Electrochemical Sensing for Vitamins
Abstract
1. Introduction
2. Vitamins and Their Properties
2.1. Water-Soluble Vitamins
2.2. Fat-Soluble Vitamins
3. Electrochemical Methods and Working Electrodes
3.1. Electrochemical Methods
3.2. Electrode Materials
3.2.1. Carbon-Based Electrodes
3.2.2. Metal and Metal Oxide Electrodes
3.2.3. Functional Material-Modification Electrodes
3.2.4. Other Functional Materials
4. Electrochemical Sensing of Vitamins
4.1. Electrochemical Sensors for Water-Soluble Vitamins
4.1.1. Vitamin B1 Sensors
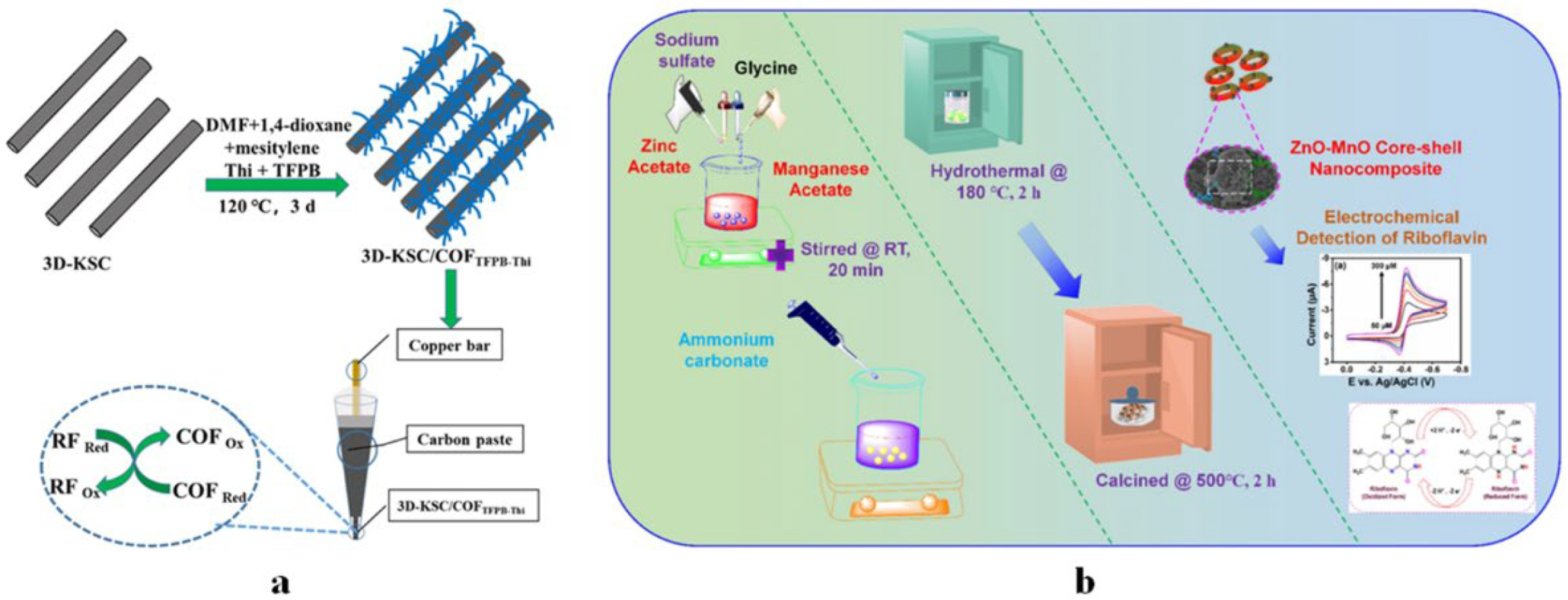
4.1.2. Vitamin B2 Sensors
4.1.3. Vitamin B3 Sensors
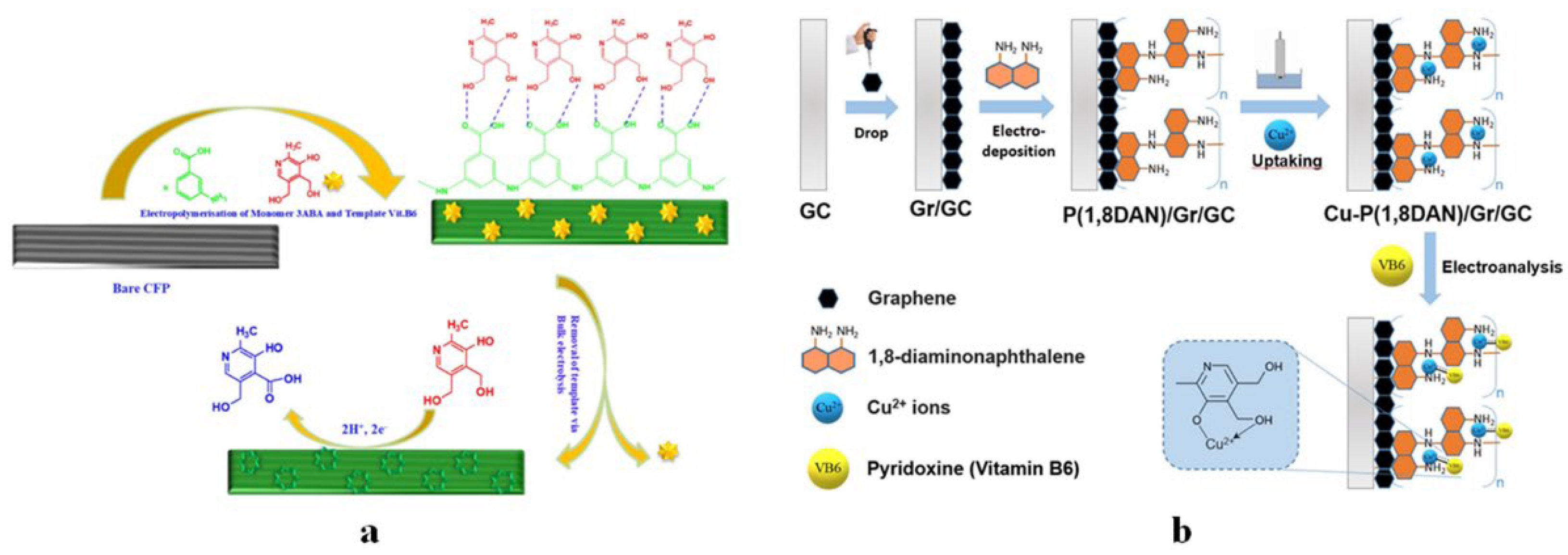
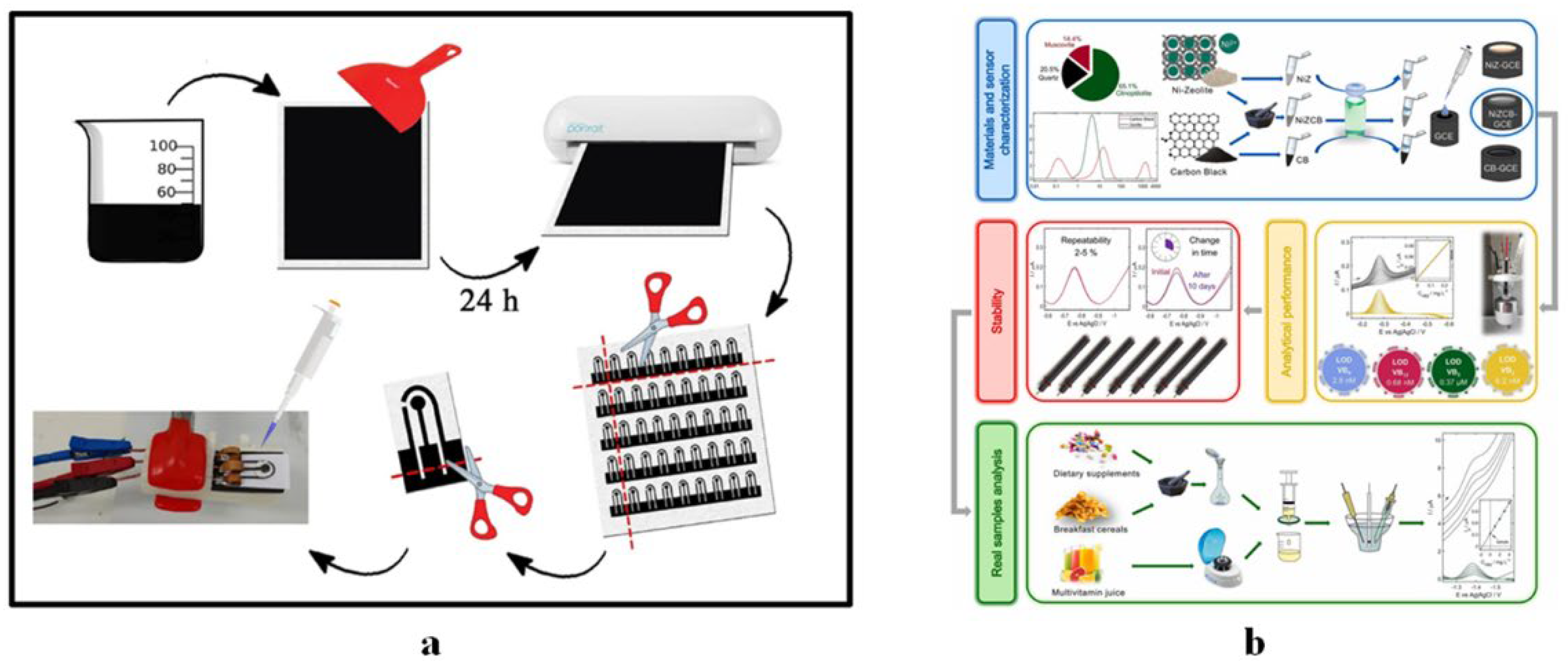
4.1.4. Vitamin B6 Sensors
4.1.5. Vitamin B9 Sensors
4.1.6. Vitamin B12 Sensors
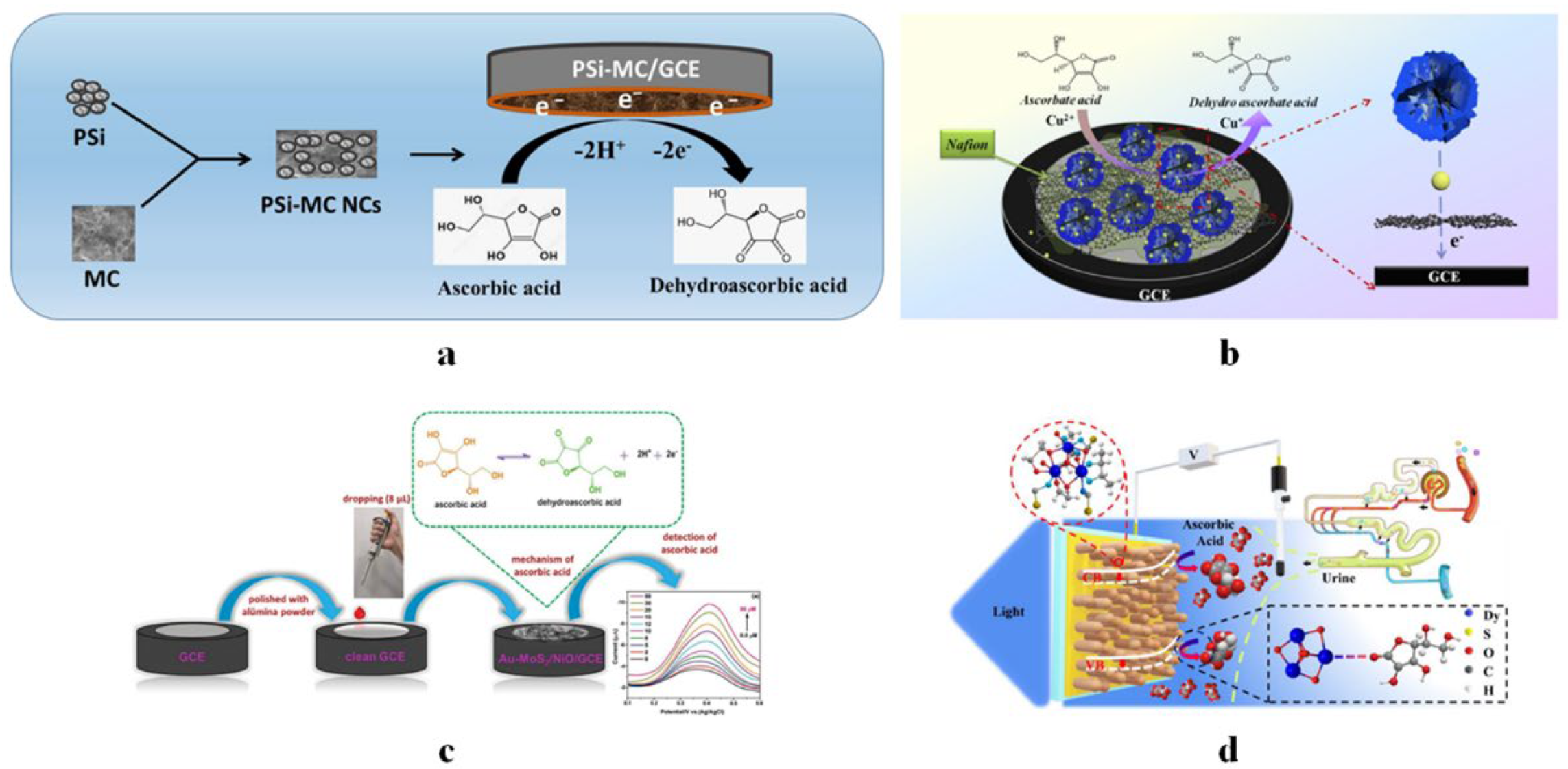
4.1.7. Vitamin C Sensors
4.1.8. Vitamin P Sensors
4.1.9. Simultaneous Analysis of Multiple Water-Soluble Vitamins
4.2. Electrochemical Sensors for Fat-Soluble Vitamins
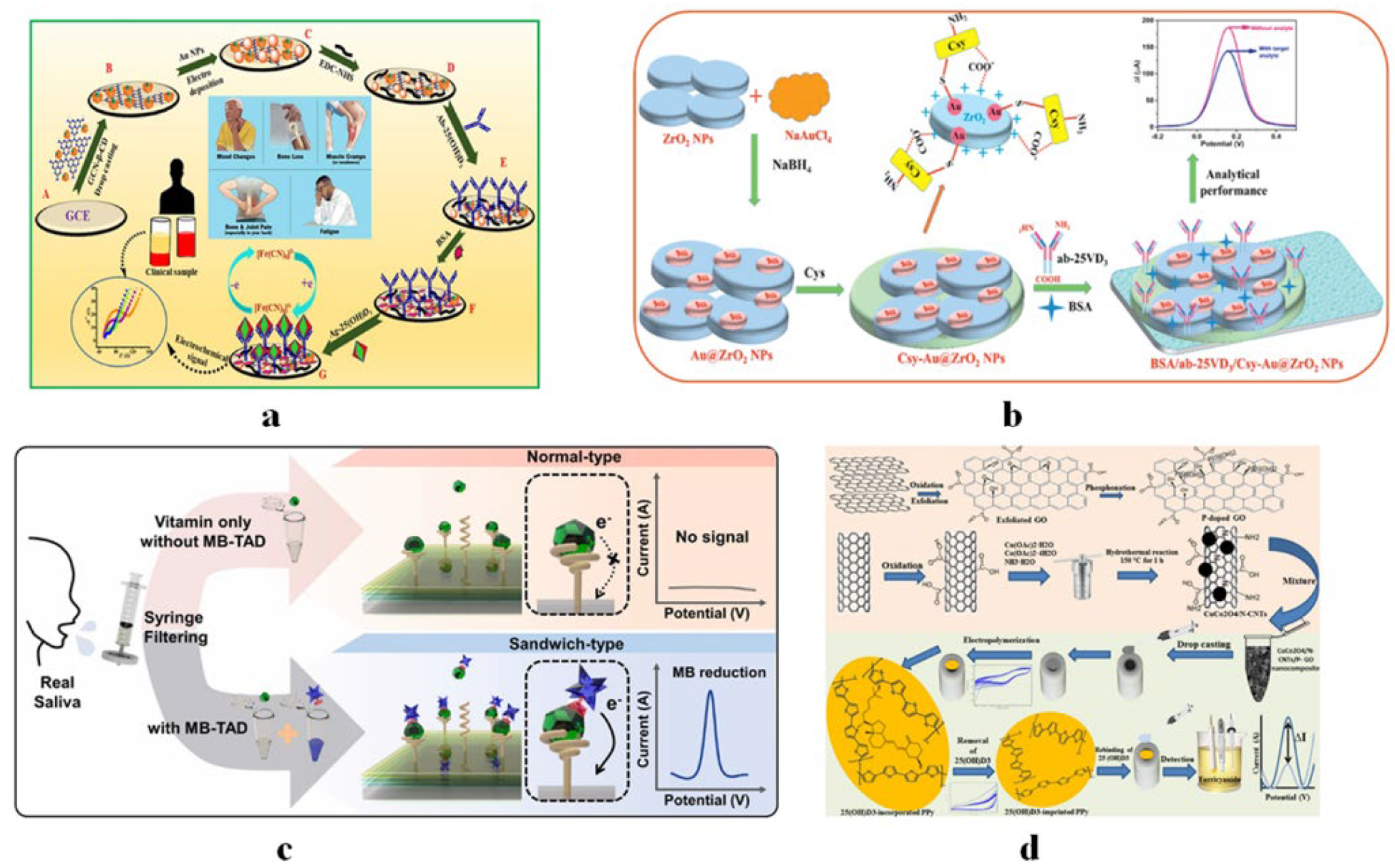
4.2.1. Vitamin D Sensors
4.2.2. Vitamin E Sensors
4.2.3. Vitamin K Sensors
4.2.4. Simultaneous Analysis of Multiple Fat-Soluble Vitamins
4.3. Simultaneous Detection of Vitamins and Non-Vitamin Substances
5. Microfabrication-Based Electrochemical Sensors for Vitamin Detection
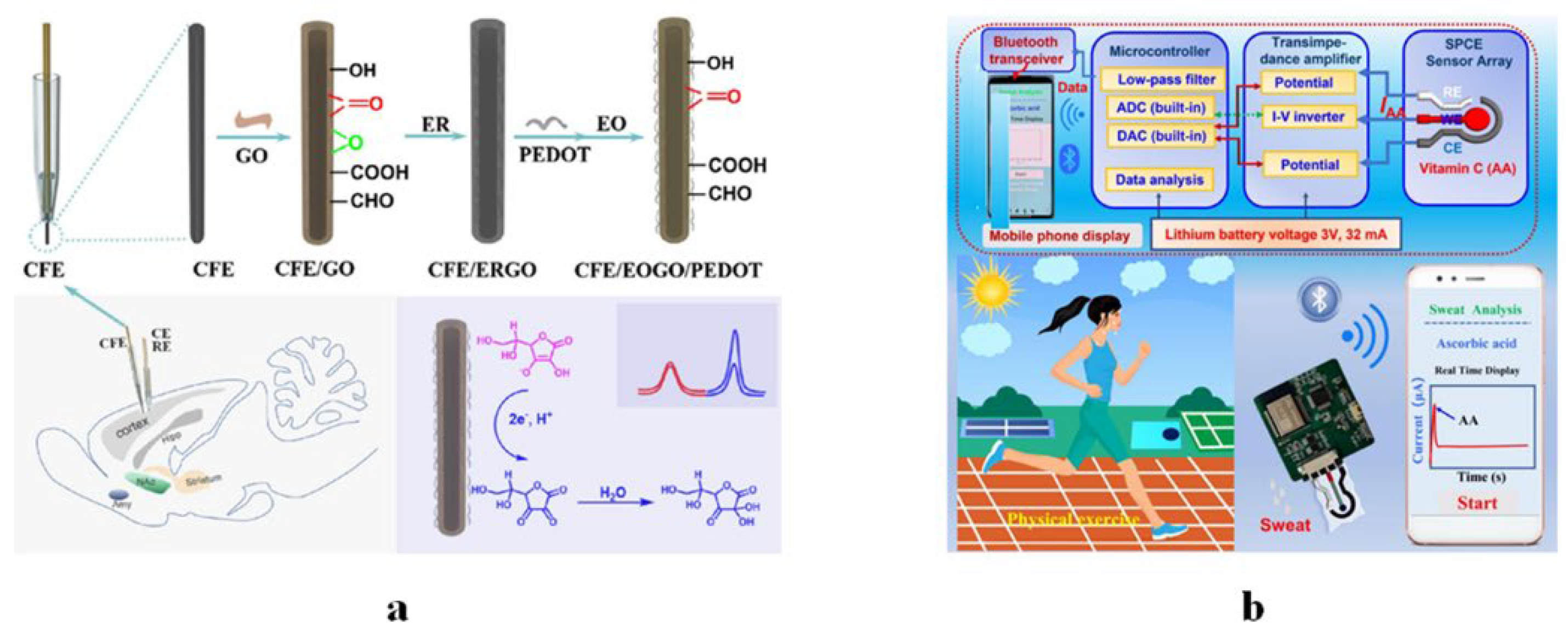
5.1. Implantable Devices
5.2. Microfluidic Devices
5.3. Portable Devices
5.4. Wearable Devices
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schnellbaecher, A.; Binder, D.; Bellmaine, S.; Zimmer, A. Vitamins in cell culture media: Stability and stabilization strategies. Biotechnol. Bioeng. 2019, 116, 1537–1555. [Google Scholar] [CrossRef] [PubMed]
- Paudics, A.; Hessz, D.; Bojtar, M.; Bitter, I.; Horvath, V.; Kallay, M.; Kubinyi, M. A pillararene-based indicator displacement assay for the fluorescence detection of vitamin B1. Sens. Actuator B-Chem. 2022, 369, 132364. [Google Scholar] [CrossRef]
- Jafari, M.; Mousavi, M.; Shirzad, K.; Hosseini, M.A.; Badiei, A.; Pourhakkak, P.; Ghasemi, J.B. A TiO2 nanotube array decorated by Ag nanoparticles for highly sensitive SERS determination and self-cleaning of vitamin B12. Microchem. J. 2022, 181, 107813. [Google Scholar] [CrossRef]
- El-Hawiet, A.; Elessawy, F.M.; El Demellawy, M.A.; El-Yazbi, A.F. Green fast and simple UPLC-ESI-MRM/MS method for determination of trace water-soluble vitamins in honey: Greenness assessment using GAPI and analytical eco-scale. Microchem. J. 2022, 181, 107625. [Google Scholar] [CrossRef]
- Prakashan, V.P.; Gejo, G.; Sanu, M.S.; Sajna, M.S.; Subin, T.; Cyriac, J.; Unnikrishnan, N.V. Novel SPR based fiber optic sensor for vitamin A using Au@Ag core-shell nanoparticles doped SiO2-TiO2-ZrO2 ternary matrix. Appl. Surf. Sci. 2019, 484, 219–227. [Google Scholar] [CrossRef]
- da Silva, T.B.V.; de Oliveira, A.; Moreira, T.F.M.; da Silva, K.C.; Zanin, R.C.; Bona, E.; Goncalves, O.H.; Shirai, M.A.; Peron, A.P.; Leimann, F.V. Analytical validation of an ultraviolet-visible procedure for determining vitamin D-3 in vitamin D-3-loaded microparticles and toxigenetic studies for incorporation into food. Food Chem. 2021, 360, 129979. [Google Scholar] [CrossRef]
- Tezcan, F.; Erim, F.B. Determination of vitamin B2 content in black, green, sage, and rosemary tea infusions by capillary electrophoresis with laser-induced fluorescence detection. Beverages 2018, 4, 86. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Lima, A.R.F.; Machado, S.A.S. A nanostructured label-free platform based on an ultrathin film for ultrasensitive detection of a secosteroid hormone. RSC Adv. 2016, 6, 34458–34467. [Google Scholar] [CrossRef]
- Porada, R.L.; Fendrych, K.; Kochana, J.; Bas, B.L. Simple and reliable determination of B group vitamins in various food matrices with the use of the voltammetric sensor based on Ni-zeolite/carbon black nanocomposite. Food Control 2022, 142, 109243. [Google Scholar] [CrossRef]
- Wu, N.; Wang, L.Y.; Xie, Y.; Du, Y.; Song, Y.H.; Wang, L. Double signal ratiometric electrochemical riboflavin sensor based on macroporous carbon/electroactive thionine-contained covalent organic framework. J. Colloid Interface Sci. 2022, 608, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.J.J.; Wang, S.F. An effective morphology controlled hydrothermal synthesis of Bi2WO6 and its application in riboflavin electrochemical sensor. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 648, 129183. [Google Scholar] [CrossRef]
- Tesfaye, G.; Negash, N.; Tessema, M. Sensitive and selective determination of vitamin B2 in non-alcoholic beverage and milk samples at poly (glutamic acid)/zinc oxide nanoparticles modified carbon paste electrode. BMC Chem. 2022, 16, 69. [Google Scholar] [CrossRef]
- Yamabe, S.; Tsuchida, N.; Yamazaki, S. A DFT study on the tautomerization of vitamin B3 (niacin). Comput. Theor. Chem. 2022, 1214, 113800. [Google Scholar] [CrossRef]
- Bourgin, M.; Kepp, O.; Kroemer, G. Immunostimulatory effects of vitamin B5 improve anticancer immunotherapy. Oncoimmunology 2022, 11, e2031500. [Google Scholar] [CrossRef] [PubMed]
- Porada, R.; Fendrych, K.; Bas, B. Electrochemical sensor based on Ni-exchanged natural zeolite/carbon black hybrid nanocomposite for determination of vitamin B-6. Microchim. Acta 2021, 188, 323. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.R.; Benny, L.; Varghese, A.; John, N.S.; Hegde, G. Molecularly imprinted scaffold based on poly (3-aminobenzoic acid) for electrochemical sensing of vitamin B-6. J. Electrochem. Soc. 2021, 168, 077512. [Google Scholar]
- Vu, T.V.; Nguyen, M.T.T.; Do, T.T.; Nguyen, H.L.; Nguyen, V.A.; Nguyen, D.T. Adsorption of copper ions onto poly(1,8-diaminonaphthalene)/graphene film for voltammetric determination of pyridoxine. Electroanalysis 2022, 34, 1478–1486. [Google Scholar] [CrossRef]
- Ranjan, R.; Kumar, M.; Swain, D.K.; Singh, S.P.; Kharche, S.D.; Chauhan, M.S. Vitamin B7 protects DNA damage and stabilizes mitochondrial transmembrane potential from cryoinjury. Small Rumin. Res. 2022, 212, 106719. [Google Scholar]
- Ali, H.; Verma, N. A hybrid UV-Vis spectroelectrochemical approach for measuring folic acid using a novel Ni-CNF/ITO electrode. Electrochim. Acta 2022, 428, 140920. [Google Scholar] [CrossRef]
- Olmo, F.; Rodriguez, A.; Colina, A.; Heras, A. UV/Vis absorption spectroelectrochemistry of folic acid. J. Solid State Electrochem. 2022, 26, 29–37. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.Y. Electrochemical sensors based on Au-PPy NPs@f-CNTs nanocomposite modified glassy carbon electrode for determination of Vitamin B12 as a key agent in human motion coordination. Int. J. Electrochem. Sci. 2021, 16, 211029. [Google Scholar] [CrossRef]
- Stojanovic, G.M.; Kojic, T.; Simic, M.; Jovanovic-Galovic, A.; Pavlovic, B.; Zurutuza, A.; Anzi, L.; Sordan, R. Rapid selective detection of ascorbic acid using graphene-based microfluidic platform. IEEE Sens. J. 2021, 21, 16744–16753. [Google Scholar] [CrossRef]
- Tortolini, C.; Tasca, F.; Venneri, M.A.; Marchese, C.; Antiochia, R. Gold nanoparticles/carbon nanotubes and gold nanoporous as novel electrochemical platforms for L-ascorbic acid detection: Comparative performance and application. Chemosensors 2021, 9, 229. [Google Scholar] [CrossRef]
- Atacan, K.; Guy, N.; Ozacar, M. Preparation of gold decorated MoS2/NiO nanocomposite in the production of a new electrochemical sensor for ascorbic acid detection. Korean J. Chem. Eng. 2022, 39, 2172–2181. [Google Scholar] [CrossRef]
- Sampathkumar, P.; Sudalaimani, S.; Giribabu, K.; Suresh, C. Nanomolar detection of vitamin C in artificial urine using a glassy carbon electrode modified with molybdenum disulfide. J. Electrochem. Soc. 2021, 168, 087507. [Google Scholar] [CrossRef]
- Senocak, A.; Korkmaz, E.; Khataee, A.; Demirbas, E. A facile and synergetic strategy for electrochemical sensing of rutin antioxidant by Ce-Cr doped magnetite@rGO. Mater. Chem. Phys. 2022, 275, 125298. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Ganesan, S.; Theyagarajan, K.; Duraisamy, M.; Thenmozhi, K.; Weng, C.H.; Lin, Y.T.; Ponnusamy, V.K. Novel biomass-derived porous-graphitic carbon coated iron oxide nanocomposite as an efficient electrocatalyst for the sensitive detection of rutin (vitamin P) in food and environmental samples. Environ. Res. 2022, 211, 113012. [Google Scholar] [CrossRef] [PubMed]
- Vallez-Gomis, V.; Carchano-Olcina, S.; Azorin, C.; Benede, J.L.; Chisvert, A.; Salvador, A. Simultaneous quantification of vitamin A and derivatives in cosmetic products by liquid chromatography with ultraviolet detection. Separations 2022, 9, 40. [Google Scholar] [CrossRef]
- Polli, F.; D’Agostino, C.; Zumpano, R.; De Martino, V.; Favero, G.; Colangelo, L.; Minisola, S.; Mazzei, F. ASu@MNPs-based electrochemical immunosensor for vitamin D-3 serum samples analysis. Talanta 2023, 251, 123755. [Google Scholar] [CrossRef]
- Goharrizi, M.; Oskuee, R.K.; Aleyaghoob, G.; Mohajeri, T.; Mohammadinejad, A.; Rezayi, M. A new molecularly imprinted polymer electrochemical sensor based on CuCo2O4/N-doped CNTs/P-doped GO nanocomposite for detection of 25-hydroxyvitamin D-3 in serum samples. Biotechnol. Appl. Biochem. 2022. [Google Scholar] [CrossRef]
- Chauhan, D.; Gupta, P.K.; Solanki, P.R. Amine functionalized noble metal: Metal oxide nanohybrid for efficient electrochemical determination of 25-hydroxy vitamin-D-3 in human serum. J. Electrochem. Soc. 2021, 168, 117508. [Google Scholar] [CrossRef]
- Jashari, G.; Kastrati, G.; Korecka, L.; Metelka, R.; Sys, M.; Ashrafi, A.M. Electrochemical behaviour of tocopherols: Possibilities of their simultaneous voltammetric detection. Appl. Sci. 2021, 11, 8095. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Oprea, B. Processes and interactions impacting the stability and compatibility of vitamin K and gold nanoparticles. Processes 2022, 10, 1805. [Google Scholar] [CrossRef]
- Jeong, I.S.; Gu, S.Y.; Park, K.H.; Lee, S.Y.; Kim, S.G. A simultaneous determination and monitoring of vitamin K-1 (phylloquinone) and vitamin K-2 (menaquinone) in vegetable drinks and natto sold on the Korean market. J. Food Meas. Charact. 2022, 16, 248–257. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, J.; Luo, Y.H.; Zhang, Y.; Cai, X.Q.; Liu, B.T.; Zhang, Q.Y.; Wu, H.Y.; Liu, Z.Z.; Zhang, D.E. Facile synthesis of bowknot-like cerous phosphate as a bifunctional sensor for ascorbic acid detection. J. Solid State Chem. 2022, 307, 122745. [Google Scholar] [CrossRef]
- Rison, S.; Mathew, A.T.; George, L.; Maiyalagan, T.; Hegde, G.; Varghese, A. Pt nanospheres decorated graphene-beta-CD modified pencil graphite electrode for the electrochemical determination of vitamin B6. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Moustafa, A.; El-Kamel, R.S.; Abdelgawad, S.; Fekry, A.M.; Shehata, M. Electrochemical determination of vitamin B-6 (pyridoxine) by reformed carbon paste electrode with iron oxide nanoparticles. Ionics 2022, 28, 4471–4484. [Google Scholar] [CrossRef]
- Ognjanovic, M.; Stankovic, D.M.; Jacimovic, Z.K.; Kosovic-Perutovic, M.; Mariano, J.; Krehula, S.; Music, S.; Antic, B. Construction of sensor for submicromolar detection of riboflavin by surface modification of SPCE with thermal degradation products of nickel acetate tetrahydrate. Electroanalysis 2022, 34, 1431–1440. [Google Scholar] [CrossRef]
- Ghanbari, H.; Chamjangali, M.A.; Faraji, M. Simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid in a variety of food, pharmaceutical, and biologic samples using a modified glassy carbon electrode with sunset yellow. Anal. Bioanal. Chem. Res. 2022, 9, 411–429. [Google Scholar]
- Yin, S.; Hossain, M.N.; Li, Y.X.; Sun, C.J.; Kraatz, H.B. Development of a novel electrochemical aptasensor based on catalytic hairpin assembly and DNA tetrahedron for the detection of 25-hydroxyvitamin D-3. Sens. Actuator B-Chem. 2022, 354, 131217. [Google Scholar] [CrossRef]
- Anusha, T.; Bhavani, K.S.; Kumar, J.V.S.; Brahman, P.K.; Hassan, R.Y.A. Fabrication of electrochemical immunosensor based on GCN-beta-CD/Au nanocomposite for the monitoring of vitamin D deficiency. Bioelectrochemistry 2022, 143, 107935. [Google Scholar] [CrossRef] [PubMed]
- Tiris, G.; Khoshnavaz, Y.; Oven, E.N.; Mehmandoust, M.; Erk, N. A sensitive voltammetric sensor for specific recognition of vitamin C in human plasma based on MAPbl(3) perovskite nanorods. J. Electrochem. Sci. Eng. 2022, 12, 175–183. [Google Scholar]
- Park, J.; Kim, M.; Kim, W.; Jo, S.; Kim, W.; Kim, C.; Park, H.; Lee, W.; Park, J. Ultrasensitive detection of 25-hydroxy vitamin D-3 in real saliva using sandwich-type electrochemical aptasensor. Sens. Actuator B-Chem. 2022, 355, 131239. [Google Scholar] [CrossRef]
- Alhazimeh, H.; Al-Fandi, M.G.; Al-Ebbini, L.M.K. Development of an inkjet-printed electrochemical nanosensor for ascorbic acid detection. Sensor Rev. 2022, 42, 342–353. [Google Scholar] [CrossRef]
- Abd-Elsabour, M.; Abd-Elsabur, K.M.; Assafand, F.H.; Hasan, I.M.A. An electrochemical sensor based on poly(methyl orange) modified glassy carbon electrode for simultaneous determination of vitamins B-2 and C in aqueous solution. Anal. Bioanal. Chem. Res. 2022, 9, 259–268. [Google Scholar]
- Luo, X.L.; Chen, L.M.; Yang, J.Y.; Li, S.T.; Li, M.T.; Mo, Q.; Li, Y.B.; Li, X.C. Electrochemically simultaneous detection of ascorbic acid, sulfite and oxalic acid on Pt-Pd nanoparticles/chitosan/nitrogen doped graphene modified glassy carbon electrode: A method for drug quality control. Microchem. J. 2021, 169, 106623. [Google Scholar] [CrossRef]
- Abo-bakr, A.M.; Abd-Elsabour, M.; Abou-Krisha, M.M. An efficient novel electrochemical sensor for simultaneous determination of vitamin C and aspirin based on a PMR/Zn-Al LDH/GCE. Electroanalysis 2021, 33, 2476–2489. [Google Scholar] [CrossRef]
- Karastogianni, S.; Girousi, S. Square wave voltammetric (SWV) determination of cyanocobalamin (vitamin B12) in pharmaceuticals and supplements on a carbon paste electrode (CPE) modified by a manganese(II) polymeric film. Anal. Lett. 2022, 55, 399–410. [Google Scholar] [CrossRef]
- Pereira, D.F.; Santana, E.R.; Spinelli, A. Electrochemical paper-based analytical devices containing magnetite nanoparticles for the determination of vitamins B-2 and B-6. Microchem. J. 2022, 179, 107588. [Google Scholar] [CrossRef]
- Roncevic, I.S.; Skroza, D.; Vrca, I.; Kondza, A.M.; Vladislavic, N. Development and optimization of electrochemical method for determination of vitamin C. Chemosensors 2022, 10, 283. [Google Scholar] [CrossRef]
- Buleandra, M.; Popa, D.E.; Popa, A.; Codreanu, N.A.M.; David, I.G. Multi-analyte sensor based on pencil graphite electrode for riboflavin and pyridoxine determination. J. Electrochem. Soc. 2022, 169, 017517. [Google Scholar] [CrossRef]
- Singh, P.; Singh, K.R.B.; Singh, J.; Prasad, P.; Singh, R.P. Bioinspired triangular ZnO nanoclusters synthesized by Argyreia nervosa nascent leaf extract for the efficient electrochemical determination of vitamin C. RSC Adv. 2021, 11, 25752–25763. [Google Scholar] [CrossRef]
- Shanmugam, R.; Koventhan, C.; Chen, S.M.; Hung, W.S. A portable Ru-decorated cobalt phosphide on graphitic carbon nitride sensor: An effective electrochemical evaluation method for vitamin B-2 in the environment and biological samples. Chem. Eng. J. 2022, 446, 136909. [Google Scholar] [CrossRef]
- Bakhsh, H.; Palabiyik, I.M.; Oad, R.K.; Qambrani, N.; Buledi, J.A.; Solangi, A.R.; Sherazi, S.T.H. SnO2 nanostructure based electroanalytical approach for simultaneous monitoring of vitamin C and vitamin B-6 in pharmaceuticals. J. Electroanal. Chem. 2022, 910, 116181. [Google Scholar] [CrossRef]
- Bora, H.; Mandal, D.; Chandra, A. High-performance, nitrogen-doped, carbon-nanotube-based electrochemical sensor for vitamin D3 detection. ACS Appl. Bio Mater. 2022, 5, 1721–1730. [Google Scholar] [CrossRef]
- Nagarajappa, H.; Manjunatha, J.G.; Al-Kahtani, A.A.; Tighezza, A.M.; Ataollahi, N. Electrochemical determination of riboflavin using a poly(titan yellow) modified carbon nanotube paste electrode in the presence of dopamine. Chemistryselect 2022, 7, e202200537. [Google Scholar] [CrossRef]
- Gao, J.J.; Liu, H.; Wu, K.X.; Yan, J.F.; Tong, C. A novel nonenzymatic ascorbic acid electrochemical sensor based on gold nanoparticals-chicken egg white-copper phosphate-graphene oxide hybrid nanoflowers. Nanotechnology 2021, 32, 325504. [Google Scholar] [CrossRef]
- Pushpanjali, P.A.; Manjunatha, J.G.; Hareesha, N.; Amrutha, B.M.; Raril, C.; Alothman, Z.A.; Alanazi, A.M.; Pandith, A. Fabrication of poly(L-Aspartic acid) Layer on graphene nanoplatelets paste electrode for riboflavin sensing. Mater. Chem. Phys. 2022, 276, 125392. [Google Scholar] [CrossRef]
- Spissu, Y.; Barberis, A.; Bazzu, G.; D’Hallewin, G.; Rocchitta, G.; Serra, P.A.; Marceddu, S.; Vineis, C.; Garroni, S.; Culeddu, N. Functionalization of screen-printed sensors with a high reactivity carbonaceous material for ascorbic acid detection in fresh-cut fruit with low vitamin C content. Chemosensors 2021, 9, 354. [Google Scholar] [CrossRef]
- Ganesamurthi, J.; Shanmugam, R.; Chen, S.M. Electrochemical evaluation of vitamin B-2 through a portable electrochemical sensor based on binary transition metal oxide in various biological and vegetable samples. J. Electrochem. Soc. 2022, 169, 096505. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Han, D.F.; Xiao, R.; Wang, T.Q.; Liang, Z.S.; Wu, Z.F.; Han, F.J.; Han, D.X.; Ma, Y.M.; Niu, L. An enzyme-free photoelectrochemical sensor platform for ascorbic acid detection in human urine. Chemosensors 2022, 10, 268. [Google Scholar] [CrossRef]
- Zhou, X.; Qu, Q.; Wang, L.; Li, L.; Li, S.L.; Xia, K. Nitrogen dozen carbon quantum dots as one dual function sensing platform for electrochemical and fluorescent detecting ascorbic acid. J. Nanopart. Res. 2020, 22, 20. [Google Scholar] [CrossRef]
- Martins, E.C.; Santana, E.R.; Spinelli, A. Nitrogen and sulfur co-doped graphene quantum dot-modified electrode for monitoring of multivitamins in energy drinks. Talanta 2023, 252, 123836. [Google Scholar] [CrossRef]
- Prinith, N.S.; Manjunatha, J.G.; Tigari, G.; Alothman, Z.A.; Alanazi, A.M.; Pandith, A. Mechanistic insights into the voltammetric determination of riboflavin at poly (serine) modified graphite and carbon nanotube composite paste electrode. Chemistryselect 2021, 6, 10746–10757. [Google Scholar] [CrossRef]
- Avan, A.A.; Filik, H. Simultaneous determination of fat-soluble vitamins by using modified glassy carbon electrode. Russ. J. Electrochem. 2021, 57, 858–871. [Google Scholar] [CrossRef]
- Qu, Z.B.; Jiang, Y.M.; Zhang, J.X.; Chen, S.; Zeng, R.J.; Zhuo, Y.; Lu, M.; Shi, G.Y.; Gu, H. Tailoring oxygen-containing groups on graphene for ratiometric electrochemical measurements of ascorbic acid in living subacute Parkinson’s disease mouse brains. Anal. Chem. 2021, 93, 16598–16607. [Google Scholar] [CrossRef]
- Ercarikci, E.; Aksu, Z.; Kiransan, K.D.; Topcu, E. Graphene paper with electrodeposited NiCo2S4 nanoparticles as a novel flexible sensor for simultaneous detection of folic acid and ascorbic acid. Diamond Relat. Mater. 2022, 121, 108713. [Google Scholar] [CrossRef]
- Yin, S.; Li, Y.X.; Hossain, M.N.; Sun, C.J.; Kraatz, H.B. Electrochemical detection of 25-hydroxyvitamin D3 using an oligonucleotide aptasensor. Sens. Actuator B-Chem. 2021, 340, 129945. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Zhang, X.J. Selective and sensitive determination of folic acid based on molecularly imprinted poly (o-aminophenol) and reduced graphene oxide decorated with Au nanoparticles. Curr. Anal. Chem. 2021, 17, 1201–1210. [Google Scholar] [CrossRef]
- Wang, Q.G.; Xiao, X.Y.; Hu, X.W.; Huang, L.B.; Li, T.; Yang, M.H. Molecularly imprinted electrochemical sensor for ascorbic acid determination based on MXene modified electrode. Mater. Lett. 2021, 285, 129158. [Google Scholar] [CrossRef]
- Singh, S.; Batra, N.; Ansari, M.; Urooj, S. Electrochemical sensing or examining vitamin D3 based on MIP using NOVA 1.7 and Autolab PGSTAT 302N. J. Clin. Diagn. Res. 2020, 14, OC17–OC20. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Miao, Q.S. Molecularly imprinted sensor for ascorbic acid based on gold nanoparticles and multiwalled carbon nanotubes. Curr. Anal. Chem. 2020, 16, 905–913. [Google Scholar] [CrossRef]
- Wang, M.Y.; Ceto, X.; del Valle, M. A novel electronic tongue using electropolymerized molecularly imprinted polymers for the simultaneous determination of active pharmaceutical ingredients. Biosens. Bioelectron. 2022, 198, 113807. [Google Scholar] [CrossRef]
- Brezo-Borjan, T.; Stojanovic, Z.; Suturovic, Z.; Kos, J.; Kravic, S.; Durovic, A. A simple adsorptive chronopotentiometric stripping method for determination of vitamin B-1 in pharmaceutical products. Monatsh. Chem. 2020, 151, 285–291. [Google Scholar] [CrossRef]
- Arul, P.; Gowthaman, N.S.K.; Narayanamoorthi, E.; John, S.A.; Huang, S.T. Synthesis of homogeneously distributed gold nanoparticles built-in metal free organic framework: Electrochemical detection of riboflavin in pharmaceutical and human fluids samples. J. Electroanal. Chem. 2021, 887, 115143. [Google Scholar] [CrossRef]
- Charithra, M.M.; Manjunatha, J.G. Fabrication of poly (Evans blue) modified graphite paste electrode as an electrochemical sensor for sensitive and instant riboflavin detection. Moroc. J. Chem. 2021, 9, 7–17. [Google Scholar]
- Dokur, E.; Gorduk, O.; Sahin, Y. Selective electrochemical sensing of riboflavin based on functionalized multi-walled carbon nanotube/gold nanoparticle/pencil graphite electrode. ECS J. Solid State Sci. Technol. 2020, 9, 121003. [Google Scholar] [CrossRef]
- Grace, A.A.; Thillaiarasi, S.; Dharuman, V. Binary metal oxide adsorbed graphene modified glassy carbon electrode for detection of riboflavin. Electroanalysis 2021, 33, 993–1006. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G. A simple and low-cost poly (DL-phenylalanine) modified carbon sensor for the improved electrochemical analysis of Riboflavin. J. Sci. 2020, 5, 502–511. [Google Scholar] [CrossRef]
- Manoj, D.; Manigandan, R.; Rajendran, S.; Ponce, L.C. Self-assembled dendrite-like 3D-CeO2 nanostructures for non-enzymatic vitamin B2 sensor. Mater. Lett. 2021, 295, 129834. [Google Scholar] [CrossRef]
- Rajkumar, C.; Kim, H. Interface engineering of ruthenium-supported sulfur-doped graphitic carbon nitride for ultrasensitive electrochemical determination of riboflavin. J. Taiwan Inst. Chem. Eng. 2022, 138, 104470. [Google Scholar] [CrossRef]
- Girija, S.; Sankar, S.S.; Kundu, S.; Wilson, J. Microfibers of embellished cobalt-zeolite imidazole framework for vitamin-B(2)detection. J. Electrochem. Soc. 2020, 167, 137511. [Google Scholar]
- Lin, J.X.; Mei, Q.W.; Duan, Y.C.; Yu, C.H.; Ding, Y.P.; Li, L. A highly sensitive electrochemical sensor based on nanoflower-like MoS2-Ag-CNF nanocomposites for the detection of VB2. J. Nanopart. Res. 2020, 22, 274. [Google Scholar] [CrossRef]
- Monnappa, A.B.; Manjunatha, J.G.G.; Bhatt, A.S.; Ananda, P.P. Fabrication of a sensitive and selective electrochemical sensing platform based on poly-l-leucine modified sensor for enhanced voltammetric determination of Riboflavin. J. Food Meas. Charact. 2020, 14, 3633–3643. [Google Scholar] [CrossRef]
- Sharma, A.; Khosla, A.; Arya, S. Synthesis of SnO2 nanowires as a reusable and flexible electrode for electrochemical detection of riboflavin. Microchem. J. 2020, 156, 104858. [Google Scholar] [CrossRef]
- Vijayaprasath, G.; Habibulla, I.; Dharuman, V.; Balasubramanian, S.; Ganesan, R. Fabrication of Gd2O3 nanosheet-modified glassy carbon electrode for nonenzymatic highly selective electrochemical detection of vitamin B2. ACS Omega 2020, 5, 17892–17899. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, C.; Negut, C.C.; Gugoasa, L.A.D.; van Staden, J.F. Sensitive voltammetric determination of riboflavin in pharmaceutical and biological samples using FSN-Zonyl-Nafion modified carbon paste electrode. Microchem. J. 2020, 155, 104729. [Google Scholar] [CrossRef]
- Derakhshan, M.; Shamspur, T.; Molaakbari, E.; Mostafavi, A.; Saljooqi, A. Fabrication of a novel electrochemical sensor for determination of riboflavin in different drink real samples. Russ. J. Electrochem. 2020, 56, 181–188. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G. Elevated and rapid voltammetric sensing of riboflavin at poly(helianthin dye) blended carbon paste electrode with heterogeneous rate constant elucidation. J. Iran. Chem. Soc. 2020, 17, 1507–1519. [Google Scholar] [CrossRef]
- Tigari, G.; Manjunatha, J.G. A surfactant enhanced novel pencil graphite and carbon nanotube composite paste material as an effective electrochemical sensor for determination of riboflavin. J. Sci. 2020, 5, 56–64. [Google Scholar] [CrossRef]
- Sangavi, R.; Keerthana, M.; Malini, T.P. Design of an electrochemical sensor for the determination of riboflavin using cobalt doped dysprosium oxide nanocubes modified glassy carbon electrode. Chemistryselect 2022, 7, e202201661. [Google Scholar] [CrossRef]
- Negut, C.C.; Stefanov, C.; Gugoasa, L.A.D.; van Staden, J.F. Rapidly renewable graphite paste electrode modified with 5,10,15,20-tetrakis(4-methoxyphenyl)-21H,23H-porphine cobalt(II) for electrochemical determination of nicotinic acid. J. Electroanal. Chem. 2020, 863, 114063. [Google Scholar] [CrossRef]
- Cui, M.; Long, L.K.; Wu, Y.H.; Gao, D.; Wang, Y.D. Electrochemical biosensor based on reduced graphene oxide and CMC/silica sol-gel hybrid membranes for the detection of VB6. Funct. Mater. 2021, 28, 605–611. [Google Scholar]
- Dokur, E.; Gorduk, O.; Sahin, Y. Cost-effective and Facile Production of a Phosphorus-doped Graphite Electrode for the Electrochemical Determination of Pyridoxine. Electroanalysis 2021, 33, 1657–1667. [Google Scholar] [CrossRef]
- Zabolestani, H.; Sarhadi, H.; Beitollahi, H. Electrochemical sensor based on modified screen printed electrode for vitamin B-6 detection. Surf. Eng. Appl. Electrochem. 2021, 57, 277–285. [Google Scholar] [CrossRef]
- Sadeghi, H.; Shahidi, S.A.; Raeisi, S.N.; Ghorbani-HasanSaraei, A.; Karimi, F. Electrochemical determination of vitamin B-6 in water and juice samples using an electrochemical sensor amplified with NiO/CNTs and Ionic liquid. Int. J. Electrochem. Sci. 2020, 15, 10488–10498. [Google Scholar] [CrossRef]
- Rejithamol, R.; Beena, S. Electrochemical quantification of pyridoxine (VB6) in human blood from other water-soluble vitamins. Chem. Pap. 2020, 74, 2011–2020. [Google Scholar] [CrossRef]
- Porada, R.; Fendrych, K.; Bas, B. Development of novel Mn-zeolite/graphite modified Screen-printed Carbon Electrode for ultrasensitive and selective determination of folic acid. Measurement 2021, 179, 109450. [Google Scholar] [CrossRef]
- Zare, M.; Sarhadi, H. A novel vitamin B9 sensor based on modified screen-printed electrode. J. Electrochem. Sci. Eng. 2021, 11, 1–9. [Google Scholar]
- Winiarski, J.P.; Rampanelli, R.; Bassani, J.C.; Mezalira, D.Z.; Jost, C.L. Multi-walled carbon nanotubes/nickel hydroxide composite applied as electrochemical sensor for folic acid (vitamin B-9) in food samples. J. Food Compos. Anal. 2020, 92, 103511. [Google Scholar] [CrossRef]
- Mani, V.; Balamurugan, T.S.T.; Huang, S.T. Rapid one-pot synthesis of polydopamine encapsulated carbon anchored with Au nanoparticles: Versatile electrocatalysts for chloramphenicol and folic acid sensors. Int. J. Mol. Sci. 2020, 21, 2853. [Google Scholar] [CrossRef]
- Antherjanam, S.; Saraswathyamma, B.; Krishnan, R.G.; Gopakumar, G.M. Electrochemical sensors as a versatile tool for the quantitative analysis of Vitamin B-12. Chem. Pap. 2021, 75, 2981–2995. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, N.; Jiang, X.Y.; Wan, D.J.; Xie, Y.Q. Facile detection of vitamin B12 with copper oxide nanocrystal graphenic composite electrode. Water 2021, 13, 1790. [Google Scholar] [CrossRef]
- Sharma, A.; Arya, S.; Chauhan, D.; Solanki, P.R.; Khajuria, S.; Khosla, A. Synthesis of Au-SnO2 nanoparticles for electrochemical determination of vitamin B-12. J. Mater. Res. Technol 2020, 9, 14321–14337. [Google Scholar] [CrossRef]
- Pereira, D.F.; Santana, E.R.; Piovesan, J.V.; Spinelli, A. A novel electrochemical strategy for determination of vitamin B-12 by Co(I/II) redox pair monitoring with boron-doped diamond electrode. Diamond Relat. Mater. 2020, 105, 107793. [Google Scholar] [CrossRef]
- Bettazzi, F.; Ingrosso, C.; Sfragano, P.S.; Pifferi, V.; Falciola, L.; Curri, M.L.; Palchetti, I. Gold nanoparticles modified graphene platforms for highly sensitive electrochemical detection of vitamin C in infant food and formulae. Food Chem. 2021, 344, 128692. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Mashhadizadeh, M.H.; Norouzi, Z. Introducing a novel nanocomposite consisting of TiO2 nanoparticles@copper oxide/reduced graphene oxide for the electrocatalytic sensing of ascorbic acid. J. Iran. Chem. Soc. 2021, 18, 1329–1341. [Google Scholar] [CrossRef]
- Ikram, M.; Sajid, M.M.; Javed, Y.; Afzal, A.M.; Shad, N.A.; Sajid, M.; Akhtar, K.; Yousaf, M.I.; Sharma, S.K.; Aslam, H.; et al. Crystalline growth of tungsten trioxide (WO3) nanorods and their development as an electrochemical sensor for selective detection of vitamin C. J. Mater. Sci.-Mater. Electron. 2021, 32, 6344–6357. [Google Scholar] [CrossRef]
- Khand, N.H.; Palabiyik, I.M.; Buledi, J.A.; Ameen, S.; Memon, A.F.; Ghumro, T.; Solangi, A.R. Functional Co3O4 nanostructure-based electrochemical sensor for direct determination of ascorbic acid in pharmaceutical samples. J. Nanostruct. Chem. 2021, 11, 455–468. [Google Scholar] [CrossRef]
- Kong, L.J.; Chen, J.H.; Peng, Z.H.; Zhang, J.W.; Gao, Y.; Yan, S.C. Synthesis of Ag-Cu alloy nanosheets for ascorbic acid detection. Mater. Express 2021, 11, 1001–1006. [Google Scholar] [CrossRef]
- Sudeep, M.; Manjunatha, C.; Aan, M.P.S.; Ashoka, S.; Suresh, R.; Ujwal, S.M. Development of nano copper sulfide (CuS) structures for electrochemical detection of vitamin C. J. Nanostruct. 2021, 11, 628–637. [Google Scholar]
- Suriyaprakash, J.; Shan, L.W.; Gupta, N.; Wang, H.; Wu, L.J. Janus 2D-carbon nanocomposite-based ascorbic acid sensing device: Experimental and theoretical approaches. Compos. Pt. B-Eng. 2022, 245, 110233. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Rahman, M.M.; Islam, M.A. Selective detection of ascorbic acid with wet-chemically prepared CdO/SnO2/V2O5 micro-sheets by electrochemical approach. SN Appl. Sci. 2020, 2, 1953. [Google Scholar] [CrossRef]
- Alam, M.M.; Balkhoyor, H.B.; Asiri, A.M.; Karim, M.R.; Chani, M.T.S.; Rahman, M.M. Fabrication of ascorbic acid sensor with Co3O4 center dot Fe2O3 nanosphere materials by electrochemical technique. Surf. Interfaces 2020, 20, 100607. [Google Scholar] [CrossRef]
- de Faria, L.V.; Lisboa, T.P.; de Farias, D.M.; Araujo, F.M.; Machado, M.M.; de Sousa, R.A.; Matos, M.A.C.; Munoz, R.A.A.; Matos, R.C. Direct analysis of ascorbic acid in food beverage samples by flow injection analysis using reduced graphene oxide sensor. Food Chem. 2020, 319, 126509. [Google Scholar] [CrossRef]
- Duzmen, S.; Baytak, A.K.; Aslanoglu, M. A novel voltammetric platform composed of poly(aminopyrazine), ZrO2 and CNTs for a rapid, sensitive and selective determination of ascorbic acid in pharmaceuticals and food samples. Mater. Chem. Phys. 2020, 252, 123170. [Google Scholar] [CrossRef]
- Gurusamy, T.; Murugan, R.; Durairaj, A.; Ramanujam, K. Confinement catalysis of non-covalently functionalized carbon nanotube in ascorbic acid sensing. Electroanalysis 2020, 32, 2481–2492. [Google Scholar] [CrossRef]
- Ibarlucea, B.; Roig, A.P.; Belyaev, D.; Baraban, L.; Cuniberti, G. Electrochemical detection of ascorbic acid in artificial sweat using a flexible alginate/CuO-modified electrode. Microchim. Acta 2020, 187, 520. [Google Scholar] [CrossRef]
- Navadeepthy, D.; Thangapandian, M.; Viswanathan, C.; Ponpandian, N. A nanocomposite of NiFe2O4-PANI as a duo active electrocatalyst toward the sensitive colorimetric and electrochemical sensing of ascorbic acid. Nanoscale Adv. 2020, 2, 3481–3493. [Google Scholar] [CrossRef]
- Shen, T.Y.; Liu, T.C.; Mo, H.Q.; Yuan, Z.C.; Cui, F.; Jin, Y.X.; Chen, X.J. Cu-based metal-organic framework HKUST-1 as effective catalyst for highly sensitive determination of ascorbic acid. RSC Adv. 2020, 10, 22881–22890. [Google Scholar] [CrossRef] [PubMed]
- Alkhawaldeh, A.K. Platinum nanoparticle electrode modified iodine using cyclic voltammetry and chronoamperometry for determination of ascorbic acid. Anal. Bioanal. Electrochem. 2020, 12, 780–792. [Google Scholar]
- Zhu, S.C.; Xie, A.J.; Wei, B.Y.; Tao, X.; Zhang, J.H.; Peng, W.H.; Liu, C.Y.; Gu, L.Y.; Xu, C.F.; Luo, S.P. Construction and application of a nonenzymatic ascorbic acid sensor based on a NiO1.0/polyaniline(3.0) hybrid. New J. Chem. 2020, 44, 9288–9297. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Bukharinova, M.A.; Stozhko, N.Y.; Sokolkov, S.V.; Tarasov, A.V.; Vidrevich, M.B. Electrochemical sensor based on a carbon veil modified by phytosynthesized gold nanoparticles for determination of ascorbic acid. Sensors 2020, 20, 1800. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.N.; Liu, J.J.; Zhou, M. Amperometric ascorbic acid sensor based on disposable facial tissues derived carbon aerogels. Chem. Res. Chin. Univ. 2020, 36, 139–144. [Google Scholar] [CrossRef]
- Kumar, A.; Furtado, V.L.; Goncalves, J.M.; Bannitz-Fernandes, R.; Netto, L.E.S.; Araki, K.; Bertotti, M. Amperometric microsensor based on nanoporous gold for ascorbic acid detection in highly acidic biological extracts. Anal. Chim. Acta 2020, 1095, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pastor, J.A.; Martinez-Sanchez, A.; Aznar-Poveda, J.; Garcia-Sanchez, A.J.; Garcia-Haro, J.; Aguayo, E. Quick and cost-effective estimation of vitamin C in multifruit juices using voltammetric methods. Sensors 2020, 20, 676. [Google Scholar] [CrossRef]
- Osman, A.M.; Abulkibash, A.M.; Atieh, M.A. Time-biased square wave differential electrolytic potentiometry for determination of ascorbic acid in a complex matrix at multi-walled carbon nanotubes modified silver electrodes. Arabian J. Chem. 2020, 13, 2955–2963. [Google Scholar] [CrossRef]
- Loguercio, L.F.; Thesing, A.; Noremberg, B.D.; Lopes, B.V.; Maron, G.K.; Machado, G.; Pope, M.A.; Carreno, N.L.V. Direct laser writing of poly(furfuryl alcohol)/graphene oxide electrodes for electrochemical determination of ascorbic acid. Chemelectrochem 2022, 9, e202200334. [Google Scholar]
- Low, S.C.; Shaimi, R. Amperometric sensor using nylon-6-film-modified carbon electrode for low-cost detection of ascorbic acid. Monatsh. Chem. 2022, 153, 551–560. [Google Scholar] [CrossRef]
- Banavath, R.; Abhinav, A.; Srivastava, R.; Bhargava, P. Highly sensitive ascorbic acid sensors from EDTA chelation derived nickel hexacyanoferrate/ graphene nanocomposites. Electrochim. Acta 2022, 419, 140335. [Google Scholar] [CrossRef]
- Li, Y.Q.; Pan, F.; Yin, S.Y.; Tong, C.Y.; Zhu, R.L.; Li, G.X. Nafion assisted preparation of prussian blue nanoparticles and its application in electrochemical analysis of L-ascorbic acid. Microchem. J. 2022, 177, 107278. [Google Scholar] [CrossRef]
- Shayanfar, F.; Sarhadi, H. Determination of vitamin C at modified screen printed electrode: Application for sensing of vitamin C in real samples. Surf. Eng. Appl. Electrochem. 2021, 57, 487–494. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsareii, S.A. Porous silicon-mesoporous carbon nanocomposite based electrochemical sensor for sensitive and selective detection of ascorbic acid in real samples. J. Taiwan Inst. Chem. Eng. 2021, 125, 360–371. [Google Scholar] [CrossRef]
- Sivam, T.; Gowthaman, N.S.K.; Lim, H.N.; Andou, Y.; Arul, P.; Narayanamoorthi, E.; John, S.A. Tunable electrochemical behavior of dicarboxylic acids anchored Co-MOF: Sensitive determination of rutin in pharmaceutical samples. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 622, 126667. [Google Scholar] [CrossRef]
- Senocak, A.; Khataee, A.; Demirbas, E.; Doustkhah, E. Ultrasensitive detection of rutin antioxidant through a magnetic micro-mesoporous graphitized carbon wrapped Co nanoarchitecture. Sens. Actuator B-Chem. 2020, 312, 127939. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Pattnaik, P.K.; Goel, S. Simultaneous detection of Vitamin B-12 and Vitamin C from real samples using miniaturized laser-induced graphene based electrochemiluminescence device with closed bipolar electrode. Sens. Actuator A-Phys. 2021, 331, 112831. [Google Scholar] [CrossRef]
- Porada, R.; Bas, B. Separation of the overlapped vitamin B1 and B3 voltammetric peaks by means of Continuous Wavelet Transform and differentiation. Monatsh. Chem. 2021, 152, 1107–1117. [Google Scholar] [CrossRef]
- Contursi, M.; Coviello, D.; Ciriello, R.; Guerrieri, A.; Palmieri, M.A.; Langerame, F.; Bianco, G.; Salvi, A.M. Surface and electrochemical characterization of a new Layered GC/betaine/Pt electrode and investigation on its performance as a densor for two B complex vitamins, B1 and B6: Preliminary results. Electroanalysis 2021, 33, 483–494. [Google Scholar] [CrossRef]
- Wang, H.M.; Zhang, X.L.; Wang, S.J.; Xiao, S.; Ma, H.Y.; Wang, X. Multianalyte electrochemical electrode for the determination of vitamins B2 and B6 in complex biosystem. Microchem. J. 2020, 158, 105233. [Google Scholar] [CrossRef]
- Yomthiangthae, P.; Kondo, T.; Chailapakul, O.; Siangproh, W. The effects of the supporting electrolyte on the simultaneous determination of vitamin B-2, vitamin B-6, and vitamin C using a modification-free screen-printed carbon electrode. New J. Chem. 2020, 44, 12603–12612. [Google Scholar] [CrossRef]
- Zeybek, B.; Uge, A. Development of carbon-based sensors for electrochemical quantification of vitamins B-2 and B-6 at nanomolar levels. Chem. Pap. 2021, 75, 1323–1339. [Google Scholar] [CrossRef]
- Dhanalakshmi, N.; Priya, T.; Karthikeyan, V.; Thinakaran, N. Binary mixture of lanthanide metal doped ZnO nanorod: F-MWCNT nanocomposite for simultaneous and selective determination of vitamins B-2 and B-6. J. Nanosci. Nanotechnol. 2020, 20, 2154–2164. [Google Scholar] [CrossRef]
- Kaur, A.; Rana, S.; Bharti, A.; Chaudhary, G.R.; Prabhakar, N. Voltammetric detection of vitamin D employing Au-MoS2 hybrid as immunosensing platform. Microchim. Acta 2021, 188, 222. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.; Kim, W.; Jo, S.; Kim, M.; Kim, C.; Park, H.; Bang, D.; Lee, W.; Park, J. Bio-inspired Ag nanovilli-based sandwich-type SERS aptasensor for ultrasensitive and selective detection of 25-hydroxy vitamin D3. Biosens. Bioelectron. 2021, 188, 113341. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kapoor, S.; Bharti, A.; Rana, S.; Chaudhary, G.R.; Prabhakar, N. Gold-platinum bimetallic nanoparticles coated 3-(aminopropyl) triethoxysilane (APTES) based electrochemical immunosensor for vitamin D estimation. J. Electroanal. Chem. 2020, 873, 114400. [Google Scholar] [CrossRef]
- Anusha, T.; Bhavani, K.S.; Kumar, J.V.S.; Bonanni, A.; Brahman, P.K. Fabrication of handmade paper sensor based on silver-cobalt doped copolymer-ionic liquid composite for monitoring of vitamin D-3 level in real samples. Microchem. J. 2021, 161, 105789. [Google Scholar] [CrossRef]
- Wadhwa, S.; John, A.T.; Nagabooshanam, S.; Mathur, A.; Narang, J. Graphene quantum dot-gold hybrid nanoparticles integrated aptasensor for ultra-sensitive detection of vitamin D-3 towards point-of-care application. Appl. Surf. Sci. 2020, 521, 146427. [Google Scholar] [CrossRef]
- Anusha, T.; Bhavani, K.S.; Kumar, J.V.S.; Brahman, P.K. Designing and fabrication of electrochemical nanosensor employing fullerene-C-60 and bimetallic nanoparticles composite film for the detection of vitamin D-3 in blood samples. Diamond Relat. Mater. 2020, 104, 107761. [Google Scholar] [CrossRef]
- Rostami-Javanroudi, S.; Babakhanian, A. New electrochemical sensor for direct quantification of vitamin K in human blood serum. Microchem. J. 2021, 163, 105716. [Google Scholar] [CrossRef]
- Kastrati, G.; Jashari, G.; Sys, M.; Svecova, B.; Arbneshi, T.; Metelka, R.; Bilkova, Z.; Korecka, L. Simultaneous determination of vitamin E and vitamin K in food supplements using adsorptive stripping square-wave voltammetry at glassy carbon electrode. Appl. Sci. 2020, 10, 4759. [Google Scholar] [CrossRef]
- Thangphatthanarungruang, J.; Yakoh, A.; Laocharoensuk, R.; Chotsuwan, C.; Chailapakul, O.; Siangproh, W. High-efficient of graphene nanocomposite: Application to rapidly simultaneous identification and quantitation of fat-soluble vitamins in different matric samples. J. Electroanal. Chem. 2020, 873, 114361. [Google Scholar] [CrossRef]
- Darabi, R.; Shabani-Nooshabadi, M.; Khoobi, A. A potential strategy for simultaneous determination of deferoxamine and vitamin C using MCR-ALS with nanostructured electrochemical sensor in serum and urine of thalassemia and diabetic patients. J. Electrochem. Soc. 2021, 168, 046514. [Google Scholar] [CrossRef]
- Kumar, M.; Wang, M.; Swamy, B.E.K.; Praveen, M.; Zhao, W. Poly (alanine)/NaOH/ MoS2/MWCNTs modified carbon paste electrode for simultaneous detection of dopamine, ascorbic acid, serotonin and guanine. Colloid Surf. B-Biointerfaces 2020, 196, 111299. [Google Scholar] [CrossRef] [PubMed]
- Arroquia, A.; Acosta, I.; Armada, M.P.G. Self-assembled gold decorated polydopamine nanospheres as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 109, 110602. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Galal, A.; El-Gohary, A.R. Crown ether modified poly(hydroquinone)/carbon nanotubes based electrochemical sensor for simultaneous determination of levodopa, uric acid, tyrosine and ascorbic acid in biological fluids. J. Electroanal. Chem. 2020, 863, 114032. [Google Scholar] [CrossRef]
- Buledi, J.A.; Ameen, S.; Khand, N.H.; Solangi, A.R.; Taqvi, I.H.; Agheem, M.H.; Wajdan, Z. CuO nanostructures based electrochemical sensor for simultaneous determination of hydroquinone and ascorbic acid. Electroanalysis 2020, 32, 1600–1607. [Google Scholar] [CrossRef]
- Swamy, B.K.; Shiprath, K.; Rakesh, G.; Ratnam, K.V.; Manjunatha, H.; Janardan, S.; Naidu, K.C.B.; Ramesh, S.; Suresh, K.; Ratnamala, A. Simultaneous detection of dopamine, tyrosine and ascorbic acid using NiO/graphene modified graphite electrode. Biointerface Res. Appl. Chem. 2020, 10, 5599–5609. [Google Scholar]
- Muhammad, H.Y.; Faizullah, A.T. Double injection-single detector flow injection spectrophotometric method for simultaneous determination of ascorbic acid and paracetamol in pharmaceutical formulations. J. Iran. Chem. Soc. 2020, 17, 111–118. [Google Scholar] [CrossRef]
- Veerapandi, G.; Meenakshi, S.; Anitta, S.; Arul, C.; Ashokkumar, P.; Sekar, C. Precise and quick detection of ascorbic acid and eugenol in fruits, pharmaceuticals and medicinal herbs using hydroxyapatite-titanium dioxide nanocomposite-based electrode. Food Chem. 2022, 382, 132251. [Google Scholar] [CrossRef]
- Rees, M.; Wright, A.G.; Holdcroft, S.; Bertoncello, P. Voltammetry at hexamethyl-P-terphenyl poly(benzimidazolium) (HMT-PMBI)-coated glassy carbon electrodes: Charge transport properties and detection of uric and ascorbic acid. Sensors 2020, 20, 443. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Xiao, X.; Li, C.C.; Luo, Y.; Chen, S.; Shi, G.Y.; Han, K.; Gu, H. Facile ratiometric electrochemical sensor for in vivo/online repetitive measurements of cerebral ascorbic acid in brain microdiaysate. Anal. Chem. 2020, 92, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Pan, L.Y.; Han, X.; Ha, M.N.; Li, K.R.; Yu, H.; Zhang, Q.H.; Li, Y.G.; Hou, C.Y.; Wang, H.Z. A portable ascorbic acid in sweat analysis system based on highly crystalline conductive nickel-based metal-organic framework (Ni-MOF). J. Colloid Interface Sci. 2022, 616, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Albin, S.; Heise, R.L.; Yadavalli, V.K. Portable, disposable, biomimetic electrochemical sensors for analyte detection in a single drop of whole blood. Chemosensors 2022, 10, 263. [Google Scholar] [CrossRef]
- Ma, J.L.; Shen, L.X.; Jiang, Y.; Ma, H.T.; Lv, F.; Liu, J.S.; Su, Y.; Zhu, N. Wearable self-powered smart sensors for portable nutrition monitoring. Anal. Chem. 2022, 94, 2333–2340. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; Silva, A.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; May, J.; et al. Epidermal enzymatic biosensors for sweat vitamin C: Toward personalized nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef]


| Vitamin | Compound | Intake | Main Roles | Source | Possible Consequence | |
|---|---|---|---|---|---|---|
| Deficiency | Excessive | |||||
| VB1 | Thiamine | 1.2 mg d−1 | Carbohydrate and amino acid metabolism | Rice, milk, eggs, meat | Metabolic diseases, beriberi, nervous system diseases | Excessive thiamine is excreted in the urine |
| VB2 | Riboflavin | 1.3 mg d−1 | Promote cell growth and regeneration | Whole-grain products, eggs, liver | Inflammation of mouth corners and oral mucosa | Oxidative damage to DNA and liver |
| VB3 | Nicotinic acid, nicotinamide | 16 mg d−1 | Main redox mediators in cell metabolism | Animal viscera, muscle tissues, fruits, and egg yolks | Skin disease, digestive system symptoms and dementia | Flushing, hypotension |
| VB5 | D-pantothenic acid | 5 mg d−1 | Amino acid catabolism, glycolysis and fatty acid metabolism | All types of animal and plant tissues | Fatigue, headache, nausea, vomiting, stomach pain | Mild diarrhea and gastrointestinal distress |
| VB6 | Pyridoxine | 1.5 mg d−1 | Cell growth and normal functional performance | Vegetables and protein rich foods | Dermatitis, glossitis, anemia, numbness, weakened immune | Dermatological lesions; photosensitivity |
| VB7 | Biotin | 30 μg d−1 | Cofactor necessary for four important carboxylases | Meats, grains and vegetables | Skin diseases, nerve problems, growth retardation | - |
| VB9 | Folic acid | 400 μg d−1 | Maintain and produce new cells, prevent DNA changes | Liver, dried beans, yolks, fruits, nuts, leafy vegetables | Neural tube defects, megaloblastic anemia, colon cancer | Impair the absorption of zinc and VB12 |
| VB12 | Cobalamin | 2.4 μg d−1 | Nerve cell growth, red blood cell formation | Liver, kidney, meat, fish, clams, eggs, milk, cheese | Neuropsychiatric, cardiovascular and hematological diseases | Renal failure, liver disease and neurotoxicity |
| VC | L-ascorbic acid | 120 mg d−1 | Antioxidant; synthesis of collagen, carnitine, adrenaline | Fresh fruits and vegetables | Scurvy, anemia, infections, cardiovascular disease, cancer | Headache, difficulty sleeping, skin redness |
| VP | Rutin | 500 mg d−1 | Anti-radiation, analgesic, anti-inflammatory, antioxidant | Fresh plants and fruits | Increase the brittleness of capillaries | Allergies and eczema |
| VA | Retinoic acid | 900 mg d−1 | Maintain normal function of human retina, bone growth | Animals (retinol); fruits, vegetables (carotenoids) | Blindness, decreased immune system efficiency, cancer | Severe headache, blurred vision, nausea, dizziness |
| VD | - | 15 μg d−1 | Promote the absorption of calcium and phosphate in the intestine | Egg yolk, mushroom, cod liver oil, fresh salmon | Osteomalacia in adults and rickets in children | Nausea, vomiting, muscle weakness, pain |
| VE | - | 15 mg d−1 | Prevent the formation of reactive oxygen species | Fruits and vegetables, nuts, lean meat, milk, eggs | Circulatory disorders, fertility disorder and Alzheimer’s disease | Hemorrhage and interrupt blood coagulation |
| VK | Naphtho-quinone compounds | 120 μg d−1 | Control calcium levels and synthesize proteins needed for blood coagulation | VK1 from Green vegetables, VK2 from eggs, cheese, liver, meat | Decrease prothrombin and the tendency of excessive bleeding | - |
| Vitamin | Electrode | Technique | Medium | Linear Range | LOD | Application | Ref. |
|---|---|---|---|---|---|---|---|
| VB2 | ZnO NPS-CPE | CV, SWV | PBS (pH 6) | 0.005–10 μM | 0.7 ± 0.01 nM | Beverage, milk | [12] |
| Bi2WO6 (PVP + NaOH)/GCE | DPV | 0.05 M PBS (pH 7.0) | 0.03–457 μM | 3.65 nM | Almond milk, soymilk | [11] | |
| Ru/S-GCN-SPCE | CV, DPV | 0.1 M PBS (pH 7.0) | 0.003–75 μM 95–260 μM | 54.3 pM | Oral solution, syrup, tablets | [81] | |
| VB6 | IONCPE | DPV | 0.1 M PBS (pH 6.0) | 8.88–1000.0 μM | 9.06 μM | Urine, pharmaceuticals | [37] |
| Pt/β-CD-GR/PGE | DPV | 0.1 M PBS (pH 7.0) | 5–205 nM | 1.2 nM | Juice | [36] | |
| P-doped/PGE | DPV | 0.1 M PBS (pH 3.0) | 0.5–300 μM | 0.219 μM | Beverage | [90] | |
| VB9 | ZnFe2O4MNPs/SPE | DPV | 0.1 M PBS (pH 7.0) | 1.0–100.0 μM | 0.3 μM | Tablets, urine | [99] |
| GCE/f-MWCNT-Ni(OH)2-Si4Pic+Cl− | DPV | PBS (pH 7.0) | 0.5–26 μM | 0.095 μM | Dietary supplement, fortifier compound, wheat flour | [100] | |
| VB12 | Copper oxide- GUITAR | LSV | Neutral (pH = 7) solutions | 0.15–7378 nM | 0.59 nM | Bacterial strains | [103] |
| Mn-CPE | SWV | Acetate buffer (pH 4.6) | 13.86–1500 ng L−1 | 4.34 ng L−1 | Tablets, dietary supplements | [48] | |
| VC | Pyrolytic graphite sheet | CV, SWV | KNO3 (pH 7.0) | 1.0–400 μM | 0.4 μM | Extract of cultivated arugula | [50] |
| Gr/NiHCF | CV, Amp * | 0.01 M PBS (pH 7.4) | 1–16,280 μM | 0.25 μM | Supplements, fruit juices | [130] | |
| PBNPs/GCE | CV, Amp | PBS (pH 5) | 1–1100 μM | 0.47 μM | Domestic water, medicine samples | [131] | |
| VP | Co-BPDC-MOF/GCE | DPV, Amp | 0.1 M PBS (pH 7) | 0.5–1000 μM | 0.03 μM | BROMEZER tablet | [134] |
| Co/ZIF-C/GCE | DPV | BR buffer (pH 2.0) | 0.1–30 μM | 22 nM | Tablets | [135] | |
| VD (25-OHD3) | Ab-25(OH)D3-Cys/Au/MoS2/FTO | DPV | 5 mM [Fe(CN)6]3−/4− + 0.9% KCl(pH 7.4) | 1 pg mL−1–100 ng mL−1 | 0.38 pg mL−1 | - | [143] |
| Glut/Au-Pt/APTES/FTO | DPV | 50 mM PBS (pH 7.4, 0.9% NaCl) + [Fe(CN)6]3−/4− | 0.1 pg mL−1–1 μg mL−1 | 0.49 pg mL−1 | Serum | [145] | |
| VD3 | Co-Ag/PANI-PPY/IL/GCE | CV, SWV, Amp | PBS (pH 7) | 0.0125–22.5 μM | 0.0073 μM | Serum, urine | [146] |
| Paper-sensor | CV, SWV, Amp | PBS (pH 7) | 0.025–0.125 μM | 0.015 μM | Serum, urine | [146] | |
| GQD-Au | EIS | 0.1 M PBS (pH 7.0) | 1–500 nM | 0.70 nM | Serum | [147] | |
| CuNPs-NiNPs@reduced-fullerene- C60/GCE | CV, SWV | PBS (pH 7.0) | 1.25–475 μM | 0.0025 μM | Serum, urine | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Hu, N.; Deng, J.; Yang, J. Electrochemical Sensing for Vitamins. Chemosensors 2022, 10, 494. https://doi.org/10.3390/chemosensors10110494
Yang Y, Hu N, Deng J, Yang J. Electrochemical Sensing for Vitamins. Chemosensors. 2022; 10(11):494. https://doi.org/10.3390/chemosensors10110494
Chicago/Turabian StyleYang, Yanting, Ning Hu, Jinan Deng, and Jun Yang. 2022. "Electrochemical Sensing for Vitamins" Chemosensors 10, no. 11: 494. https://doi.org/10.3390/chemosensors10110494
APA StyleYang, Y., Hu, N., Deng, J., & Yang, J. (2022). Electrochemical Sensing for Vitamins. Chemosensors, 10(11), 494. https://doi.org/10.3390/chemosensors10110494









