Target and Suspect Analysis with High-Resolution Mass Spectrometry for the Exhaustive Monitoring of PCBs and Pesticides in Posidonia oceanica Meadows and Sediments
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Study Area and Sampling
2.3. Sample Pretreatment
2.4. Extraction Procedure
2.5. GC-Q-Orbitrap MS Parameters
2.6. Method Validation
2.7. Analysis of Organic Contaminants: Target and Suspect Screenings
3. Results
3.1. Extraction Procedure Optimization and Validation
3.2. Application: Occurrence and Compartmentation of POPs
3.2.1. Target Analysis: PCBs
3.2.2. Target Analysis: Priority Pesticides
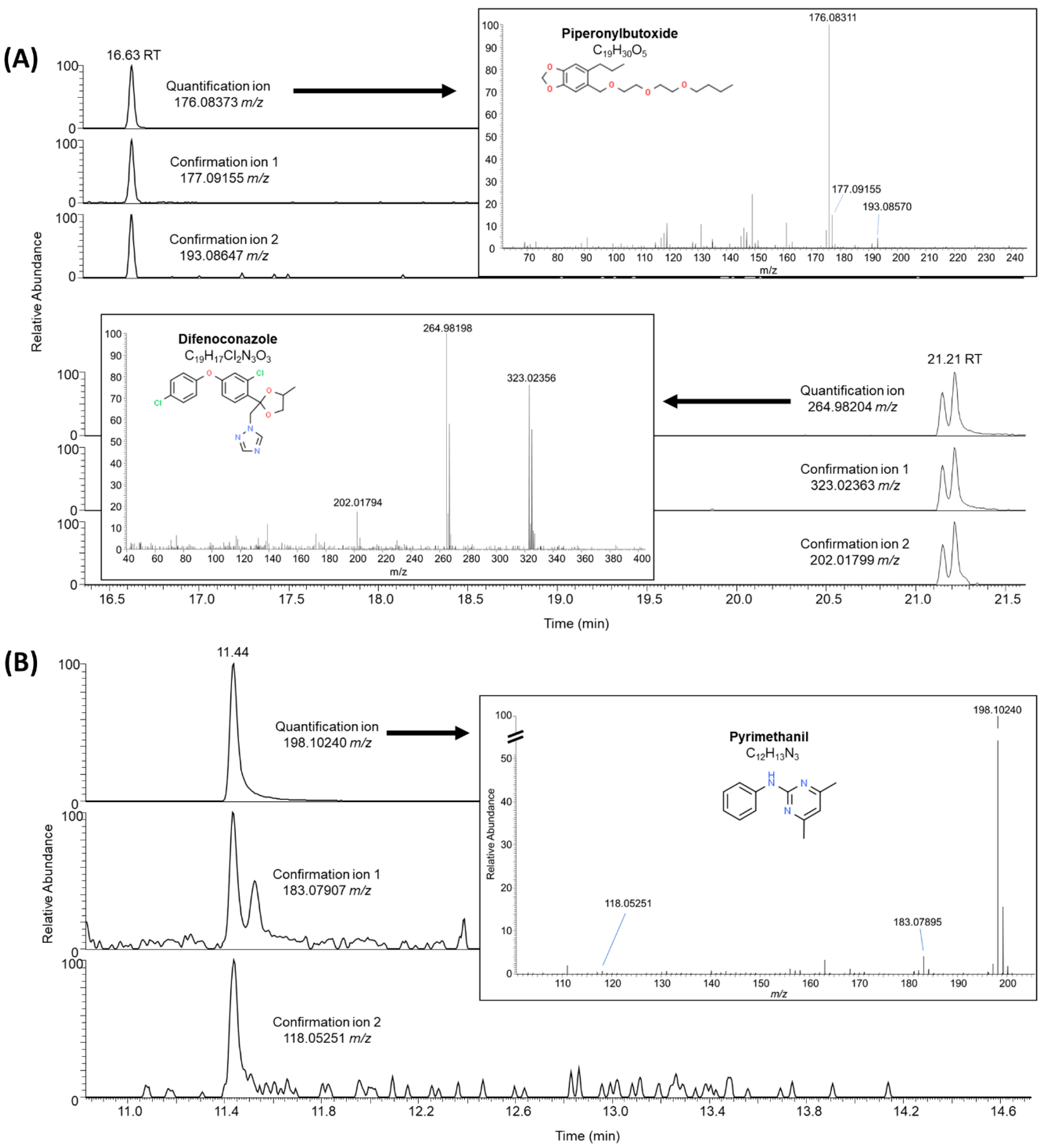
3.2.3. Suspect Analysis: Current-Use Pesticides
4. Discussion
4.1. Extraction Procedure
4.2. Target Analysis: PCBs
4.3. Target Analysis: Priority Pesticides
4.4. Suspect Analysis: Current-Use Pesticides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, R.; Bu, D.; Liu, G.; Zheng, M.; Lammel, G.; Fu, J.; Yang, L.; Li, C.; Habib, A.; Yang, Y.; et al. New classes of organic pollutants in the remote continental environment—Chlorinated and brominated polycyclic aromatic hydrocarbons on the Tibetan Plateau. Environ. Int. 2020, 137, 105574. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- 2455/2001/EC. Decision No 2455/2001/EC of the European Parliament and of the Council of 20 November 2001 Establishing the List of Priority Substances in the Field of Water Policy and Amending Directive 2000/60/EC. 2001, Volume 331. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:331:0001:0005:EN:PDF (accessed on 11 November 2022).
- Bilcke, C. Vanden The Stockholm Convention on Persistent Organic Pollutants. Am. J. Int. Law 2001, 95, 692–708. [Google Scholar] [CrossRef]
- UNEP. Report of the Meeting of the MED POL National Coordinators. In Facts Sheets on Marine Pollution Indicators; UNEP: Barcelona, Spain, 2005. [Google Scholar]
- Law, R.; Hanke, G.; Angelidis, M.O.; Batty, J.; Bignert, A.; Dachs, J.; Davies, I.; Denga, Y.; Duffek, A.; Herut, B.; et al. Marine Strategy Framework Directive. Task Group 8 Report: Contaminants and pollution effects. EUR 24335 EN—Joint Research Centre Scientific and Technical Reports; European Commission: Luxembourg, 2010. [Google Scholar]
- UNEP/RAMOGE. Manual on the Biomarkers Recommended for the MED POL Biomonitoring Programme; UNEP: Athens, Greece, 1999; ISBN 928071788X. [Google Scholar]
- Pergent-Martini, C.; Pergent, G. Marine phanerogams as a tool in the evaluation of marine trace-metal contamination: An example from the Mediterranean. Int. J. Environ. Pollut. 2000, 13, 126–147. [Google Scholar] [CrossRef]
- Pergent, G. Les indicateurs écologiques de la qualité du milieu marin en Méditerranée. Oceanis 1991, 17, 341–350. [Google Scholar]
- Bonanno, G.; Veneziano, V.; Orlando-Bonaca, M. Comparative assessment of trace element accumulation and biomonitoring in seaweed Ulva lactuca and seagrass Posidonia oceanica. Sci. Total Environ. 2020, 718, 137413. [Google Scholar] [CrossRef]
- Moreno, D.; Aguilera, P.A.; Castro, H. Assessment of the conservation status of seagrass (Posidonia oceanica) meadows: Implications for monitoring strategy and the decision-making process. Biol. Conserv. 2001, 102, 325–332. [Google Scholar] [CrossRef]
- Directive, H. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. 1992, 206, 7–50. [Google Scholar]
- Vassallo, P.; Paoli, C.; Rovere, A.; Montefalcone, M.; Morri, C.; Bianchi, C.N. The value of the seagrass Posidonia oceanica: A natural capital assessment. Mar. Pollut. Bull. 2013, 75, 157–167. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Pergent, G.; Monnier, B.; Boudouresque, C.-F.; Mori, C.; Valette-Sansevin, A. Contribution of Posidonia oceanica meadows in the context of climate change mitigation in the Mediterranean Sea. Mar. Environ. Res. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Jebara, A.; Lo Turco, V.; Potortì, A.G.; Bartolomeo, G.; Ben Mansour, H.; Di Bella, G. Organic pollutants in marine samples from Tunisian coast: Occurrence and associated human health risks. Environ. Pollut. 2021, 271, 116266. [Google Scholar] [CrossRef]
- Mauro, L.; Paola, G.; Margherita, V.; Rugiada, R.; Francesca, B.; Primo, M.; Duccio, S.; Enrica, F. Human impact on a small barrier reef meadow of Posidonia oceanica (L.) Delile on the north Tyrrhenian coast (Italy). Mar. Pollut. Bull. 2013, 77, 45–54. [Google Scholar] [CrossRef]
- Pergent, G.; Labbe, C.; Lafabrie, C.; Kantin, R.; Pergent-Martini, C. Organic and inorganic human-induced contamination of Posidonia oceanica meadows. Ecol. Eng. 2011, 37, 999–1002. [Google Scholar] [CrossRef]
- Apostolopoulou, M.V.; Monteyne, E.; Krikonis, K.; Pavlopoulos, K.; Roose, P.; Dehairs, F. Monitoring polycyclic aromatic hydrocarbons in the Northeast Aegean Sea using Posidonia oceanica seagrass and synthetic passive samplers. Mar. Pollut. Bull. 2014, 87, 338–344. [Google Scholar] [CrossRef]
- Pergent, G.; Boudouresque, C.-F.; Crouzet, A.; Meinesz, A. Cyclic Changes along Posidonia oceanica rhizomes (Lepidochronology): Present State and Perspectives. Mar. Ecol. 1989, 10, 221–230. [Google Scholar] [CrossRef]
- Pergent-Martini, C. Posidonia oceanica: A biological indicator of past and present mercury contamination in the Mediterranean Sea. Mar. Environ. Res. 1998, 45, 101–111. [Google Scholar] [CrossRef]
- Bucalossi, D.; Leonzio, C.; Casini, S.; Fossi, M.C.; Marsili, L.; Ancora, S.; Wang, W.; Scali, M. Application of a suite of biomarkers in Posidonia oceanica (L.) Delile to assess the ecotoxicological impact on the coastal environment. Mar. Environ. Res. 2006, 62, S327–S331. [Google Scholar] [CrossRef]
- Gil-Solsona, R.; Álvarez-Muñoz, D.; Serra-Compte, A.; Rodríguez-Mozaz, S. (Xeno)metabolomics for the evaluation of aquatic organism’s exposure to field contaminated water. Trends Environ. Anal. Chem. 2021, 31, e00132. [Google Scholar] [CrossRef]
- Goto, A.; Tue, N.M.; Isobe, T.; Takahashi, S.; Tanabe, S.; Kunisue, T. Nontarget and Target Screening of Organohalogen Compounds in Mussels and Sediment from Hiroshima Bay, Japan: Occurrence of Novel Bioaccumulative Substances. Environ. Sci. Technol. 2020, 54, 5480–5488. [Google Scholar] [CrossRef]
- Sanganyado, E.; Bi, R.; Teta, C.; Buruaem Moreira, L.; Yu, X.; Yajing, S.; Dalu, T.; Rajput, I.R.; Liu, W. Toward an integrated framework for assessing micropollutants in marine mammals: Challenges, progress, and opportunities. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2824–2871. [Google Scholar] [CrossRef]
- Gómez-Gutiérrez, A.; Garnacho, E.; Bayona, J.M.; Albaigés, J. Assessment of the Mediterranean sediments contamination by persistent organic pollutants. Environ. Pollut. 2007, 148, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Astudillo-Pascual, M.; Domínguez, I.; Aguilera, P.A.; Garrido Frenich, A. New Phenolic Compounds in Posidonia oceanica Seagrass: A Comprehensive Array Using High Resolution Mass Spectrometry. Plants 2021, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Analytical quality control and method validation procedures for pesticides residues analysis in food and feed (SANTE 11312/2021). Eur. Comm. 2021, 1–55. [Google Scholar]
- Commission Directive 2009/90/EC. Directive 2009/90/EC of 31 July 2009 Laying Down, Pursuant to Directive 2000/60/EC of the European Parliament and of the Council, Technical Specifications for Chemical Analysis and Monitoring of Water Status. Off. J. Eur. 2009, L201, 36–38. [Google Scholar]
- Vargas-Pérez, M.; Domínguez, I.; González, F.J.E.; Frenich, A.G. Application of full scan gas chromatography high resolution mass spectrometry data to quantify targeted-pesticide residues and to screen for additional substances of concern in fresh-food commodities. J. Chromatogr. A 2020, 1622, 461118. [Google Scholar] [CrossRef]
- Barco-Bonilla, N.; Nieto-García, A.J.; Romero-González, R.; Martínez Vidal, J.L.; Frenich, A.G. Simultaneous and highly sensitive determination of PCBs and PBDEs in environmental water and sediments by gas chromatography coupled to high resolution magnetic sector mass spectrometry. Anal. Methods 2015, 7, 3036–3047. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. In-cell clean-up pressurized liquid extraction and gas chromatography–tandem mass spectrometry determination of hydrophobic persistent and emerging organic pollutants in coastal sediments. J. Chromatogr. A 2016, 1429, 107–118. [Google Scholar] [CrossRef]
- Domínguez, I.; Arrebola, F.J.; Romero-González, R.; Nieto-García, A.; Martínez Vidal, J.L.; Garrido Frenich, A. Solid phase microextraction and gas chromatography coupled to magnetic sector high resolution mass spectrometry for the ultra-trace determination of contaminants in surface water. J. Chromatogr. A 2017, 1518, 15–24. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Maldonado-Reina, A.J.; López-Ruiz, R.; Garrido Frenich, A.; Arrebola, F.J.; Romero-González, R. Co-formulants in plant protection products: An analytical approach to their determination by gas chromatography–high resolution mass accuracy spectrometry. Talanta 2021, 234, 122641. [Google Scholar] [CrossRef]
- Garrido Frenich, A.; Martínez Vidal, J.L.; Fernández Moreno, J.L.; Romero-González, R. Compensation for matrix effects in gas chromatography-tandem mass spectrometry using a single point standard addition. J. Chromatogr. A 2009, 1216, 4798–4808. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Lewis, M.A.; Dantin, D.D.; Chancy, C.A.; Abel, K.C.; Lewis, C.G. Florida seagrass habitat evaluation: A comparative survey for chemical quality. Environ. Pollut. 2007, 146, 206–218. [Google Scholar] [CrossRef]
- Qiu, Y.W.; Qiu, H.L.; Zhang, G.; Li, J. Bioaccumulation and cycling of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in three mangrove reserves of south China. Chemosphere 2019, 217, 195–203. [Google Scholar] [CrossRef]
- Robinson, C.D.; Webster, L.; Martínez-Gómez, C.; Burgeot, T.; Gubbins, M.J.; Thain, J.E.; Vethaak, A.D.; McIntosh, A.D.; Hylland, K. Assessment of contaminant concentrations in sediments, fish and mussels sampled from the North Atlantic and European regional seas within the ICON project. Mar. Environ. Res. 2017, 124, 21–31. [Google Scholar] [CrossRef]
- León, V.M.; Viñas, L.; Concha-Graña, E.; Fernández-González, V.; Salgueiro-González, N.; Moscoso-Pérez, C.; Muniategui-Lorenzo, S.; Campillo, J.A. Identification of contaminants of emerging concern with potential environmental risk in Spanish continental shelf sediments. Sci. Total Environ. 2020, 742, 140505. [Google Scholar] [CrossRef]
- Abbassy, M.M.S. Distribution pattern of persistent organic pollutants in aquatic ecosystem at the Rosetta Nile branch estuary into the Mediterranean Sea, North of Delta, Egypt. Mar. Pollut. Bull. 2018, 131, 115–121. [Google Scholar] [CrossRef]
- Montuori, P.; Aurino, S.; Garzonio, F.; Triassi, M. Polychlorinated biphenyls and organochlorine pesticides in Tiber River and Estuary: Occurrence, distribution and ecological risk. Sci. Total Environ. 2016, 571, 1001–1016. [Google Scholar] [CrossRef]
- Moschino, V.; Del Negro, P.; De Vittor, C.; Da Ros, L. Biomonitoring of a polluted coastal area (Bay of Muggia, Northern Adriatic Sea): A five-year study using transplanted mussels. Ecotoxicol. Environ. Saf. 2016, 128, 1–10. [Google Scholar] [CrossRef]
- Benedicto, J.; Campillo, J.A.; Fernández, B.; Martínez-Gómez, C.; León, V.M. Estrategia Marina Demarcación Marina Levantino-Balear Parte IV. Descriptores del Buen Estado Ambiental. Descriptor 8: Contaminantes y sus efectos. Evaluación inicial y Buen Estado Ambiental. [Marine Strategy for the Levantine—Balearic Marine Demarcation. Descriptors of Good Environmental Status. Descriptor 8: Pollutants and their Effects. Initial Evaluation and Good Environmental Status; Ministry of Agriculture, Food and Environment, Government of Spain: Madrid, Spain, 2012.
- Benedicto, J.; Campillo, J.A.; Fernández, B.; Martínez-Gómez, C.; León, V.M. Estrategia Marina Demarcación Marina Estrecho y Alborán. Descriptores del Buen Estado Ambiental. Descriptor 8: Contaminantes y sus efectos. Evaluación Inicial y Buen Estado Ambiental. [Marine Strategy for the Strait and Alboran Marine Demarcation. Descriptors of Good Environmental Status. Descriptor 8: Pollutants and their Effects. Initial Evaluation and Good Environmental Status; Ministry of Agriculture, Food and Environment, Government of Spain: Madrid, Spain, 2012.
- Bajt, O.; Ramšak, A.; Milun, V.; Andral, B.; Romanelli, G.; Scarpato, A.; Mitrić, M.; Kupusović, T.; Kljajić, Z.; Angelidis, M.; et al. Assessing chemical contamination in the coastal waters of the Adriatic Sea using active mussel biomonitoring with Mytilus galloprovincialis. Mar. Pollut. Bull. 2019, 141, 283–298. [Google Scholar] [CrossRef]
- Moreno-González, R.; León, V.M. Presence and distribution of current-use pesticides in surface marine sediments from a Mediterranean coastal lagoon (SE Spain). Environ. Sci. Pollut. Res. 2017, 24, 8033–8048. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Estarellas, F.; Deudero, S. Microplastics in the Mediterranean Sea: Deposition in coastal shallow sediments, spatial variation and preferential grain size. Mar. Environ. Res. 2016, 115, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.C. Persistent Organic Pollutants (POPs) and Related Chemicals in the Global Environment: Some Personal Reflections. Environ. Sci. Technol. 2021, 55, 9400–9412. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, I.; Sintes, T.; Bouma, T.; Duarte, C. Experimental assessment and modeling evaluation of the effects of the seagrass Posidonia oceanica on flow and particle trapping. Mar. Ecol. Prog. Ser. 2008, 356, 163–173. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuar. Coast. Shelf Sci. 2014, 140, 14–21. [Google Scholar] [CrossRef]
- de los Santos, C.B.; Krång, A.-S.; Infantes, E. Microplastic retention by marine vegetated canopies: Simulations with seagrass meadows in a hydraulic flume. Environ. Pollut. 2021, 269, 116050. [Google Scholar] [CrossRef]
- Gacia, E.; Granata, T.C.; Duarte, C.M. An approach to measurement of particle flux and sediment retention within seagrass (Posidonia oceanica) meadows. Aquat. Bot. 1999, 65, 255–268. [Google Scholar] [CrossRef]
- Gerstenbacher, C.M.; Finzi, A.C.; Rotjan, R.D.; Novak, A.B. A review of microplastic impacts on seagrasses, epiphytes, and associated sediment communities. Environ. Pollut. 2022, 303, 119108. [Google Scholar] [CrossRef]
- Guigue, C.; Tedetti, M.; Dang, D.H.; Mullot, J.-U.; Garnier, C.; Goutx, M. Remobilization of polycyclic aromatic hydrocarbons and organic matter in seawater during sediment resuspension experiments from a polluted coastal environment: Insights from Toulon Bay (France). Environ. Pollut. 2017, 229, 627–638. [Google Scholar] [CrossRef]
| LEAF | RHIZOME | SEDIMENT | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | LOQ (µg kg−1) | Linear Working Range (µg kg−1) | Linearity (R2) | Recovery | Inter-Day Precision (RSD%) | LOQ (µg kg−1) | Linear Working Range (µg kg−1) | Linearity (R2) | Recovery | Inter-Day Precision (RSD%) | LOQ (µg kg−1) | Linear Working Range (µg kg−1) | Linearity (R2) | Recovery | Inter-Day Precision (RSD%) | ||||||
| R (%) b | R (%) b | R (%) b | |||||||||||||||||||
| VL1 | VL2 | VL1 | VL2 | VL1 | VL2 | VL1 | VL2 | VL1 | VL2 | VL1 | VL2 | ||||||||||
| PCBs | |||||||||||||||||||||
| PCB 18 | 0.266 | 10–1000 | 0.9922 | 98(7) | 108(10) | 18 | 15 | 0.168 | 10–400 | 0.9986 | 97(9) | 98(3) | 17 | 6 | 0.017 | 1–200 | 0.9977 | 104(9) | 98(3) | 11 | 7 |
| PCB 28+31 | 0.516 | 20–2000 | 0.9978 | 104(5) | 99(0) | 6 | 17 | 0.378 | 10–400 | 0.9988 | 93(3) | 99(8) | 1 | 7 | 0.023 | 1–200 | 0.9989 | 99(2) | 108(3) | 7 | 5 |
| PCB 52 | 0.404 | 10–400 | 0.9966 | 110(5) | 99(1) | 17 | 13 | 0.378 | 10–400 | 0.9979 | 101(5) | 100(5) | 4 | 3 | 0.013 | 1–200 | 0.9991 | 111(4) | 100(1) | 5 | 3 |
| PCB 44 | 0.015 | 20–2000 | 0.9998 | 99(0) | 100(0) | 8 | 17 | 0.127 | 10–1000 | 0.9982 | 105(4) | 99(2) | 5 | 6 | 0.009 | 1–200 | 0.9986 | 118(2) | 98(3) | 3 | 3 |
| PCB 66 | 0.151 | 20–2000 | 0.9993 | 120(4) | 101(1) | 17 | 9 | 0.534 | 10–200 | 0.9930 | 115(17) | 95(8) | 18 | 5 | 0.027 | 1–200 | 0.9984 | 108(8) | 98(4) | 15 | 8 |
| PCB 101 | 0.753 | 20–2000 | 0.9998 | 99(2) | 100(0) | 18 | 17 | 0.210 | 10–400 | 0.9894 | 94(11) | 106(10) | 2 | 4 | 0.040 | 1–200 | 0.9951 | 114(9) | 97(8) | 13 | 9 |
| PCB 81 | 0.485 | 10–1000 | 0.9997 | 99(5) | 99(2) | 10 | 15 | 0.061 | 10–1000 | 0.9982 | 80(3) | 99(2) | 8 | 4 | 0.004 | 0.2–200 | 0.9926 | 102(7) | 108(9) | 10 | 10 |
| PCB 77 | 0.018 | 10–2000 | 0.9997 | 106(7) | 100(1) | 8 | 14 | 0.162 | 10–200 | 0.9916 | 106(7) | 101(1) | 9 | 3 | 0.030 | 1–200 | 0.9964 | 94(10) | 105(7) | 12 | 10 |
| PCB 123 | 0.363 | 20–1000 | 0.9996 | 104(1) | 99(2) | 8 | 15 | 0.009 | 10–400 | 0.9987 | 93(1) | 95(13) | 12 | 2 | 0.015 | 1–40 | 0.9975 | 103(7) | 100(9) | 8 | 9 |
| PCB 118 | 0.121 | 20–1000 | 0.9979 | 101(2) | 100(1) | 5 | 14 | 0.037 | 10–400 | 0.9986 | 99(2) | 91(8) | 14 | 3 | 0.004 | 0.2–200 | 0.9926 | 102(5) | 93(10) | 7 | 6 |
| PCB 114 | 0.032 | 10–400 | 0.9991 | 101(2) | 102(3) | 5 | 6 | 0.074 | 10–400 | 0.9984 | 98(8) | 94(10) | 18 | 9 | 0.046 | 1–200 | 0.9965 | 118(11) | 96(7) | 17 | 8 |
| PCB 153 | 0.368 | 20–400 | 0.9951 | 100(6) | 100(3) | 11 | 18 | 0.035 | 10–400 | 0.9988 | 93(2) | 99(10) | 11 | 3 | 0.093 | 2–200 | 0.9989 | 97(10) | 100(1) | 15 | 5 |
| PCB 105 | 0.608 | 20–1000 | 0.9973 | 97(4) | 101(1) | 10 | 18 | 0.075 | 10–400 | 0.9987 | 101(3) | 98(10) | 14 | 11 | 0.006 | 2–200 | 0.9936 | 102(13) | 95(9) | 15 | 9 |
| PCB 138 | 0.267 | 10–1000 | 0.9970 | 99(0) | 100(2) | 3 | 14 | 0.261 | 10–1000 | 0.9803 | 93(15) | 97(4) | 10 | 5 | 0.004 | 1–200 | 0.9984 | 118(2) | 99(2) | 2 | 1 |
| PCB 126 | 0.136 | 20–2000 | 0.9998 | 94(2) | 100(0) | 4 | 14 | 0.021 | 10–2000 | 0.9996 | 107(1) | 100(1) | 16 | 12 | 0.016 | 2–40 | 0.9760 | 85(5) | 109(15) | 15 | 7 |
| PCB 128 | 0.022 | 20–400 | 0.9908 | 107(1) | 101(1) | 11 | 12 | 0.121 | 10–400 | 0.9979 | 99(12) | 99(8) | 1 | 3 | 0.019 | 2–200 | 0.9987 | 90(12) | 100(5) | 13 | 9 |
| PCB 167 | 0.023 | 10–1000 | 0.9982 | 102(1) | 100(1) | 4 | 11 | 0.130 | 10–400 | 0.9988 | 92(7) | 99(10) | 3 | 1 | 0.046 | 1–200 | 0.9987 | 113(10) | 100(1) | 15 | 8 |
| PCB 156 | 0.177 | 20–2000 | 0.9985 | 101(1) | 109(2) | 4 | 14 | 0.050 | 10–2000 | 0.9994 | 80(3) | 100(1) | 5 | 10 | 0.010 | 1–200 | 0.9990 | 85(7) | 99(2) | 18 | 7 |
| PCB 157 | 0.265 | 20–1000 | 0.9934 | 100(1) | 102(1) | 6 | 15 | 0.035 | 10–400 | 0.9988 | 86(2) | 99(10) | 2 | 4 | 0.024 | 2–200 | 0.9995 | 84(4) | 100(1) | 18 | 10 |
| PCB 180 | 0.206 | 20–1000 | 0.9965 | 108(9) | 100(0) | 9 | 17 | 0.182 | 10–400 | 0.9976 | 88(16) | 98(4) | 18 | 8 | 0.028 | 2–200 | 0.9997 | 101(16) | 100(3) | 18 | 5 |
| PCB 169 | 0.171 | 10–1000 | 0.9977 | 100(1) | 100(1) | 10 | 15 | 0.052 | 10–2000 | 0.9996 | 102(1) | 100(0) | 13 | 8 | 0.048 | 2–200 | 0.9984 | 115(7) | 101(0) | 10 | 5 |
| PCB 170 | 0.253 | 20–1000 | 0.9976 | 95(1) | 99(2) | 8 | 14 | 0.057 | 10–400 | 0.9987 | 91(4) | 99(9) | 5 | 4 | 0.001 | 1–200 | 0.9981 | 115(1) | 99(3) | 12 | 10 |
| PCB 189 | 0.253 | 20–1000 | 0.9978 | 99(8) | 100(1) | 15 | 15 | 0.094 | 10–2000 | 0.9991 | 98(8) | 101(2) | 9 | 12 | 0.017 | 2–200 | 0.9981 | 82(6) | 103(4) | 10 | 10 |
| PCB 194 | 0.078 | 20–2000 | 0.9986 | 97(5) | 99(0) | 5 | 16 | 0.069 | 10–2000 | 0.9993 | 90(8) | 100(1) | 10 | 5 | 0.070 | 2–40 | 0.9801 | 101(14) | 102(7) | 17 | 5 |
| PCB 206 | 0.212 | 20–1000 | 0.9986 | 83(8) | 99(2) | 14 | 14 | 0.018 | 10–1000 | 0.9992 | 120(1) | 100(1) | 10 | 5 | 0.005 | 1- 40 | 0.9948 | 81(9) | 98(5) | 13 | 9 |
| Pesticides | |||||||||||||||||||||
| Pentachloro-benzene | 0.286 | 2–1000 | 0.9993 | 85(17) | 102(3) | 18 | 5 | 0.070 | 2–400 | 0.9996 | 99(3) | 100(1) | 13 | 10 | 0.001 | 0.2–40 | 0.9995 | 104(0) | 100(1) | 5 | 2 |
| Trifluralin | 0.123 | 10–1000 | 0.9998 | 100(2) | 102(0) | 6 | 3 | 0.305 | 10–400 | 0.9980 | 106(5) | 99(7) | 7 | 2 | 0.050 | 1–40 | 0.9950 | 117(4) | 101(1) | 7 | 4 |
| Hexachloro-benzene | 1.131 | 10–2000 | 0.9985 | 97(10) | 98(2) | 5 | 18 | 0.359 | 10–200 | 0.9969 | 120(8) | 101(0) | 3 | 8 | 0.205 | 2–40 | 0.9966 | 114(10) | 99(2) | 4 | 5 |
| Simazine | 0.550 | 20–1000 | 0.9991 | 101(4) | 102(2) | 10 | 3 | 0.011 | 10–200 | 0.9978 | 119(8) | 107(1) | 5 | 2 | 0.019 | 2–40 | 0.9875 | 91(1) | 103(6) | 4 | 9 |
| Atrazine | 0.376 | 10–2000 | 0.9994 | 102(6) | 102(2) | 5 | 2 | 0.777 | 10–400 | 0.9981 | 108(8) | 94(12) | 4 | 10 | 0.047 | 1–40 | 0.9981 | 102(4) | 101(4) | 5 | 11 |
| Chlorpyrifos | 0.076 | 10–2000 | 0.9974 | 101(2) | 97(4) | 6 | 3 | 0.158 | 10–400 | 0.9863 | 96(5) | 109(12) | 3 | 5 | 0.103 | 2–40 | 0.9940 | 87(9) | 101(3) | 10 | 16 |
| Aldrin | 0.216 | 20–1000 | 0.9993 | 85(5) | 101(1) | 2 | 1 | 0.635 | 10–2000 | 0.9997 | 112(3) | 99(2) | 4 | 6 | 0.025 | 2–40 | 0.9958 | 81(4) | 100(2) | 6 | 9 |
| Isodrin | 0.756 | 10–2000 | 0.9976 | 97(15) | 100(2) | 5 | 1 | 0.221 | 10–1000 | 0.9997 | 90(5) | 99(2) | 20 | 10 | 0.098 | 2–100 | 0.9991 | 100(6) | 100(2) | 9 | 9 |
| Dieldrin | 0.126 | 20–1000 | 0.9959 | 82(3) | 101(3) | 4 | 5 | 0.621 | 10–200 | 1.0000 | 108(13) | 101(3) | 9 | 6 | 0.002 | 1–100 | 0.9989 | 87(1) | 99(1) | 14 | 6 |
| Endrin | 0.946 | 10–400 | 0.9805 | 89(1) | 104(2) | 9 | 10 | 1.819 | 10–200 | 0.9964 | 107(11) | 99(1) | 12 | 6 | 0.002 | 1–100 | 0.9873 | 87(1) | 100(2) | 13 | 4 |
| o,p’-DDT | 5.348 | 20–1000 | 0.9882 | 92(16) | 100(1) | 18 | 15 | 9.785 | 10–400 | 0.9991 | 115(15) | 110(6) | 17 | 10 | 0.130 | 1–40 | 0.9973 | 91(9) | 102(3) | 11 | 9 |
| p,p’-DDD | 0.482 | 10–400 | 0.9923 | 110(12) | 106(9) | 15 | 10 | 1.982 | 10–200 | 0.9991 | 107(13) | 100(1) | 16 | 8 | 0.048 | 1–40 | 0.9923 | 92(12) | 94(9) | 13 | 6 |
| p,p’-DDT | 4.725 | 10–400 | 0.9982 | 118(15) | 100(9) | 15 | 13 | 0.419 | 10–400 | 0.9996 | 110(13) | 100(6) | 19 | 8 | 0.015 | 2–100 | 0.9996 | 112(4) | 99(2) | 8 | 9 |
| Matrix | Site | PCB 18 | PCB 28+31 | PCB 52 | PCB 44 | PCB 101 | PCB 81 | PCB 77 | PCB 123 | PCB 118 | PCB 114 | PCB 153 | PCB 105 | PCB 138 | PCB 128 | PCB 167 | PCB 157 | PCB 180 | PCB 170 | ƩPCBs | ∑7 PCBs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhizome | ALI5 | -- | 10.7 | 1.6 | 11.2 | -- | 9.7 | 11.1 | -- | -- | -- | 2.0 | -- | -- | -- | 8.7 | -- | -- | -- | 55.0 | 14.3 |
| ALI6 | -- | 1.8 | 0.6 | 1.9 | -- | -- | 1.3 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 5.6 | 2.4 | |
| ALI7 | -- | 2.6 | 0.6 | 1.6 | -- | 1.1 | 1.6 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 7.5 | 3.2 | |
| V-sed | ALI5 | 1.4 | 1.5 | 1.1 | 1.7 | 1.2 | 0.8 | 0.9 | 1.1 | 0.9 | 0.9 | 0.9 | 0.9 | 1.6 | 1.0 | 1.4 | 1.5 | 1.1 | 1.5 | 21.5 | 8.3 |
| Matrix | Site | Trifluralin | Chlorpyrifos | Isodrin | o,p´-DDT | ƩPesticides |
|---|---|---|---|---|---|---|
| Rhizome | ALI5 | 3.9 | 3.2 | 1.9 | * | 9.0 |
| ALI6 | 2.0 | 1.3 | 1.7 | -- | 5.0 | |
| ALI7 | -- | -- | 1.0 | -- | 1.0 |
| Almeria Region | Alicante Region | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix | Compound | EE2 | EE4 | RM4 | RM6 | ALM1 | ALM3 | V1 | V2 | CG2 | CG3 | CG4 | C2 | ALI1 | ALI2 | ALI3 | ALI7 | ALI5 | ALI6 | ALI4 |
| Leaf | 1,4-Dimethyl naphthalene | 3.22 | 8.97 | -- | n.s. | -- | -- | n.s. | 28.96 | -- | 16.87 | -- | -- | -- | -- | -- | 10.02 | 14.31 | 16.32 | 9.88 |
| 2-Phenylphenol | -- | -- | 2.89 | n.s. | -- | -- | n.s. | -- | -- | -- | -- | -- | -- | 11.03 | 8.43 | 9.18 | 7.16 | 9.6 | ||
| Terbutryn | -- | -- | -- | n.s. | -- | -- | n.s. | -- | -- | -- | -- | -- | 0.21 | 0.11 | -- | -- | -- | -- | -- | |
| Tetraconazole | -- | -- | -- | n.s. | -- | -- | n.s. | -- | -- | -- | -- | -- | -- | -- | -- | 1.12 | -- | -- | -- | |
| Piperonylbutoxide | -- | -- | -- | n.s. | -- | -- | n.s. | -- | -- | 84.39 | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Difenoconazole | -- | -- | -- | n.s. | -- | -- | n.s. | -- | -- | 265.24 | -- | -- | -- | -- | -- | 10.05 | -- | -- | -- | |
| Mean regional values | 82.11 | 15.35 | ||||||||||||||||||
| Rhizome | 2,4,6-trichlorophenol | -- | -- | -- | n.s. | -- | 0.88 | n.s. | -- | -- | -- | 0.36 | -- | -- | -- | -- | -- | -- | -- | -- |
| 1,4-Dimethyl naphthalene | -- | -- | -- | n.s. | -- | 5.33 | n.s. | -- | -- | -- | 4.46 | -- | -- | -- | -- | -- | -- | -- | -- | |
| Lindane | -- | -- | -- | n.s. | -- | -- | n.s. | -- | -- | -- | -- | -- | -- | -- | -- | 0.22 | 0.51 | 0.11 | -- | |
| Pyrimethanil | -- | -- | -- | n.s. | -- | 40.66 | n.s. | -- | -- | -- | 29.29 | -- | 0.13 | -- | -- | -- | -- | -- | ||
| Penconazole | -- | -- | -- | n.s. | -- | 0.28 | n.s. | -- | -- | -- | 0.13 | -- | -- | -- | -- | -- | -- | -- | -- | |
| Fludioxonil | -- | -- | -- | n.s. | -- | -- | n.s. | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 2.85 | 7.93 | |
| Fenbuconazole | -- | 6.16 | 8.73 | n.s. | 6.59 | -- | n.s. | -- | 6.84 | 7.11 | -- | -- | 4.08 | -- | -- | 3.15 | -- | -- | -- | |
| Mean regional values | 16.69 | 3.63 | ||||||||||||||||||
| NV-Sed | Prallethrin | -- | -- | -- | 5.78 | -- | -- | 7.45; 5.52 | n.s. | -- | -- | -- | 6.9 | 4.23 | n.s. | n.s. | 7.31 | -- | -- | -- |
| V-Sed | -- | -- | -- | n.s. | -- | -- | n.s. | n.s. | -- | -- | -- | 5.28 | 5.01 | 6.01; 4.27 | 7.53; -- | 5.74 | -- | -- | -- | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astudillo-Pascual, M.; Aguilera, P.A.; Garrido Frenich, A.; Domínguez, I. Target and Suspect Analysis with High-Resolution Mass Spectrometry for the Exhaustive Monitoring of PCBs and Pesticides in Posidonia oceanica Meadows and Sediments. Chemosensors 2022, 10, 531. https://doi.org/10.3390/chemosensors10120531
Astudillo-Pascual M, Aguilera PA, Garrido Frenich A, Domínguez I. Target and Suspect Analysis with High-Resolution Mass Spectrometry for the Exhaustive Monitoring of PCBs and Pesticides in Posidonia oceanica Meadows and Sediments. Chemosensors. 2022; 10(12):531. https://doi.org/10.3390/chemosensors10120531
Chicago/Turabian StyleAstudillo-Pascual, Marina, Pedro A. Aguilera, Antonia Garrido Frenich, and Irene Domínguez. 2022. "Target and Suspect Analysis with High-Resolution Mass Spectrometry for the Exhaustive Monitoring of PCBs and Pesticides in Posidonia oceanica Meadows and Sediments" Chemosensors 10, no. 12: 531. https://doi.org/10.3390/chemosensors10120531
APA StyleAstudillo-Pascual, M., Aguilera, P. A., Garrido Frenich, A., & Domínguez, I. (2022). Target and Suspect Analysis with High-Resolution Mass Spectrometry for the Exhaustive Monitoring of PCBs and Pesticides in Posidonia oceanica Meadows and Sediments. Chemosensors, 10(12), 531. https://doi.org/10.3390/chemosensors10120531








