Simulation Calculation Verification of Graphene Oxide-Decorated Silver Nanoparticles Growing on Titania Nanotube Array as SERS Sensor Substrate
Abstract
1. Introduction
2. Experimental Measurement and Theoretical Calculation Section
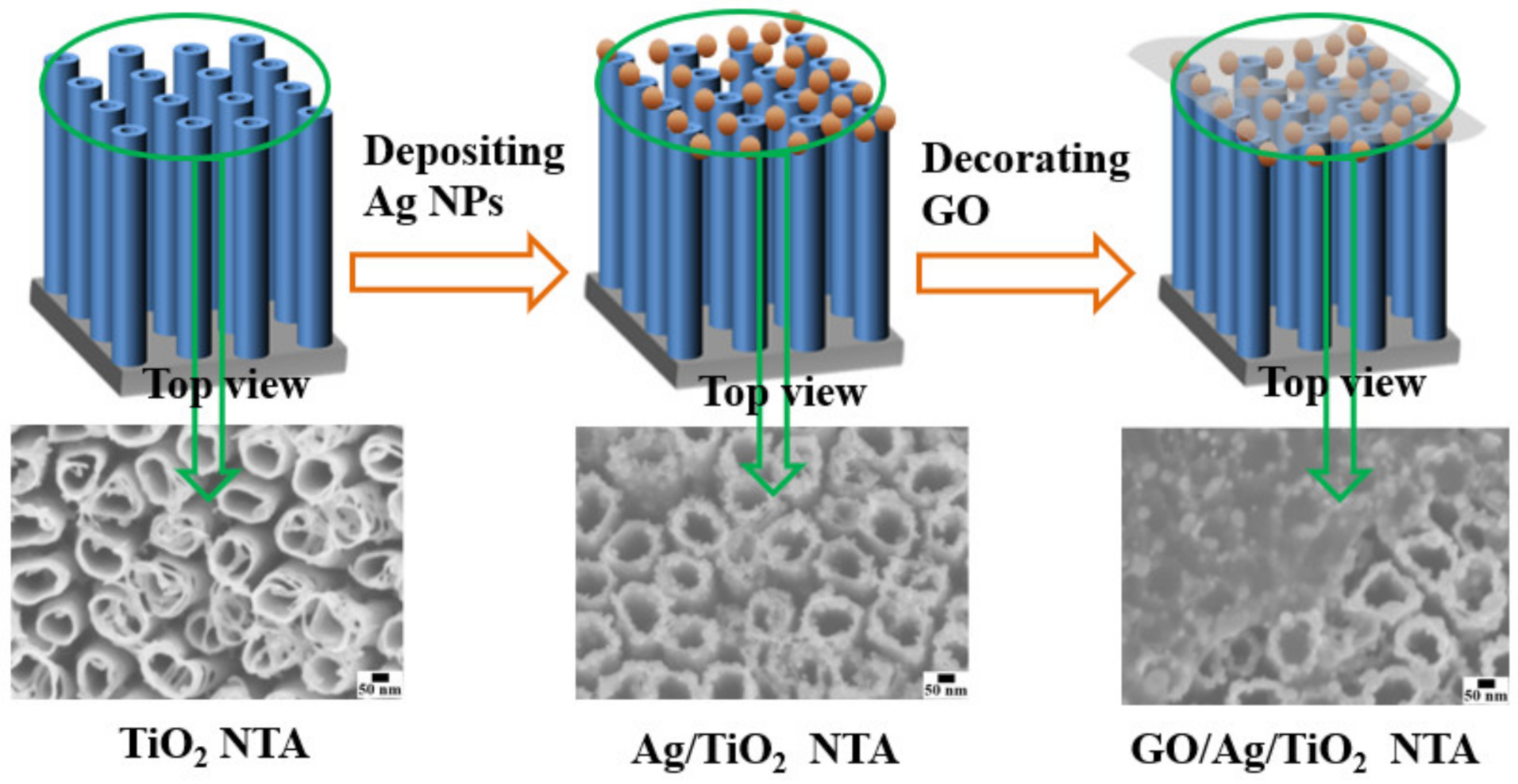
2.1. Experimental Measurement and Characterization
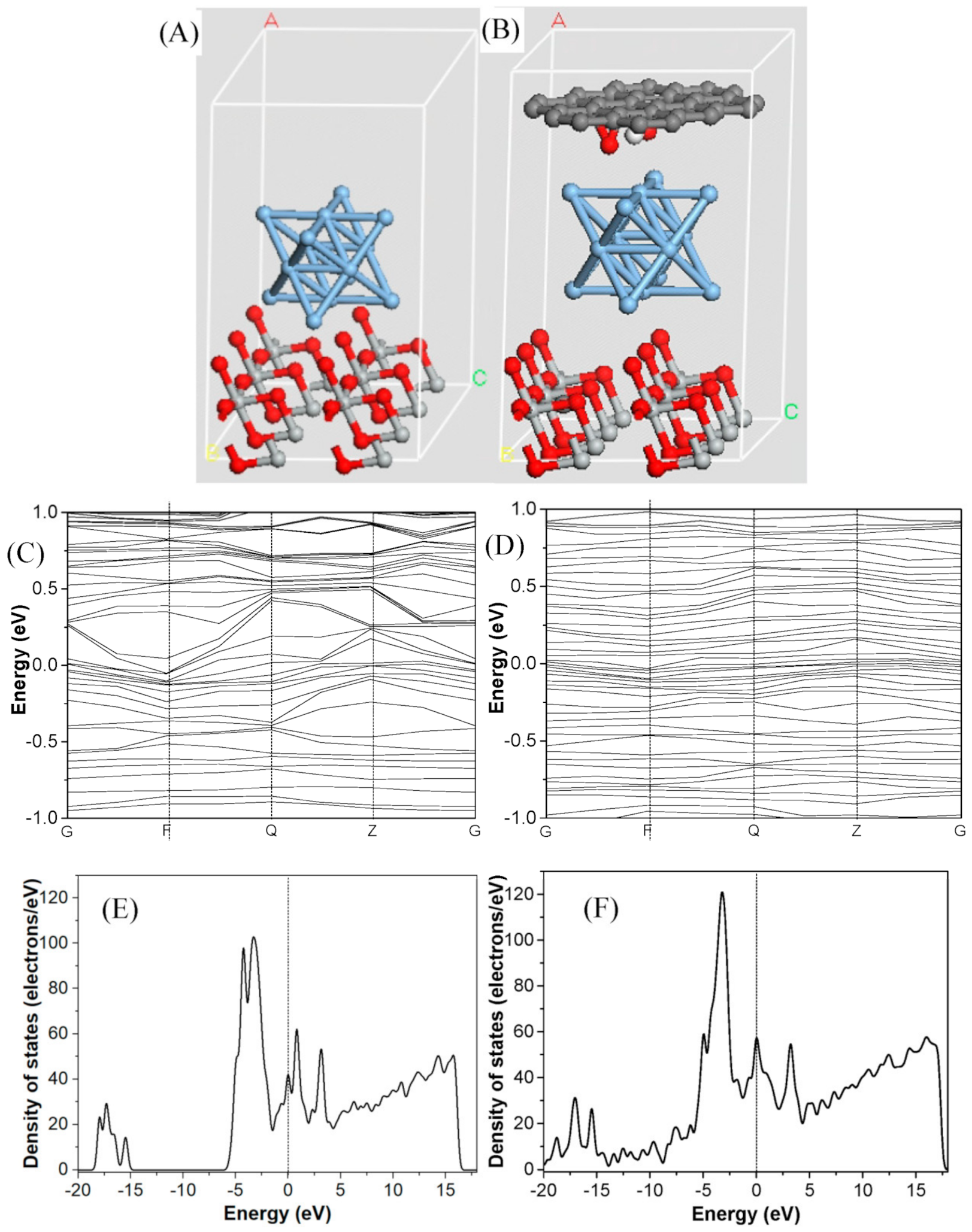
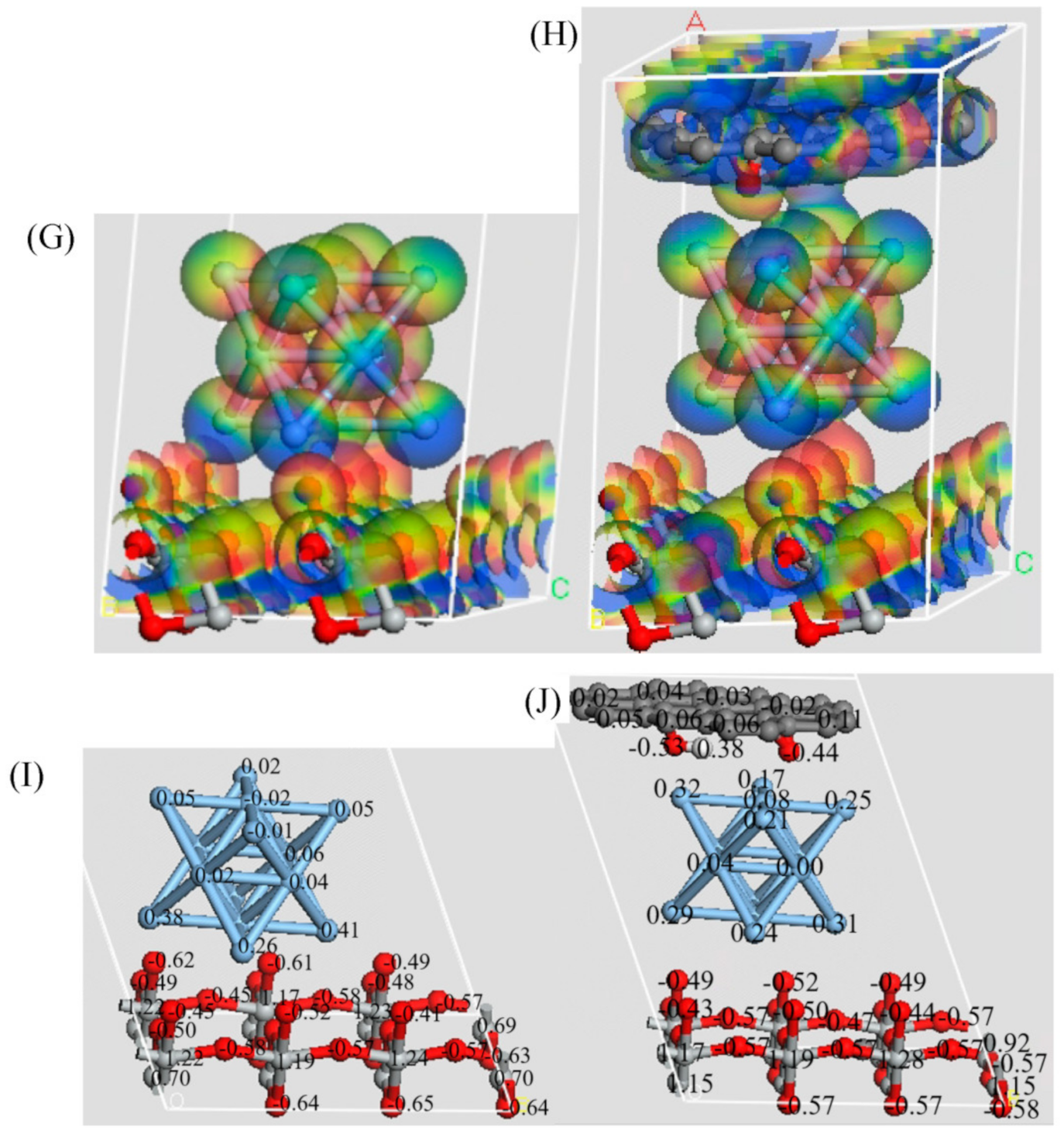
2.2. Theoretical Calculation Section
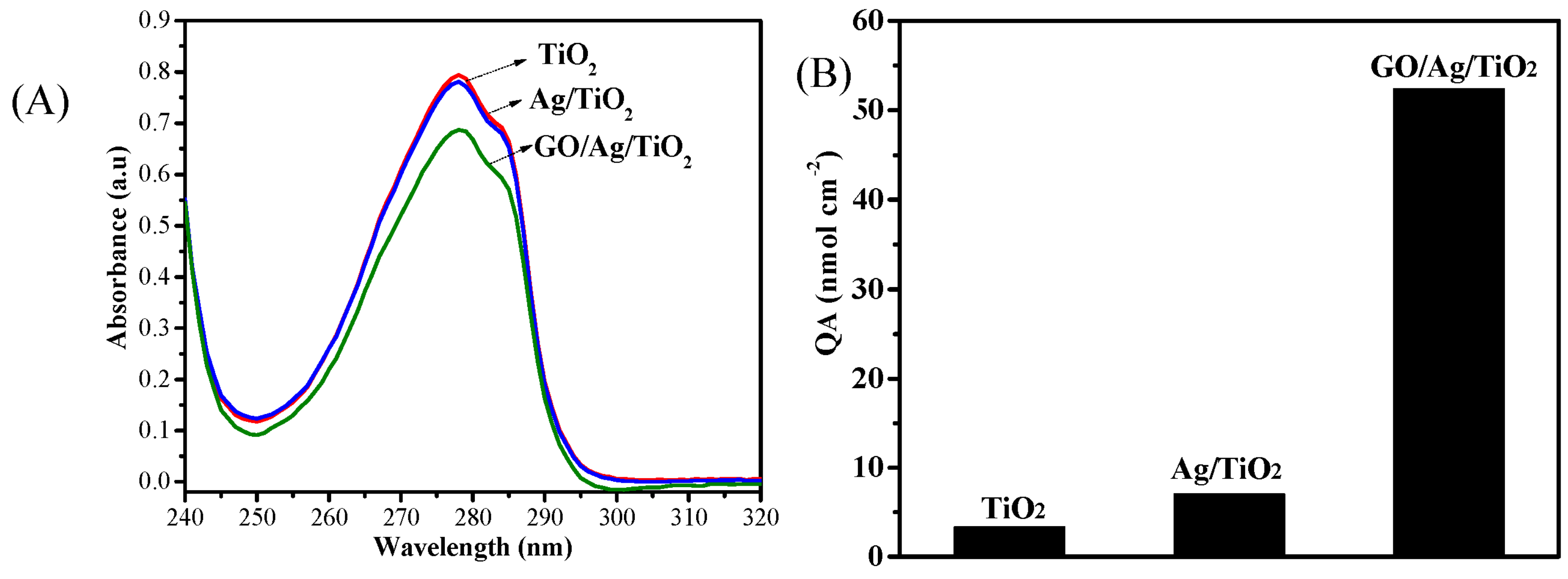
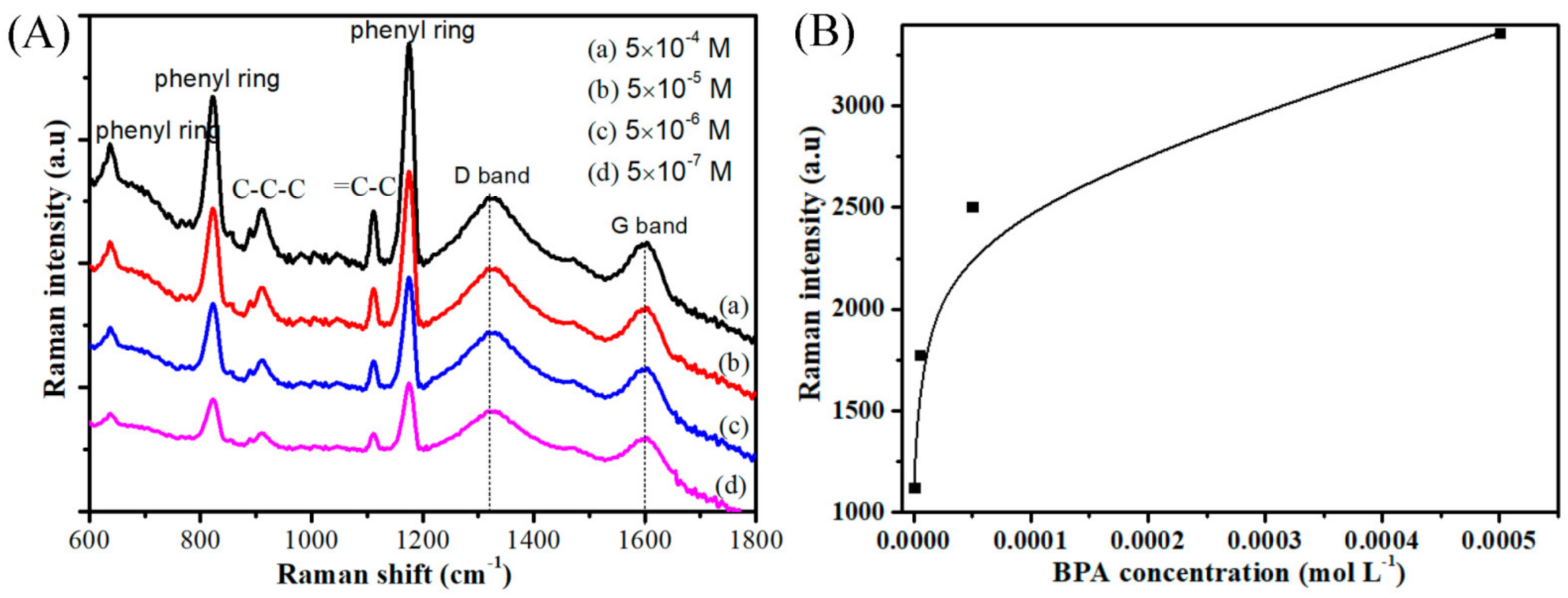
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Anderson, W.; Li, J.; Lin, L.L.; Wang, Y.; Trau, M. A High-Resolution Study of in Situ Surface-Enhanced Raman Scattering Nanotag Behavior in Biological Systems. J. Colloid Interface Sci. 2019, 537, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, L.; Wang, Y.; Liu, P.; Jiang, H.; Xu, Z.; Ma, Z.; Oren, S.; Chow, E.K.C.; Lu, M.; et al. Tunable Optical Nanoantennas Incorporating Bowtie Nanoantenna Arrays with Stimuli-Responsive Polymer. Sci. Rep. 2015, 5, 18567. [Google Scholar] [CrossRef] [PubMed]
- Kozhina, E.P.; Bedin, S.A.; Nechaeva, N.L.; Podoynitsyn, S.N.; Tarakanov, V.P.; Andreev, S.N.; Grigoriev, Y.V.; Naumov, A.V. Ag-Nanowire Bundles with Gap Hot Spots Synthesized in Track-Etched Membranes as Effective Sers-Substrates. Appl. Sci. 2021, 11, 1375. [Google Scholar] [CrossRef]
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.; Hu, X.; Wang, X.; Huang, Z.; Li, F.; Wu, N. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, M.; Zanchi, C.; Lucotti, A.; Bombelli, A.; Villa, N.S.; Casazza, M.; Ciusani, E.; de Grazia, U.; Santoro, M.; Fazio, E.; et al. Laser-Synthesized Sers Substrates as Sensors toward Therapeutic Drug Monitoring. Nanomaterials 2019, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Kozhina, E.P.; Andreev, S.N.; Tarakanov, V.P.; Bedin, S.A.; Doludenko, I.M.; Naumov, A.V. Study of Local Fields of Dendrite Nanostructures in Hot Spots Formed on Sers-Active Substrates Produced Via Template-Assisted Synthesis. Bull. Russ. Acad. Sci. Phys. 2020, 84, 1465–1468. [Google Scholar] [CrossRef]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic Theories of Surface-Enhanced Raman Spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- McNeely, J.; Ingraham, H.M.; Premasiri, W.R.; Ziegler, L.D. Chemical Enhancement Effects on Protoporphyrin Ix Surface-Enhanced Raman Spectra: Metal Substrate Dependence and a Vibronic Theory Analysis. J. Raman Spectrosc. 2021, 52, 323–338. [Google Scholar] [CrossRef]
- Xie, Y. Electrochemical Properties of Sodium Manganese Oxide/Nickel Foam Supercapacitor Electrode Material. Inorg. Nano-Met. Chem. 2022, 52, 548–555. [Google Scholar] [CrossRef]
- Harris, R.A.; Prakash, J. Surface Enhanced Raman Scattering with Methyl-Orange on Ag-TiO2 Nanocomposites: A Computational Investigation. J. Mol. Graph. Modell. 2019, 87, 220–226. [Google Scholar] [CrossRef]
- Xie, Y. Enhancement Effect of Silver Nanoparticles Decorated Titania Nanotube Array Acting as Active Sers Substrate. Inorg. Nano-Met. Chem. 2021. Published Online. [Google Scholar] [CrossRef]
- Xie, Y. Electrochemical Performance of Polyaniline Support on Electrochemical Activated Carbon Fiber. J. Mater. Eng. Perform. 2022, 31, 1949–1955. [Google Scholar] [CrossRef]
- Xie, Y. Electrochemical and Hydrothermal Activation of Carbon Fiber Supercapacitor Electrode. Fibers Polym. 2022, 23, 10–17. [Google Scholar] [CrossRef]
- Lin, L.-K.; Stanciu, L.A. Bisphenol a Detection Using Gold Nanostars in a Sers Improved Lateral Flow Immunochromatographic Assay. Sens. Actuators B Chem. 2018, 276, 222–229. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y. Sandwich-Structured Polypyrrole Layer/Kcl-Polyacrylamide-Gelatin Hydrogel/Polypyrrole Layer as All-in-One Polymer Self-Healing Supercapacitor. Electrochim. Acta 2022, 435, 141371. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y. Hydrogen Bond Enforced Polyaniline Grown on Activated Carbon Fibers Substrate for Wearable Bracelet Supercapacitor. J. Energy Storage 2022, 52, 105042. [Google Scholar] [CrossRef]
- Xie, Y. Electrochemical Investigation of Free-Standing Reduced Graphene Oxide Hydrogel. Fuller. Nanotub. Carbon Nanostructures 2022, 30, 619–625. [Google Scholar] [CrossRef]
- Yan, H.; Wu, H.; Li, K.; Wang, Y.W.; Tao, X.; Yang, H.; Li, A.M.; Cheng, R.S. Influence of the Surface Structure of Graphene Oxide on the Adsorption of Aromatic Organic Compounds from Water. ACS Appl. Mater. Interfaces 2015, 7, 6690–6697. [Google Scholar] [CrossRef]
- Almohammed, S.; Zhang, F.; Rodriguez, B.J.; Rice, J.H. Electric Field-Induced Chemical Surface-Enhanced Raman Spectroscopy Enhancement from Aligned Peptide Nanotube-Graphene Oxide Templates for Universal Trace Detection of Biomolecules. J. Phys. Chem. Lett. 2019, 10, 1878–1887. [Google Scholar] [CrossRef]
- Kostadinova, T.; Politakos, N.; Trajcheva, A.; Blazevska-Gilev, J.; Tomovska, R. Effect of Graphene Characteristics on Morphology and Performance of Composite Noble Metal-Reduced Graphene Oxide Sers Substrate. Molecules 2021, 26, 4775. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, C.X.; Liu, L.; Zhao, Y.M.; Xu, H.J. Recyclable Three-Dimensional Ag Nanoparticle-Decorated TiO2 Nanorod Arrays for Surface-Enhanced Raman Scattering. Biosens. Bioelectron. 2015, 64, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Samodelova, M.V.; Kapitanova, O.O.; Evdokimov, P.V.; Eremina, O.E.; Goodilin, E.A.; Veselova, I.A. Plasmonic Features of Free-Standing Chitosan Nanocomposite Film with Silver and Graphene Oxide for Sers Applications. Nanotechnology 2022, 33, 335501. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Meng, Y. Sers Performance of Graphene Oxide Decorated Silver Nanoparticle/Titania Nanotube Array. RSC Adv. 2014, 4, 41734–41743. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Wang, J.; Shao, Y.; Mei, L. A Recyclable Graphene/Ag/TiO2 Sers Substrate with High Stability and Reproducibility for Detection of Dye Molecules. New J. Chem. 2022, 46, 18787–18795. [Google Scholar] [CrossRef]
- Doan, M.Q.; Anh, N.H.; Van Tuan, H.; Tu, N.C.; Lam, N.H.; Khi, N.T.; Phan, V.N.; Thang, P.D.; Le, A.-T. Improving Sers Sensing Efficiency and Catalytic Reduction Activity in Multifunctional Ternary Ag-TiO2-Go Nanostructures: Roles of Electron Transfer Process on Performance Enhancement. Adsorpt. Sci. Technol. 2021, 2021, 1169599. [Google Scholar] [CrossRef]
- Deriu, C.; Morozov, A.N.; Mebel, A.M. Direct and Water-Mediated Adsorption of Stabilizers on Sers-Active Colloidal Bimetallic Plasmonic Nanomaterials: Insight into Citrate-Auag Interactions from Dft Calculations. J. Phys. Chem. A 2022, 126, 5236–5251. [Google Scholar] [CrossRef]
- Muniz-Miranda, F.; Pedone, A.; Menziani, M.C.; Muniz-Miranda, M. Dft and Td-Dft Study of the Chemical Effect in the Sers Spectra of Piperidine Adsorbed on Silver Colloidal Nanoparticles. Nanomaterials 2022, 12, 2907. [Google Scholar] [CrossRef]
- Premkumar, R.; Hussain, S.; Jayram, N.D.; Koyambo-Konzapa, S.-J.; Revathy, M.S.; Mathavan, T.; Benial, A.M.F. Adsorption and Orientation Characteristics of 1-Methylpyrrole-2-Carbonyl Chloride Using Sers and Dft Investigations. J. Mol. Struct. 2022, 1253, 132201. [Google Scholar] [CrossRef]
- Wu, X.; Vega Canamares, M.; Kakoulli, I.; Sanchez-Cortes, S. Chemical Characterization and Molecular Dynamics Simulations of Bufotenine by Surface-Enhanced Raman Scattering (Sers) and Density Functional Theory (Dft). J. Phys. Chem. Lett. 2022, 13, 5831–5837. [Google Scholar] [CrossRef]
- Ricci, M.; Becucci, M.; Castellucci, E.M. Chemical Enhancement in the Sers Spectra of Indigo: Dft Calculation of the Raman Spectra of Indigo-Ag14 Complexes. Vib. Spectrosc. 2019, 100, 159–166. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Ye, R.; Huang, D.; Wang, Y.; Chen, S. Fabrication of a Novel Electrochemical Sensor Using Conductive Mof Cu-Cat Anchored on Reduced Graphene Oxide for Bpa Detection. J. Appl. Electrochem. 2022, 52, 1617–1628. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Shi, C.; Yan, B.; Gong, L.; Chen, J.; Xiang, L.; Xu, H.; Liu, Q.; Zeng, H. Unraveling the Molecular Interaction Mechanism between Graphene Oxide and Aromatic Organic Compounds with Implications on Wastewater Treatment. Chem. Eng. J. 2019, 358, 842–849. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Zhu, Y.F. Decontamination of Bisphenol a from Aqueous Solution by Graphene Adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, R.; Ma, Y.; Yu, Q.; Kannegulla, A.; Wu, B.; Fan, H.; Wang, A.X.; Kong, X. Plasmonic Cellulose Textile Fiber from Waste Paper for Bpa Sensing by Sers. Spectrochim. Acta Part A 2020, 227, 117664. [Google Scholar] [CrossRef]

 oxygen atom,
oxygen atom,  hydrogen atom,
hydrogen atom,  carbon atom).
carbon atom).
 oxygen atom,
oxygen atom,  hydrogen atom,
hydrogen atom,  carbon atom).
carbon atom).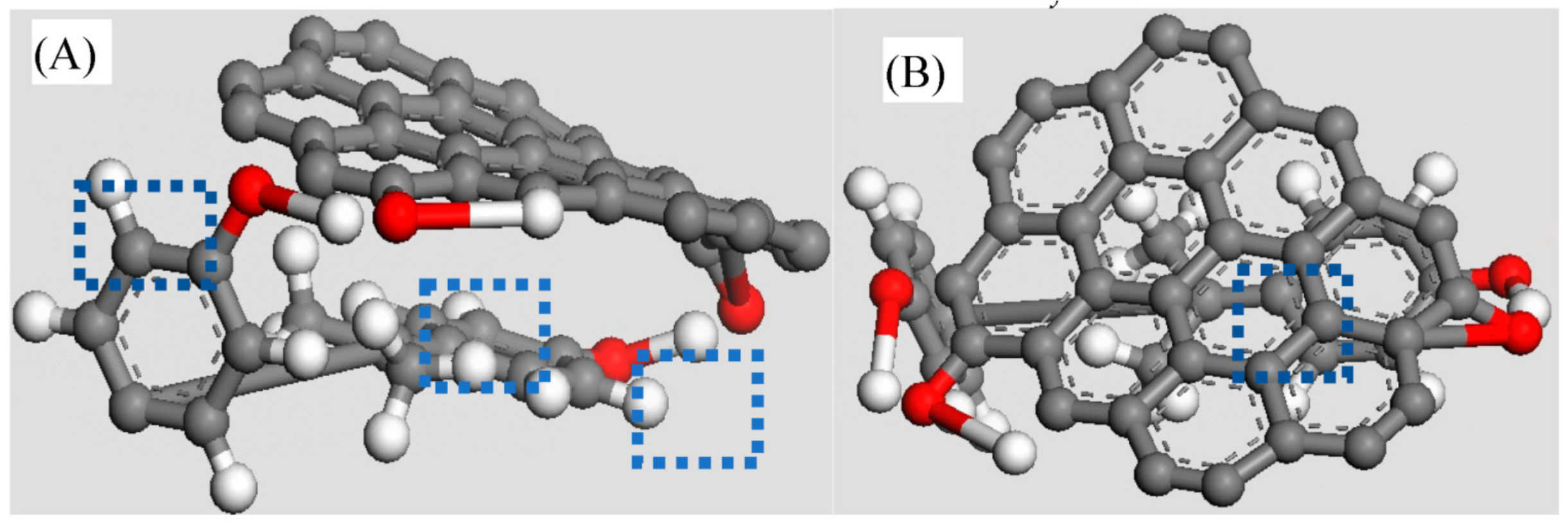
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y. Simulation Calculation Verification of Graphene Oxide-Decorated Silver Nanoparticles Growing on Titania Nanotube Array as SERS Sensor Substrate. Chemosensors 2022, 10, 507. https://doi.org/10.3390/chemosensors10120507
Xie Y. Simulation Calculation Verification of Graphene Oxide-Decorated Silver Nanoparticles Growing on Titania Nanotube Array as SERS Sensor Substrate. Chemosensors. 2022; 10(12):507. https://doi.org/10.3390/chemosensors10120507
Chicago/Turabian StyleXie, Yibing. 2022. "Simulation Calculation Verification of Graphene Oxide-Decorated Silver Nanoparticles Growing on Titania Nanotube Array as SERS Sensor Substrate" Chemosensors 10, no. 12: 507. https://doi.org/10.3390/chemosensors10120507
APA StyleXie, Y. (2022). Simulation Calculation Verification of Graphene Oxide-Decorated Silver Nanoparticles Growing on Titania Nanotube Array as SERS Sensor Substrate. Chemosensors, 10(12), 507. https://doi.org/10.3390/chemosensors10120507






