environmental microplastics are tyre-wear particles (TWP) (MP). The ecology is threatened by the TWP that will unavoidably be discharged during daily traffic. Using the Py-GC/MS (pyrolysis–gas chromatography–mass spectrometry) approach, TWP was identified and quantified. According to Goßmann et al., a method for differentiating between tyre wear on cars and trucks and quantifying their respective mass loads was developed [48]. Py-GC/MS was used to examine various complex environmental materials, including road dust, freshwater and marine sediments, blue mussels, and marine salts. The findings highlight how car-tyre-wear mass loads predominate over truck-tyre-wear mass loads in all examined samples. TWP concentrations in road dust were significantly higher than “conventional” MP concentrations (5 g TWP vs. 0.3 g MP per kilogram of dry weight of road dust [48]). Few empirical investigations report discovering tyre wear, despite desk-based research suggesting that tyre-wear particles constitute a sizable amount of MP emissions to the environment. Three entry points into the marine ecosystem were sampled: air deposition, wastewater effluent after treatment, and untreated surface runoff. Benzothiazole, a chemical marker for tyres, was found using pyrolysis in conjunction with GC-MS. Microplastics (MPs) may be present in the lagooning sludge (LS) used as a soil supplement in Morocco. The chemicals from plastics were found using pyrolysis GC/MS spectrometry, and fluorescent particles thought to be plastics were found using Nile Red staining. After density fractionation, GC-MS allowed for the detection of MP particles [49].
NASA et al. [50] demonstrated the capability of double-shot Py-GC-MS and microwave-assisted solvent extraction to gather qualitative and quantitative data on polystyrene and phthalate plasticizers in environmental samples. The method was verified, with recoveries of more than 96% and detection limits for phthalates and polystyrene of 1 ng and 1 g, respectively. The method was applied by the authors to analyze sand samples taken from a Tuscany (central Italy) beach in order to determine the concentrations of phthalate and polystyrene at various depths and separations from the coast. The use of TED-GC-MS for the investigation of polymers and their degradation processes was reported by Duemichen et al. in [51]. The gaseous decomposition products from a sample are first broken down in a thermo-gravimetric analyzer (TGA), and they are then captured on a solid-phase adsorber. The solid-phase adsorber was next examined using mass spectrometry and thermal desorption–gas chromatography (TDU-GC-MS). It has now been established that automated TED-GC-MS is a novel, versatile, multifunctional approach for thorough polymer investigations. For the detection of MPs and NPs in water samples, pyrolysis—gas chromatography time of flight mass spectrometry (Py-GCToF) has been utilized in conjunction with PTFE membranes as sample support. This makes it possible to identify smaller particle sizes (>0.1 m) in water samples. The technique was examined against a range of standards, including those that contained known MP concentrations and allowed for the detection of PVC and PS [52]. Table 1 summarizes the GC-MS-based detection of MPs.

Table 1.
GC-MS as microplastic sensors.
3. LC-MS-Based Analysis of the Effects of MPs
3.1. LC-MS-Based Analysis on the Effects of MPs on Aquatic Creatures and in Water Sources
Polyethylene MPs have been found by authors in zebrafish embryos. The embryos were exposed to MPs ranging in size from 1 to 4 mm at 0, 10, 100, and 1000 mg/L concentrations for 7 days. A total of 59 phospholipid-related chemicals showed significant changes in larval fish treated with 1000 mg/L MPs, according to LC/MS-based nontargeted metabolomics study. Clearly altered mRNA levels were also seen for genes involved in phospholipid metabolism [73].
A multi-residue analytical approach based on high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) has been developed for the identification of 21 plastic additives in river water. Phthalates, benzophenone, bisphenol A, and long- and short-chain alkylphenols (APs) are often used in the plastics sector—these were among the substances that were examined. The leached plastics and MPs enter river water from wastewater treatment plants. Nonylphenol, octylphenol, and bis (2-ethylhexyl) phthalate had detection limits that fell below environmental quality norms, although other substances were successfully detected at trace concentrations. These authors were the first to describe MPs that were di-, tri-, and o-substituted. The washing of garments releases various microfibers, including microplastic fibers (MPFs), and the authors also undertook a pilot survey to find the plastic additives in river waters near the city of Barcelona [74]. Despite the fact that MPFs in laundry wastewater significantly contribute to microplastics (MPs) in wastewater treatment plants (WWTPs), there is little quantitative data on their effects. The most significant textile fiber is polyester, and the polymer polyethylene terephthalate (PET) has been quantified by LC-MS/MS. Simulated trials were used to quantify the release of MPFs from polyester clothes during washing, and LC-MS/MS and microscopic counting were used to determine the MPF levels in two WWTPs [75].
The engineering plastic bisphenol A polycarbonate (BPA-PC), which has been overused in the creation of plastic trash, presents a significant risk of chemical re-release through outdoor weathering. Authors have thoroughly examined PC MPs photoaging behavior in aquatic environments and assessed the possible risk of released intermediates. According to LC-TOF-MS analysis, these organic chemicals that make up MP-derived dissolved organic matter (MPs DOM) were partially made up of the estrogenic substances methyparaben (MeP), p-hydroxybenzoic acid (p-HBA), and 4,4′-dihydroxybenzophenone (DHB) [76].
The potential of MS for the investigation of MPs and NPs has only been thoroughly researched by a few authors. For the investigation of polystyrene (PS) MPs and NPs in natural waters, the effectiveness of various techniques such as matrix-assisted laser desorption ionization (MALDI) coupled to time-of-flight mass spectrometry (TOF-MS), liquid chromatography coupled to high-resolution mass spectrometry (LC-HRMS), and ambient ionization approaches such as desorption electrospray ionization (DESI) was analyzed. For the quantitative investigation of PS MPLs and NPLs in natural waters, a method based on LC-HRMS, equipped with an atmospheric pressure photoionization source (APPI), operating in negative circumstances, was devised. Toluene isocratic was used as the mobile phase in an advanced polymer chromatographic (APC) column to facilitate the chromatographic separation. It has been observed that samples from rivers and the marine coast have effective recoveries of 60 and 70%, respectively, while the instrumental limit of detection (ILOD) is 20 pg and the technique limits of detection and quantification are around 30 pg L−1 and 100 pg L−1, respectively. On samples of natural seawater and those that had been fortified, the approach was validated [77].
A method for quantifying nylon MPs using LC-MS/MS was published in another investigation. In order to be identified by LC-MS/MS, PA6 and PA66 were successfully depolymerized to 6-aminocaproic acid and adipic acid, respectively. In ambient samples, the effective recovery of spiked PA6 and PA66 MPs ranged from 90.8% to 98.8%. With quantities of 0.725–321 mg/kg, PA MPs were found in indoor dust, sludge, marine sediment, freshwater sediment, fisheries sediment, and fish guts and gills. The highest PA66 MP concentrations have been found in fish guts and gills as well as interior dust, which indicates a severe danger of human exposure through ingesting dust and food intake [78].
The invention and validation of a novel method for the detection of phthalates in marine invertebrates using biocompatible solid-phase microextraction (BioSPME) and LC-MS are described by Saliu et al. in [79,80]. Small amounts of the biological components (150 mg) were sampled in glass vials with aluminum lids. The biological components were extracted using ultrasonication in acetone, dilution in ultrapure water, and BioSPME clean-up, which was then followed by electrospray (ESI) LC-MS/MS. As part of microplastic pollution biomonitoring research, tests on samples from three different phyla—Cnidaria, Porifera, and Mollusca—obtained from Maldivian coral reef environments were conducted. Good sensitivity and repeatability were reported [79], along with very little back contamination of the blanks. Saliu et al. [81] reported a novel method for phthalate determination from marine invertebrates, including Porites lobata (the scleractinian coral), Petrosia sp. (a sponge), Tridacna maxima (a clam), and Denditheca dendritrica (colonial hydrozoan), which was developed and validated. This method used biocompatible solid-phase microextraction coupled to liquid chromatography. This application’s significance depends on the fact that marine species are being used as bioindicators for microplastic contamination by the detection of phthalates in their tissues.
3.2. Detection of MPs in Miscellaneous Sources
Rats were given fibrous and granular MP and nanoplastic (NP) made of the nylon polymer polyamide 66 (PA66), and the excretory behavior of the ingested PA66 was measured using LC-MS-MS and microscopic examination. According to the LC-MS investigation, most of the PA66-MP or PA66-NP consumed was excreted within 48 h, while three other forms of PA66 were still present in the rats’ systems even after seven days of excretion. The findings showed that about 30% of the ingested PA66-NP could not be found in feces, and it was shown that PA66-NP was present in rat serum after PA66-NP consumption. According to these findings, PA66-NP can cross the gastrointestinal barrier and reach the bloodstream [81].
Zang et al. [82] used an LC-MS MS/MS method to evaluate the degree of MP degradation/mineralized waste from a landfill, and the research revealed that the presence of PET and PC were the most often found MPs. Additionally, the MP sorption compounds in the environment were studied by LC-MS. The MPs (PE, PP, PS, and PVC) were exposed for three weeks in a unique setup in a natural surface water stream before the authors enabled detection with GC and LC-ESI MS. The investigation found 34 distinct substances that have a negative impact on both animal and human life. Further research by Xu et al. [83], utilizing LC-MS/MS to examine the release of dissolved organic matter from MPs in response to UV irradiation, revealed the existence of several compounds with reactive oxygen species. Table 2 summarizes the LC-MS-based applications as microplastic sensors.

Table 2.
LC-MS as microplastic sensor.
4. Other MS-Based Microplastic Sensors
Even though GC-MS and LC-MS have largely been employed for MP detection, there have also been reports of other MS-based techniques. Time-of-flight secondary ion mass spectrometry (ToF-SIMS) was employed by Jungnickel et al. [99] for the detection, analysis, and imaging of tiny polyethylene particles (PE). Regarding imaging mass spectrometry methodology, only a few analytical methods could detect MPs smaller than 10 μm. The PE-microplastic particles were found by the authors directly in the Ottawa sand model system following exposure to sea surf simulation. Prior to that, they used a standard sample of ground polymers to improve the detection technique for identifying PE. Then, Ottawa sand was used to test the optimized procedure [99].
There have been reports of the accumulation of plastic in the sediments, thus, it is important to investigate the effects of microplastic particles on benthic freshwater animals. Pedersen et al. [100] investigation focused on the toxicity of plastic particles and their ingestion by benthic filter-feeding quagga mussels (Dreissena bugensis). Microplastic inclusion was discovered using the matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS) technique as a sensor. We measured the number of quagga mussels in the size range of 10–45 μm that were exposed to various doses of high-density fluorescent red polyethylene powder over 24 h. A few micrograms of microplastics in the digestive tract could be successfully identified using MALDI-IMS, and the method validated the finding that 95% of the microplastics consumed remained in the mussels after 24 h.
Polystyrene (PS) particles were used as a model MNP in Lin et al. [101] report of a straightforward, quick, and efficient method for identifying and quantifying micro-/nanoplastics (MPs/NPs) based on thermal fragmentation (at 380 °C) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). The signature mass prints were quantified at m/z 315.3 and reported in the high-mass regions (repeated peaks with m/z 104 in the m/z range 350–5000) as well as low-mass regions (m/z 90, 104, 128, 130, and 312–318). Additionally, polyethylene terephthalate was used to show this technique (PET).
5. Challenges and Future Perspectives
Microplastics have become, in the past few decades, the talk of environmentalists, researchers, social media, as well as the public. A lot of enthusiasm is evident as evidenced by the increasing number of research articles in this area. However, the enthusiasm is much lower compared to their magnitude of damage. The key word search on PubMed emphasizes this notion, where the search using the keywords ‘microplastics’ hit 7913 articles (Figure 4a), while the hit on ‘mass spectrometry and microplastics’ yielded 182 hits (Figure 4b). Microplastics have huge popularity, but their detection, identification, and mitigation still have low-key research publicity. Microplastic research has been worked upon in a randomized, scattered manner, with articles reporting MPs in water, sediments, air, fish, animals, humans and the like—these have increased the quantity of the research articles in this area. However, other than these scattered reports, nothing much has really progressed. For example, a keyword search on the terms, ‘detection of microplastics’ yielded 1260 hits (Figure 4c), while the search term ‘mass spectrometry and microplastics’ yielded only 182 hits (Figure 4b) on PubMed.
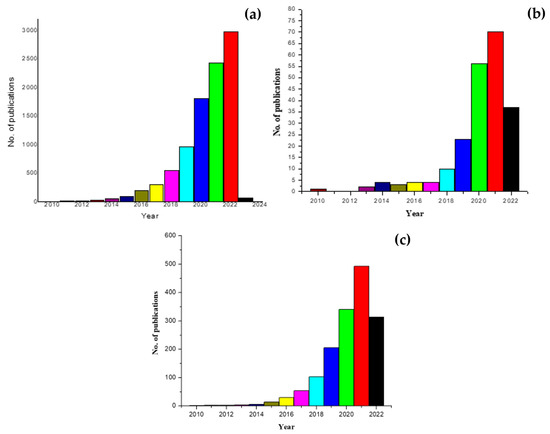
Figure 4.
Results of PubMed search on keywords such as, (a) ‘microplastics’, (b) ‘detection of microplastics’, and (c) ‘mass spectrometry and microplastics’.
To be more specific, chicken was indeed the sole terrestrial species studied for microplastics. In this investigation, it was discovered that chicken gizzards prepared and consumed by local Mexicans contained microplastics [102]. However, this study was unique in that it focused on hens that were living in gardens that were heavily polluted with plastic garbage, and it was conducted in a specific Mexican hamlet. The likelihood that the chickens consumed plastic fragments while foraging on the ground is quite significant. A single study with a tiny sample size is not adequate to represent the true problem of meat contamination. To reach any conclusions regarding meat contamination, more research including larger sample sizes on a variety of farm animals intended for human consumption should have been conducted, which, of course, has never occurred. There are many similar lone studies like this that have not been supported. Similarly, 35% of plankton-eating fish collected in the North Pacific Gyre had plastic shards the size of centimeters in their stomachs. Each fish had an average of 2.1 pieces. Similar observations were made in Brazilian estuaries, where it was discovered that plastic particles were present in the stomachs of 18% to 33% of catfish that were captured. Unstudied topics include the human consumption of fish contaminated with microplastics and determining the MP levels in human blood. There is a counterargument that the MPs were typically found in the fish intestines, which are typically not intended for human consumption (with exceptions where in some populations, whole fish are consumed, and the entrails are consumed too as delicacies). In contrast, the processing of fish and shellfish does not yield over 60% of waste as byproducts. When it comes to animals that are contaminated with MPs, the use of fish guts for the preparation of animal feed (for example, poultry production and pig raising) can be of concern. This makes it impossible to rule out the possibility of micro- and nanoplastic contamination of animal feed. However, there are no studies on the contamination of farm animals through feeding, nor are there any on the effects on animal health or the quality of meat intended for human consumption. We want to draw attention to the fact that the downstream flow of MPs into food chains has not yet been mapped. This needs to be addressed right away because there are clear gaps in the research on MPs that need to be filled and accurately correlated. The entry level of the MPs into the system is another complicated series. They could enter directly from the environment, they can be introduced during food processing, or they might be also get introduced from food packaging, from the food packaging industries. Potential challenges include judging whether the micro-/nanoplastic particles are already in the food before processing or if their presence is the consequence of the processing phase. Such questions need more focused and systematic research, which are needed to be able to bring about resolving MP pollution. Additionally, phases of MP degradation as well as the transport of plastics from specific foods and beverages to animals/humans still lack scientific understanding.
Although tremendous efforts have been made in the last decade to identify microplastics in food, standardized experimental protocols have not been attained. Among many experimental protocols attempted, the most common and reliable methods are oxidative digestion, filtering, and spectroscopic confirmation with FT-IR when the particle size is greater than 50 μm. This review emphasizes the importance of the application of proper analytical methods for detecting/sensing MPs. Detection is the fundamental step in devising mitigation methods. As highlighted in this review, there is high potential from MS-based applications. Optimized techniques, the inclusion of combinatorial techniques, and the incorporation of state-of-the-art MS methodologies are what will lead to furtherance in this area. Of the mass spectrometric techniques studied, GC-MS is the most worked on and MALDI TOF MS is the least worked on. This review prompts more attention in this direction.
Among the mass spectrometric methods, GC-MS has been used more extensively for the detection and analysis of the polymer compounds, plasticizers, and other additives of MPs. Due to their high temperature pyrolysis and EI-based hard ionization methods, GC-MS provided accurate data regarding the molecular composition of MPs and became an unavoidable tool in MP research. Although the polymers in microplastics are thermally stable, GC-MS would be the better choice for MP analysis; however, for the identification of plastic additives and the intact identification of thermolabile, adsorbed environmental compounds would be its limitation [46]. Therefore, using GC-MS as a wholesome instrument for MP research is a big challenge. In addition, time consumption and labor-intensive protocols of GC-MS emphasize the development of alternative soft ionization-based mass spectrometric methods such as MALDI MS, ESI MS, and DESI MS. These methods are very rapid and require less sample preparation protocols and will provide precise information about the molecules due to their soft ionization capacity. The DESI MS method is direct and rapid in providing molecular detail of the surfaces studied; however, it requires the development and optimization of novel ionization strategies for the ionization of tough ionizing molecules. LDI MS techniques have evolved to use nanomaterials to assist in fine-tuning the technique [103,104,105,106,107,108]; applications of this technique could certainly prove beneficial with respect to MP detection. MS-based techniques are still confronting reproducibility issues and lapses in quantification methodologies—these need to be overcome in order to fully utilize these techniques.
In terms of mitigation methods for avoiding microplastics in the environment, the ideal method is that prevention is better than cure. Why would we release microplastics into the environment and then gather or detect them using sophisticated methods? The reduced use of plastics is unequivocally the ideal solution. Once released into the environment, MPs are hard to detect—the wise and smart handling of plastics is the only solution. We have become too intertwined with plastics; we need to find a way to minimize their use. Banning plastics seems near impossible, however, recycling them could be the ideal best-case scenario (Figure 5).
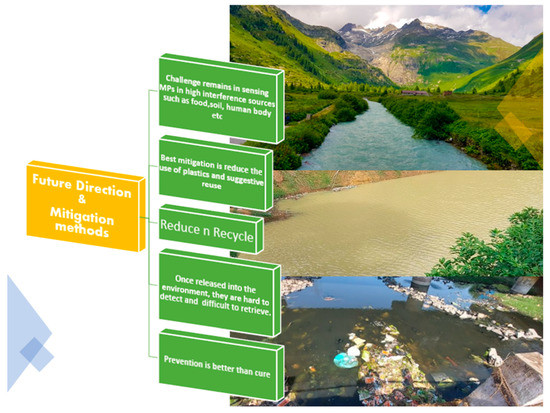
Figure 5.
Major challenges and mitigation methods confronting microplastic detection in the environment.
6. Conclusions
For the first time, we have exclusively reviewed the use of mass spectrometry as microplastic sensors. The advantages of using mass spectrometry have been elaborately discussed and the confronting challenges have been presented. Directions for future perspectives, based on what is currently lacking in this area of research, have been put forth.
Author Contributions
J.G. and M.M., preparation of original draft, review and revisions; S.C., participated in review and revisions and funding support. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, K.P.; Sharma, D.; Saha, S.; Satapathy, B.K. From outbreak of COVID-19 to launching of vaccination drive: Invigorating single-use plastics, mitigation strategies, and way forward. Environ. Sci. Pollut. Res. 2021, 28, 55811–55845. [Google Scholar] [CrossRef] [PubMed]
- Vaverková, M.D. Landfill Impacts on the Environment—Review. Geosciences 2019, 9, 431. [Google Scholar] [CrossRef]
- Henry, B.; Laitala, K.; Klepp, I.G. Microfibres from apparel and home textiles: Prospects for including microplastics in environmental sustainability assessment. Sci. Total. Environ. 2019, 652, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Schöpel, B.; Stamminger, R. A Comprehensive Literature Study on Microfibres from Washing Machines. Tenside Surfactants Deterg. 2019, 56, 94–104. [Google Scholar] [CrossRef]
- Verschoor, A.; De Poorter, L.; Dröge, R.; Kuenen, J.; de Valk, E. Emission of Microplastics and Potential Mitigation Measures: Abrasive Cleaning Agents, Paints and Tyre Wear; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2016. [Google Scholar]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Krokosz, A.; Sicińska, P. Oxidative Properties of Polystyrene Nanoparticles with Different Diameters in Human Peripheral Blood Mononuclear Cells (In Vitro Study). Int. J. Mol. Sci. 2021, 22, 4406. [Google Scholar] [CrossRef]
- Wu, X.; Pan, J.; Li, M.; Li, Y.; Bartlam, M.; Wang, Y. Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res. 2019, 165, 114979. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total. Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Primpke, S.; Christiansen, S.H.; Cowger, C.W.; De Frond, H.; Deshpande, A.; Fischer, M.; Holland, E.B.; Meyns, M.; O’Donnell, B.A.; Ossmann, B.E.; et al. Critical Assessment of Analytical Methods for the Harmonized and Cost-Efficient Analysis of Microplastics. Appl. Spectrosc. 2020, 74, 1012–1047. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Araújo, A.P.D.C.; de Melo, N.F.S.; Junior, A.G.D.O.; Rodrigues, F.P.; Fernandes, T.; Vieira, J.E.D.A.; Rocha, T.L.; Malafaia, G. How much are microplastics harmful to the health of amphibians? A study with pristine polyethylene microplastics and Physalaemus cuvieri. J. Hazard. Mater. 2020, 382, 121066. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Kang, B.; Cho, S.K.; Park, J.; Lee, Y.; Kim, W.-I.; Marunga, J.; Hwang, I.; Kim, J. Identification of Pseudomonas syringae pv. syringae causing bacterial leaf blight of Miscanthus sinensis. J. Plant Dis. Prot. 2017, 124, 97–100. [Google Scholar] [CrossRef]
- Manikandan, M.; Hua, P.-Y.; Wu, H.-F. Rapid endophytic bacterial detection by enzyme incorporated MALDI MS. RSC Adv. 2014, 4, 50233–50240. [Google Scholar] [CrossRef]
- Chou, L.; Mahaffy, P.; Trainer, M.; Eigenbrode, J.; Arevalo, R.; Brinckerhoff, W.; Getty, S.; Grefenstette, N.; Da Poian, V.; Fricke, G.M.; et al. Planetary Mass Spectrometry for Agnostic Life Detection in the Solar System. Front. Astron. Space Sci. 2021, 8, 755100. [Google Scholar] [CrossRef]
- Peters, C.A.; Hendrickson, E.; Minor, E.C.; Schreiner, K.; Halbur, J.; Bratton, S.P. Pyr-GC/MS analysis of microplastics extracted from the stomach content of benthivore fish from the Texas Gulf Coast. Mar. Pollut. Bull. 2018, 137, 91–95. [Google Scholar] [CrossRef]
- Hanslik, L.; Sommer, C.; Huppertsberg, S.; Dittmar, S.; Knepper, T.P.; Braunbeck, T. Microplastic-associated trophic transfer of benzo(k)fluoranthene in a limnic food web: Effects in two freshwater invertebrates (Daphnia magna, Chironomus riparius) and zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108849. [Google Scholar] [CrossRef]
- Hanslik, L.; Seiwert, B.; Huppertsberg, S.; Knepper, T.P.; Reemtsma, T.; Braunbeck, T. Biomarker responses in zebrafish (Danio rerio) following long-term exposure to microplastic-associated chlorpyrifos and benzo(k)fluoranthene. Aquat. Toxicol. 2022, 245, 106120. [Google Scholar] [CrossRef]
- Schrank, I.; Trotter, B.; Dummert, J.; Scholz-Böttcher, B.M.; Löder, M.G.; Laforsch, C. Effects of microplastic particles and leaching additive on the life history and morphology of Daphnia magna. Environ. Pollut. 2019, 255, 113233. [Google Scholar] [CrossRef]
- Andreas; Hadibarata, T.; Sathishkumar, P.; Prasetia, H.; Hikmat; Pusfitasari, E.D.; Tasfiyati, A.N.; Muzdalifah, D.; Waluyo, J.; Randy, A.; et al. Microplastic contamination in the Skipjack Tuna (Euthynnus affinis) collected from Southern Coast of Java, Indonesia. Chemosphere 2021, 276, 130185. [Google Scholar] [CrossRef]
- Nakano, R.; Gürses, R.K.; Tanaka, Y.; Ishida, Y.; Kimoto, T.; Kitagawa, S.; Iiguni, Y.; Ohtani, H. Pyrolysis-GC–MS analysis of ingested polystyrene microsphere content in individual Daphnia magna. Sci. Total Environ. 2022, 817, 152981. [Google Scholar] [CrossRef]
- Uribe-Echeverría, T.; Beiras, R. Acute toxicity of bioplastic leachates to Paracentrotus lividus sea urchin larvae. Mar. Environ. Res. 2022, 176, 105605. [Google Scholar] [CrossRef] [PubMed]
- Saliu, F.; Biale, G.; Raguso, C.; La Nasa, J.; Degano, I.; Seveso, D.; Galli, P.; Lasagni, M.; Modugno, F. Detection of plastic particles in marine sponges by a combined infrared micro-spectroscopy and pyrolysis-gas chromatography-mass spectrometry approach. Sci. Total. Environ. 2022, 819, 152965. [Google Scholar] [CrossRef] [PubMed]
- Brutto, S.L.; Iaciofano, D.; Turco, V.L.; Potortì, A.; Rando, R.; Arizza, V.; Di Stefano, V. First Assessment of Plasticizers in Marine Coastal Litter-Feeder Fauna in the Mediterranean Sea. Toxics 2021, 9, 31. [Google Scholar] [CrossRef]
- Halbach, M.; Vogel, M.; Tammen, J.K.; Rüdel, H.; Koschorreck, J.; Scholz-Böttcher, B.M. 30 years trends of microplastic pollution: Mass-quantitative analysis of archived mussel samples from the North and Baltic Seas. Sci. Total Environ. 2022, 826, 154179. [Google Scholar] [CrossRef] [PubMed]
- Pojar, I.; Stănică, A.; Stock, F.; Kochleus, C.; Schultz, M.; Bradley, C. Sedimentary microplastic concentrations from the Romanian Danube River to the Black Sea. Sci. Rep. 2021, 11, 2000. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, P.; Shi, H.; Wang, H.; Huang, H.; Shi, Y.; Gao, S. Photo aging and fragmentation of polypropylene food packaging materials in artificial seawater. Water Res. 2021, 188, 116456. [Google Scholar] [CrossRef] [PubMed]
- Biale, G.; La Nasa, J.; Mattonai, M.; Corti, A.; Castelvetro, V.; Modugno, F. Seeping plastics: Potentially harmful molecular fragments leaching out from microplastics during accelerated ageing in seawater. Water Res. 2022, 219, 118521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, J.; Li, X.; Peng, Q.; Yuan, H.; Li, N.; Duan, L.; Ma, J. Concentrations and distribution of phthalate esters in the seamount area of the Tropical Western Pacific Ocean. Mar. Pollut. Bull. 2019, 140, 107–115. [Google Scholar] [CrossRef]
- Doyen, P.; Hermabessiere, L.; Dehaut, A.; Himber, C.; Decodts, M.; Degraeve, T.; Delord, L.; Gaboriaud, M.; Moné, P.; Sacco, J.; et al. Occurrence and identification of microplastics in beach sediments from the Hauts-de-France region. Environ. Sci. Pollut. Res. 2019, 26, 28010–28021. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Rochman, C.M. Microwave-Assisted Extraction for Quantification of Microplastics Using Pyrolysis–Gas Chromatography/Mass Spectrometry. Environ. Toxicol. Chem. 2021, 40, 2733–2741. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Himber, C.; Boricaud, B.; Kazour, M.; Amara, R.; Cassone, A.-L.; Laurentie, M.; Paul-Pont, I.; Soudant, P.; Dehaut, A.; et al. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal. Bioanal. Chem. 2018, 410, 6663–6676. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-C.; Lai, Y.-J.; Yu, S.-J.; Li, P.; Zhou, X.-X.; Dong, L.-J.; Liu, X.; Yao, Z.-W.; Liu, J.-F. Sequential Isolation of Microplastics and Nanoplastics in Environmental Waters by Membrane Filtration, Followed by Cloud-Point Extraction. Anal. Chem. 2021, 93, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, E.; Minor, E.C.; Schreiner, K. Microplastic Abundance and Composition in Western Lake Superior As Determined via Microscopy, Pyr-GC/MS, and FTIR. Environ. Sci. Technol. 2018, 52, 1787–1796. [Google Scholar] [CrossRef]
- Roscher, L.; Halbach, M.; Nguyen, M.T.; Hebeler, M.; Luschtinetz, F.; Scholz-Böttcher, B.M.; Primpke, S.; Gerdts, G. Microplastics in two German wastewater treatment plants: Year-long effluent analysis with FTIR and Py-GC/MS. Sci. Total Environ. 2022, 817, 152619. [Google Scholar] [CrossRef]
- Funck, M.; Yildirim, A.; Nickel, C.; Schram, J.; Schmidt, T.C.; Tuerk, J. Identification of microplastics in wastewater after cascade filtration using Pyrolysis-GC–MS. MethodsX 2020, 7, 100778. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Hamzah, S.R.; Khalik, W.M.A.W.M.; Yusof, K.M.K.K.; Anuar, S.T. Spatiotemporal microplastic occurrence study of Setiu Wetland, South China Sea. Sci. Total. Environ. 2021, 788, 147809. [Google Scholar] [CrossRef] [PubMed]
- Takdastan, A.; Niari, M.H.; Babaei, A.; Dobaradaran, S.; Jorfi, S.; Ahmadi, M. Occurrence and distribution of microplastic particles and the concentration of Di 2-ethyl hexyl phthalate (DEHP) in microplastics and wastewater in the wastewater treatment plant. J. Environ. Manag. 2021, 280, 111851. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Yu, K.; Li, N.; Liu, Y.; Liu, X.; Zhang, H.; Yang, B.; Wu, W.; Gao, J.; et al. Rapid Monitoring Approach for Microplastics Using Portable Pyrolysis-Mass Spectrometry. Anal. Chem. 2020, 92, 4656–4662. [Google Scholar] [CrossRef]
- Castelvetro, V.; Corti, A.; Biale, G.; Ceccarini, A.; Degano, I.; La Nasa, J.; Lomonaco, T.; Manariti, A.; Manco, E.; Modugno, F.; et al. New methodologies for the detection, identification, and quantification of microplastics and their environmental degradation by-products. Environ. Sci. Pollut. Res. 2021, 28, 46764–46780. [Google Scholar] [CrossRef]
- Gomiero, A.; Øysæd, K.B.; Palmas, L.; Skogerbø, G. Application of GCMS-pyrolysis to estimate the levels of microplastics in a drinking water supply system. J. Hazard. Mater. 2021, 416, 125708. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Böttcher, B.M. Simultaneous Trace Identification and Quantification of Common Types of Microplastics in Environmental Samples by Pyrolysis-Gas Chromatography–Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut. Res. 2021, 28, 16925–16947. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Gago, J.; Otero, V.; Sobral, P. Microplastics in coastal sediments from Southern Portuguese shelf waters. Mar. Environ. Res. 2016, 114, 24–30. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.-T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Biale, G.; La Nasa, J.; Mattonai, M.; Corti, A.; Vinciguerra, V.; Castelvetro, V.; Modugno, F. A Systematic Study on the Degradation Products Generated from Artificially Aged Microplastics. Polymers 2021, 13, 1997. [Google Scholar] [CrossRef]
- Goßmann, I.; Halbach, M.; Scholz-Böttcher, B.M. Car and truck tire wear particles in complex environmental samples – A quantitative comparison with “traditional” microplastic polymer mass loads. Sci. Total Environ. 2021, 773, 145667. [Google Scholar] [CrossRef] [PubMed]
- El Hayany, B.; El Fels, L.; Quénéa, K.; Dignac, M.F.; Rumpel, C.; Gupta, V.K.; Hafidi, M. Microplastics from lagooning sludge to composts as revealed by fluorescent staining- image analysis, Raman spectroscopy and pyrolysis-GC/MS. J. Environ. Manag. 2020, 275, 111249. [Google Scholar] [CrossRef]
- La Nasa, J.; Biale, G.; Mattonai, M.; Modugno, F. Microwave-assisted solvent extraction and double-shot analytical pyrolysis for the quali-quantitation of plasticizers and microplastics in beach sand samples. J. Hazard. Mater. 2021, 401, 123287. [Google Scholar] [CrossRef]
- Duemichen, E.; Eisentraut, P.; Celina, M.; Braun, U. Automated thermal extraction-desorption gas chromatography mass spectrometry: A multifunctional tool for comprehensive characterization of polymers and their degradation products. J. Chromatogr. A 2019, 1592, 133–142. [Google Scholar] [CrossRef]
- Sullivan, G.L.; Gallardo, J.D.; Jones, E.W.; Hollliman, P.J.; Watson, T.M.; Sarp, S. Detection of trace sub-micron (nano) plastics in water samples using pyrolysis-gas chromatography time of flight mass spectrometry (PY-GCToF). Chemosphere 2020, 249, 126179. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Fischer, M.; Lorenz, C.; Gerdts, G.; Scholz-Böttcher, B.M. Comparison of pyrolysis gas chromatography/mass spectrometry and hyperspectral FTIR imaging spectroscopy for the analysis of microplastics. Anal. Bioanal. Chem. 2020, 412, 8283–8298. [Google Scholar] [CrossRef] [PubMed]
- Dibke, C.; Fischer, M.; Scholz-Böttcher, B.M. Microplastic Mass Concentrations and Distribution in German Bight Waters by Pyrolysis–Gas Chromatography–Mass Spectrometry/Thermochemolysis Reveal Potential Impact of Marine Coatings: Do Ships Leave Skid Marks? Environ. Sci. Technol. 2021, 55, 2285–2295. [Google Scholar] [CrossRef]
- Bouzid, N.; Anquetil, C.; Dris, R.; Gasperi, J.; Tassin, B.; Derenne, S. Quantification of Microplastics by Pyrolysis Coupled with Gas Chromatography and Mass Spectrometry in Sediments: Challenges and Implications. Microplastics 2022, 1, 229–239. [Google Scholar] [CrossRef]
- Lim, S.J.; Park, Y.-K.; Kim, H.; Kwon, J.; Moon, H.M.; Lee, Y.; Watanabe, A.; Teramae, N.; Ohtani, H.; Kim, Y.-M. Selective solvent extraction and quantification of synthetic microfibers in textile laundry wastewater using pyrolysis-gas chromatography/mass spectrometry. Chem. Eng. J. 2022, 434, 134653. [Google Scholar] [CrossRef]
- Ishimura, T.; Iwai, I.; Matsui, K.; Mattonai, M.; Watanabe, A.; Robberson, W.; Cook, A.-M.; Allen, H.L.; Pipkin, W.; Teramae, N.; et al. Qualitative and quantitative analysis of mixtures of microplastics in the presence of calcium carbonate by pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2021, 157, 105188. [Google Scholar] [CrossRef]
- Funck, M.; Al-Azzawi, M.S.; Yildirim, A.; Knoop, O.; Schmidt, T.C.; Drewes, J.E.; Tuerk, J. Release of microplastic particles to the aquatic environment via wastewater treatment plants: The impact of sand filters as tertiary treatment. Chem. Eng. J. 2021, 426, 130933. [Google Scholar] [CrossRef]
- Youn, J.-S.; Kim, Y.-M.; Siddiqui, M.Z.; Watanabe, A.; Han, S.; Jeong, S.; Jung, Y.-W.; Jeon, K.-J. Quantification of tire wear particles in road dust from industrial and residential areas in Seoul, Korea. Sci. Total Environ. 2021, 784, 147177. [Google Scholar] [CrossRef]
- Nel, H.A.; Chetwynd, A.J.; Kelly, C.A.; Stark, C.; Valsami-Jones, E.; Krause, S.; Lynch, I. An Untargeted Thermogravimetric Analysis-Fourier Transform Infrared-Gas Chromatography-Mass Spectrometry Approach for Plastic Polymer Identification. Environ. Sci. Technol. 2021, 55, 8721–8729. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Yu, J.; Ni, F.; Sheng, Y.; Scircle, A.; Cizdziel, J.V.; Zhou, Y. Separation and identification of microplastics in marine organisms by TGA-FTIR-GC/MS: A case study of mussels from coastal China. Environ. Pollut. 2021, 272, 115946. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Altmann, K.; Sommerfeld, T.; Braun, U. Quantification of microplastics in a freshwater suspended organic matter using different thermoanalytical methods—Outcome of an interlaboratory comparison. J. Anal. Appl. Pyrolysis 2020, 148, 104829. [Google Scholar] [CrossRef]
- Ravit, B.; Cooper, K.; Moreno, G.; Buckley, B.; Yang, I.; Deshpande, A.; Meola, S.; Jones, D.; Hsieh, A. Microplastics in urban New Jersey freshwaters: Distribution, chemical identification, and biological affects. AIMS Environ. Sci. 2017, 4, 809–826. [Google Scholar] [CrossRef]
- O’Brien, S.; Okoffo, E.D.; Rauert, C.; O’Brien, J.W.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Wang, X.; Thomas, K.V. Quantification of selected microplastics in Australian urban road dust. J. Hazard. Mater. 2021, 416, 125811. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, X.; Liang, S.; Li, H.; Sun, L. Pyr-GC-MS analysis of microplastics extracted from farmland soils. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Bannick, C.G.; Szewzyk, R.; Ricking, M.; Schniegler, S.; Obermaier, N.; Barthel, A.K.; Altmann, K.; Eisentraut, P.; Braun, U. Development and testing of a fractionated filtration for sampling of microplastics in water. Water Res. 2019, 149, 650–658. [Google Scholar] [CrossRef]
- Braun, U.; Altmann, K.; Herper, D.; Knefel, M.; Bednarz, M.; Bannick, C.G. Smart filters for the analysis of microplastic in beverages filled in plastic bottles. Food Addit. Contam. Part A 2021, 38, 691–700. [Google Scholar] [CrossRef]
- Dekiff, J.H.; Remy, D.; Klasmeier, J.; Fries, E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Scherer, C.; Weber, A.; Stock, F.; Vurusic, S.; Egerci, H.; Kochleus, C.; Arendt, N.; Foeldi, C.; Dierkes, G.; Wagner, M.; et al. Comparative assessment of microplastics in water and sediment of a large European river. Sci. Total. Environ. 2020, 738, 139866. [Google Scholar] [CrossRef]
- Vilakati, B.; Sivasankar, V.; Nyoni, H.; Mamba, B.B.; Omine, K.; Msagati, T.A. The Py-GC-TOF-MS analysis and characterization of microplastics (MPs) in a wastewater treatment plant in Gauteng Province, South Africa. Ecotoxicol. Environ. Saf. 2021, 222, 112478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, Z.; Huang, Z.; Bao, Z.; Luo, T.; Jin, Y. Effects of Polyethylene Microplastics on the Microbiome and Metabolism in Larval Zebrafish. Environ. Pollut. 2021, 282, 117039. [Google Scholar] [CrossRef] [PubMed]
- Bolívar-Subirats, G.; Cortina-Puig, M.; Lacorte, S. Multiresidue Method for the Determination of High Production Volume Plastic Additives in River Waters. Environ. Sci. Pollut. Res. 2020, 27, 41314–41325. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, Z.; Zhang, J.; Wang, Z.; Zhu, Y.; Wang, P.; Zhang, T.; Pu, J.; Sun, H.; Wang, L. An Innovative Evaluation Method Based on Polymer Mass Detection to Evaluate the Contribution of Microfibers from Laundry Process to Municipal Wastewater. J. Hazard. Mater. 2021, 407, 124861. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, P.; Wu, X.; Shi, H.; Huang, H.; Wang, H.; Gao, S. Insight into Chain Scission and Release Profiles from Photodegradation of Polycarbonate Microplastics. Water Res. 2021, 195, 116980. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, G.F.; Llorca, M.; Seró, R.; Moyano, E.; Barceló, D.; Abad, E.; Farré, M. Trace Analysis of Polystyrene Microplastics in Natural Waters. Chemosphere 2019, 236, 124321. [Google Scholar] [CrossRef]
- Peng, C.; Tang, X.; Gong, X.; Dai, Y.; Sun, H.; Wang, L. Development and Application of a Mass Spectrometry Method for Quantifying Nylon Microplastics in Environment. Anal Chem. 2020, 92, 13930–13935. [Google Scholar] [CrossRef]
- Saliu, F.; Montano, S.; Hoeksema, B.W.; Lasagni, M.; Galli, P. A Non-Lethal SPME-LC/MS Method for the Analysis of Plastic-Associated Contaminants in Coral Reef Invertebrates. Anal. Methods 2020, 12, 1935–1942. [Google Scholar] [CrossRef]
- Saliu, F.; Montano, S.; Lasagni, M.; Galli, P. Biocompatible Solid-Phase Microextraction Coupled to Liquid Chromatography Triple Quadrupole Mass Spectrometry Analysis for the Determination of Phthalates in Marine Invertebrate. J. Chromatogr. A 2020, 1618, 460852. [Google Scholar] [CrossRef]
- Peng, C.; He, N.; Wu, Y.; Lu, Y.; Sun, H.; Wang, L. Excretion Characteristics of Nylon Microplastics and Absorption Risk of Nanoplastics in Rats. Ecotoxicol. Environ. Saf. 2022, 238, 113586. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, Y.; Song, K.; Du, W.; Huang, W.; Gu, Z.; Feng, Z. Microplastics in Different Tissues of Wild Crabs at Three Important Fishing Grounds in China. Chemosphere 2021, 271, 129479. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, D.; Liu, P.; Ouyang, Z.; Jia, H.; Guo, X. The Characteristics of Dis-solved Organic Matter Release from UV-Aged Microplastics and Its Cytotoxicity on Human Colonic Adenocarcinoma Cells. Sci. Total Environ. 2022, 826, 154177. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Hou, S.; Sun, H. A Simple Method for Quantifying Polycarbonate and Polyethylene Terephthalate Microplastics in Environmental Samples by Liquid Chromatography–Tandem Mass Spectrometry. Environ. Sci. Technol. Lett. 2017, 4, 530–534. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Zhang, Y.; Wang, L.; Deng, J.; Gao, Y.; Yu, L.; Zhang, J.; Sun, H. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ. Int. 2019, 128, 116–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Peng, C.; Wang, P.; Lu, Y.; He, X.; Wang, L. Comparison of Detection Methods of Microplastics in Landfill Mineralized Refuse and Selection of Degradation Degree Indexes. Environ. Sci. Technol. 2021, 55, 13802–13811. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Skoczynska, E.; Siddhanti, D.; van Putten, R.-J.; Leslie, H.A.; Gruter, G.-J.M. Quantification of polyethylene terephthalate microplastics and nanoplastics in sands, indoor dust and sludge using a simplified in-matrix depolymerization method. Mar. Pollut. Bull. 2022, 175, 113403. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, Y.; Xu, Y.; Zhang, J.; Liu, C.; Tang, X.; Lu, Y.; Sun, H. Earthworms’ Degradable Bioplastic Diet of Polylactic Acid: Easy to Break Down and Slow to Excrete. Environ. Sci. Technol. 2022, 56, 5020–5028. [Google Scholar] [CrossRef]
- Yan, M.; Yang, J.; Sun, H.; Liu, C.; Wang, L. Occurrence and distribution of microplastics in sediments of a man-made lake receiving reclaimed water. Sci. Total Environ. 2022, 813, 152430. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, S.; Zhao, L.; Duan, X.; Xie, S.; Zhang, H.; Liu, Y.; Peng, Y.; Liu, C.; Wang, L. Microplastics in Yellow River Delta wetland: Occurrence, characteristics, human influences, and marker. Environ. Pollut. 2020, 258, 113232. [Google Scholar] [CrossRef]
- Panio, A.; Corsarini, S.F.; Bruno, A.; Lasagni, M.; Labra, M.; Saliu, F. Determination of phthalates in fish fillets by liquid chromatography tandem mass spectrometry (LC-MS/MS): A comparison of direct immersion solid phase microextraction (SPME) versus ultrasonic assisted solvent extraction (UASE). Chemosphere 2020, 255, 127034. [Google Scholar] [CrossRef]
- Montano, S.; Seveso, D.; Maggioni, D.; Galli, P.; Corsarini, S.; Saliu, F. Spatial variability of phthalates contamination in the reef-building corals Porites lutea, Pocillopora verrucosa and Pavona varians. Mar. Pollut. Bull. 2020, 155, 111117. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.; Abrahamsson, D.P.; Song, W.; Yang, C.; Huang, Q.; Wang, J. Non-targeted analysis for organic components of microplastic leachates. Sci. Total Environ. 2022, 816, 151598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Halden, R.U.; Kannan, K. Polyethylene Terephthalate and Polycarbonate Microplastics in Sewage Sludge Collected from the United States. Environ. Sci. Technol. Lett. 2019, 6, 650–655. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Kannan, K. Microplastics in house dust from 12 countries and associated human exposure. Environ. Int. 2020, 134, 105314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Kannan, K. Polyethylene Terephthalate and Polycarbonate Microplastics in Pet Food and Feces from the United States. Environ. Sci. Technol. 2019, 53, 12035–12042. [Google Scholar] [CrossRef]
- Di Renzo, L.; Mascilongo, G.; Berti, M.; Bogdanović, T.; Listeš, E.; Brkljača, M.; Notarstefano, V.; Gioacchini, G.; Giorgini, E.; Olivieri, V.; et al. Potential Impact of Microplastics and Additives on the Health Status of Loggerhead Turtles (Caretta caretta) Stranded along the Central Adriatic Coast. Water Air Soil Pollut. 2021, 232, 98. [Google Scholar] [CrossRef]
- Raguso, C.; Saliu, F.; Lasagni, M.; Galli, P.; Clemenza, M.; Montano, S. First detection of microplastics in reef-building corals from a Maldivian atoll. Mar. Pollut. Bull. 2022, 180, 113773. [Google Scholar] [CrossRef]
- Jungnickel, H.; Pund, R.; Tentschert, J.; Reichardt, P.; Laux, P.; Harbach, H.; Luch, A. Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS)-Based Analysis and Imaging of Polyethylene Microplastics Formation during Sea Surf Simulation. Sci. Total Environ. 2016, 563–564, 261–266. [Google Scholar] [CrossRef]
- Pedersen, A.F.; Gopalakrishnan, K.; Boegehold, A.G.; Peraino, N.J.; Westrick, J.A.; Kashian, D.R. Microplastic Ingestion by Quagga Mussels, Dreissena Bugensis, and Its Effects on Physiological Processes. Environ. Pollut. 2020, 260, 113964. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, X.; Liu, Q.; Lin, Z.; Jiang, G. Thermal Fragmentation Enhanced Identification and Quantification of Polystyrene Micro/Nanoplastics in Complex Me-dia. Talanta 2020, 208, 120478. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.S.; Xu, L.; Cai, J.; Vedarethinam, V.; Tang, Y.; Guo, Q.; Huang, H.; Shen, N.; Di, W.; Ding, H.; et al. Zirconia Hybrid Nanoshells for Nutrient and Toxin Detection. Small 2020, 16, 2003902. [Google Scholar] [CrossRef] [PubMed]
- Guinan, T.; Ronci, M.; Vasani, R.; Kobus, H.; Voelcker, N.H. Comparison of the performance of different silicon-based SALDI substrates for illicit drug detection. Talanta 2015, 132, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Guinan, T.; Della Vedova, C.; Kobus, H.; Voelcker, N.H. Mass spectrometry imaging of fingerprint sweat on nanostructured silicon. Chem. Commun. 2015, 51, 6088–6091. [Google Scholar] [CrossRef]
- Korte, A.R.; Stopka, S.A.; Morris, N.; Razunguzwa, T.; Vertes, A. Large-Scale Metabolite Analysis of Standards and Human Serum by Laser Desorption Ionization Mass Spectrometry from Silicon Nanopost Arrays. Anal. Chem. 2016, 88, 8989–8996. [Google Scholar] [CrossRef]
- Liu, P.; Hu, Y.; Chen, J.; Yang, Q. Direct detection of the anti-cancer drug 9-phenylacridine in tissues by graphite rod laser desorption vacuum-ultraviolet post-ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 1328–1334. [Google Scholar] [CrossRef]
- Wu, B.S.; Gopal, J.; Hua, P.-Y.; Wu, H.-F. Graphene nanosheet mediated MALDI-MS (GN-MALDI-MS) for rapid, in situ detection of intact incipient biofilm on material surfaces. Mater. Sci. Eng. C 2016, 66, 285–296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
