Enhanced Gas Detection by Altering Gate Voltage Polarity of Polypyrrole/Graphene Field-Effect Transistor Sensor
Abstract
1. Introduction
2. Materials and Methods
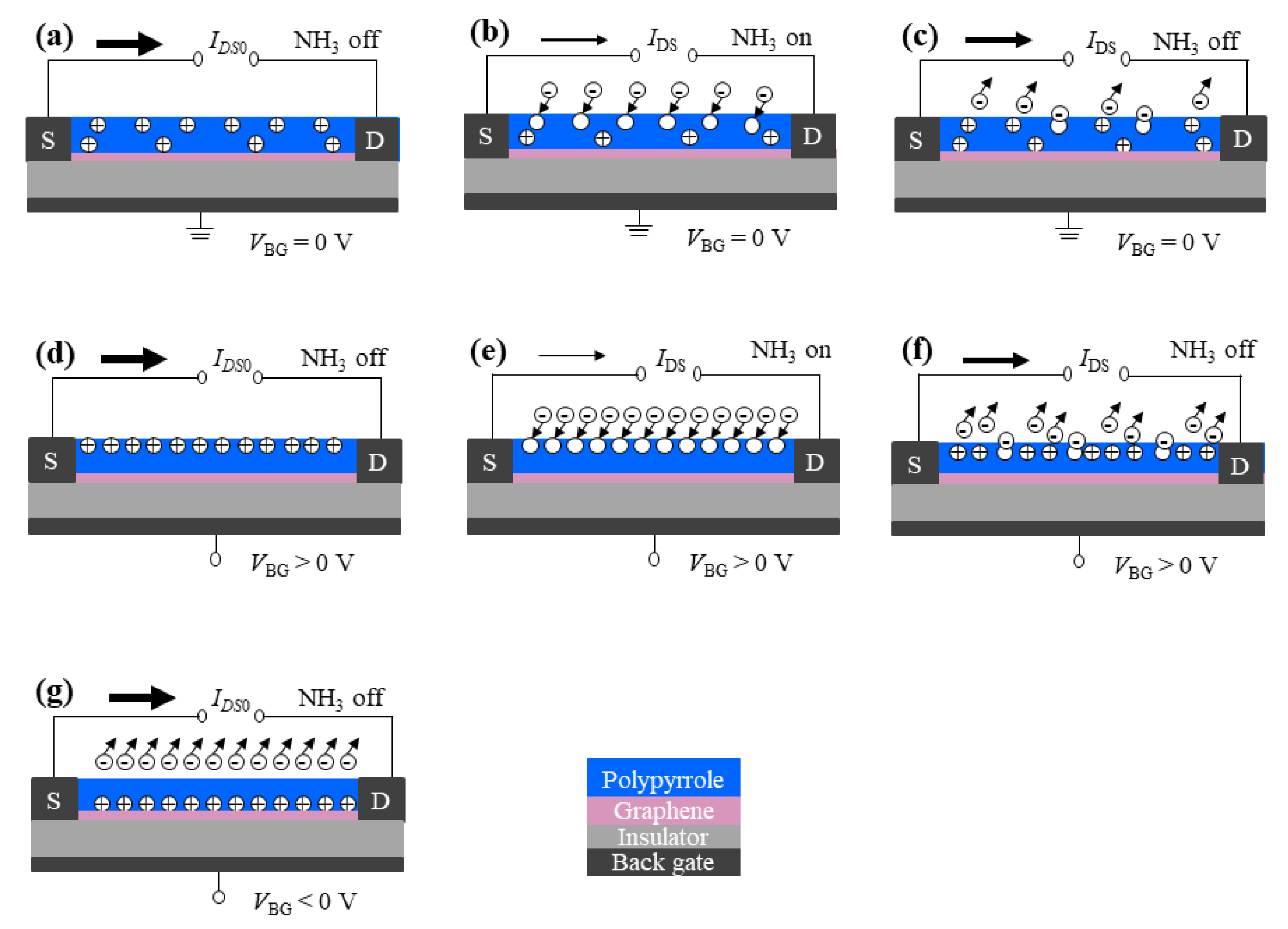
2.1. Measurement Principle
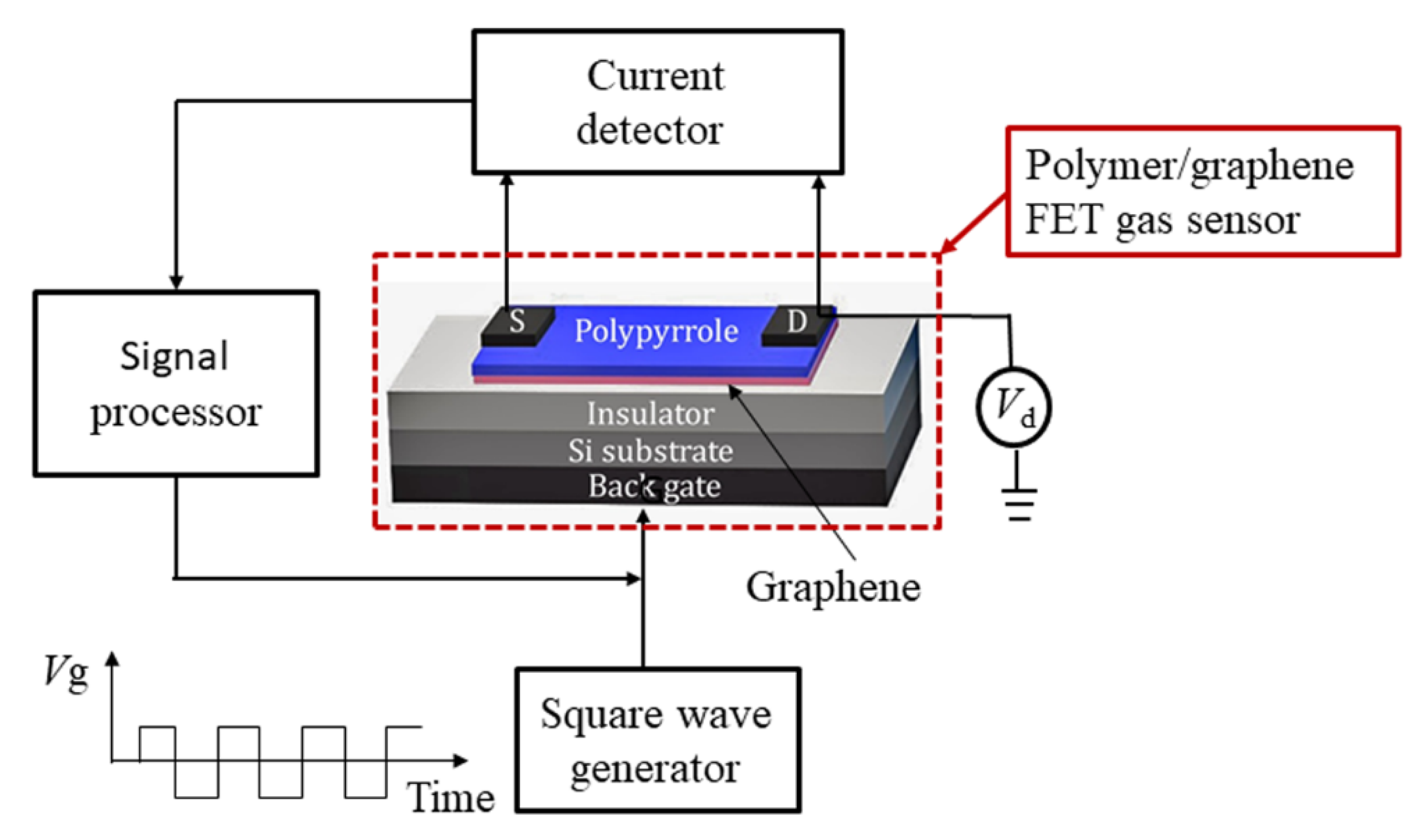
2.2. Measurement Setup
2.3. Sensor Fabrication and Measurement
3. Results and Discussion
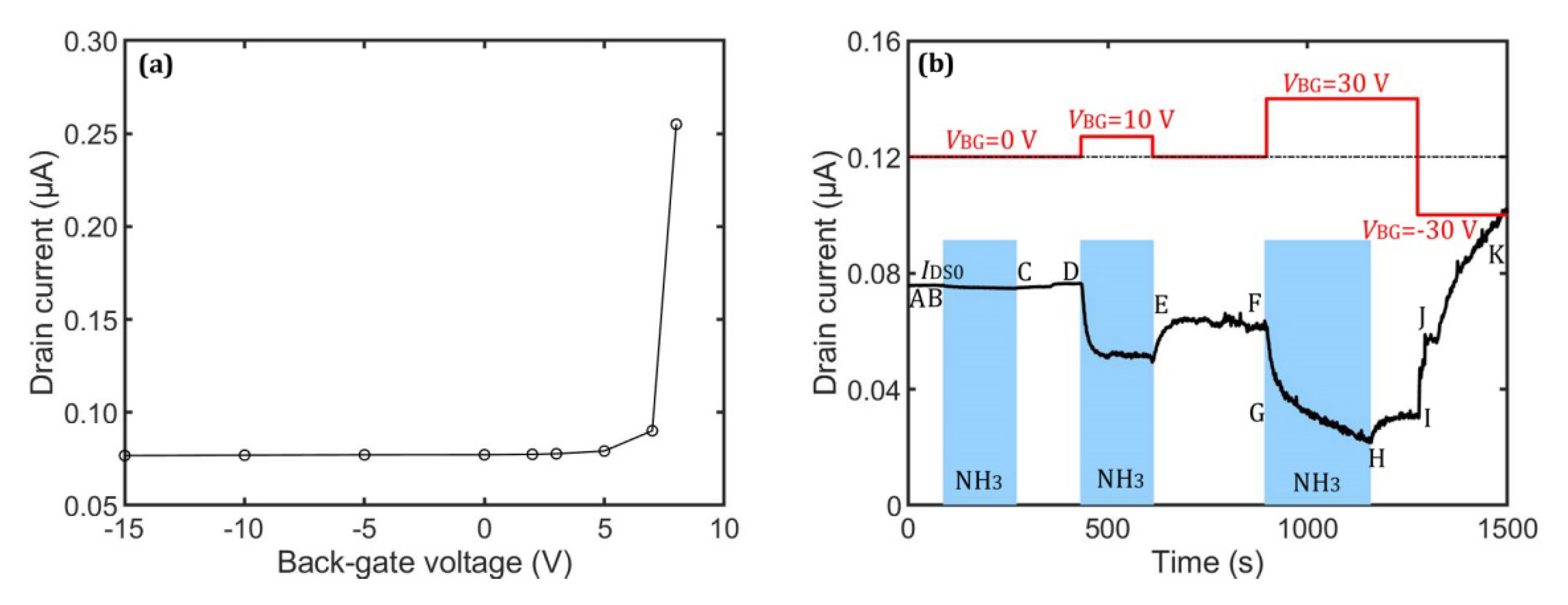
3.1. Experiment Results
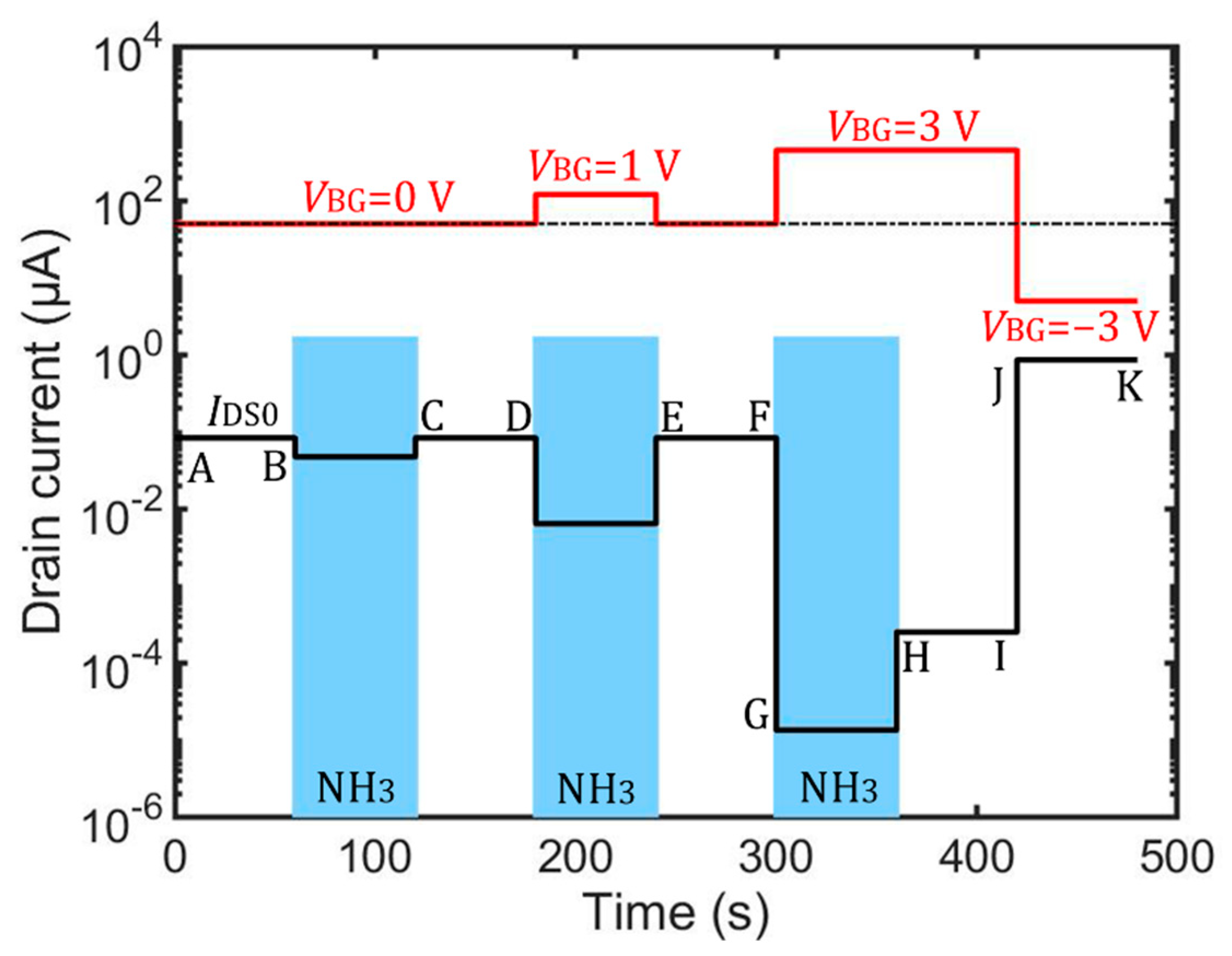
3.2. Simulation Results
3.3. Calculation Results Based on a Sensor Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Shi, W.; Song, J.; Jang, H.-J.; Dailey, J.; Yu, J.; Katz, H.E. Chemical and Biomolecule Sensing with Organic Field-Effect Transistors. Chem. Rev. 2019, 119, 3–35. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Hu, W. Organic field-effect transistor-based gas sensors. Chem. Soc. Rev. 2015, 44, 2087–2107. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Miyahara, Y. Field-Effect Transistors for Gas Sensing. In Different Types of Field-Effect Transistors—Theory and Applications; Pejovic, M.M., Pejovic, M.M., Eds.; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.; Lin, L. A versatile gas sensor with selectivity using a single graphene transistor. In Proceedings of the 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 961–964. [Google Scholar] [CrossRef]
- Tang, X.; Debliquy, M.; Lahem, D.; Yan, Y.; Raskin, J.-P. A Review on Functionalized Graphene Sensors for Detection of Ammonia. Sensors 2021, 21, 1443. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Dua, V.; Surwade, S.P.; Ammu, S.; Agnihotra, S.R.; Jain, S.; Roberts, K.E.; Park, S.; Ruoff, R.S.; Manohar, S.K. All-Organic Vapor Sensor Using Inkjet-Printed Reduced Graphene Oxide. Angew. Chem. Int. Ed. 2010, 49, 2154–2157. [Google Scholar] [CrossRef]
- Tabata, H.; Sato, Y.; Oi, K.; Kubo, O.; Katayama, M. Bias- and Gate-Tunable Gas Sensor Response Originating from Modulation in the Schottky Barrier Height of a Graphene/MoS2 van der Waals Heterojunction. ACS Appl. Mater. Interfaces 2018, 10, 38387–38393. [Google Scholar] [CrossRef]
- Kumar, B.; Min, K.; Bashirzadeh, M.; Farimani, A.B.; Bae, M.-H.; Estrada, D.; Kim, Y.D.; Yasaei, P.; Park, Y.D.; Pop, E.; et al. The Role of External Defects in Chemical Sensing of Graphene Field-Effect Transistors. Nano Lett. 2013, 13, 1962–1968. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Cong, C.; Shang, J.; Yu, T. Hysteresis of Electronic Transport in Graphene Transistors. ACS Nano 2010, 4, 7221–7228. [Google Scholar] [CrossRef]
- Kulkarni, G.S.; Reddy, K.; Zhong, Z.; Fan, X. Graphene nanoelectronic heterodyne sensor for rapid and sensitive vapour detection. Nat Commun. 2014, 5, 4376. [Google Scholar] [CrossRef]
- Mackin, C.; Schroeder, V.; Zurutuza, A.; Su, C.; Kong, J.; Swager, T.M.; Palacios, T. Chemiresistive Graphene Sensors for Ammonia Detection. ACS Appl. Mater. Interfaces 2018, 10, 16169–16176. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.; Olivier, M.; Liao, H.; Debliquy, M. Solution precursor plasma-sprayed tungsten oxide coatings for nitrogen dioxide detection. Ceram. Int. 2014, 40, 11427–11431. [Google Scholar] [CrossRef]
- Geng, X.; Luo, Y.; Zheng, B.; Zhang, C. Photon assisted room-temperature hydrogen sensors using PdO loaded WO3 nanohybrids. Int. J. Hydrogen Energy 2017, 42, 6425–6434. [Google Scholar] [CrossRef]
- Ahmed, A.Y.; Baskaran, F.M.F.; Rabih, A.A.S.; Dennis, J.O.; Khir, M.H.M.; Elmaleeh, M.A.A. Design, Modeling and Simulation of Microhotplate for Application in Gas Detection. In Proceedings of the 2018 International Conference on Intelligent and Advanced System (ICIAS), Kuala Lumpur, Malaysia, 13–14 August 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Prades, J.D.; Jimenez-Diaz, R.; Hernandez-Ramirez, F.; Barth, S.; Cirera, A.; Romano-Rodriguez, A.; Mathur, S.; Morante, J.R. Equivalence between thermal and room temperature UV light-modulated responses of gas sensors based on individual SnO2 nanowires. Sens. Actuators B Chem. 2009, 140, 337–341. [Google Scholar] [CrossRef]
- Salehi-Khojin, A.; Lin, K.Y.; Field, C.R.; Masel, R.I. Nonthermal Current-Stimulated Desorption of Gases from Carbon Nanotubes. Science 2010, 329, 1327–1330. [Google Scholar] [CrossRef]
- Novak, J.P.; Snow, E.S.; Houser, E.J.; Park, D.; Stepnowski, J.L.; McGill, R.A. Nerve agent detection using networks of single-walled carbon nanotubes. Appl. Phys. Lett. 2003, 83, 4026–4028. [Google Scholar] [CrossRef]
- Rumyantsev, S.; Liu, G.; Shur, M.S.; Potyrailo, R.A.; Balandin, A.A. Selective Gas Sensing with a Single Pristine Graphene Transistor. Nano Lett. 2012, 12, 2294–2298. [Google Scholar] [CrossRef]
- Snow, E.S. Chemical Detection with a Single-Walled Carbon Nanotube Capacitor. Science 2005, 307, 1942–1945. [Google Scholar] [CrossRef]
- Kulkarni, G.S.; Zang, W.; Zhong, Z. Nanoelectronic Heterodyne Sensor: A New Electronic Sensing Paradigm. Acc. Chem. Res. 2016, 49, 2578–2586. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Chu, Y.; Hayasaka, T.; Joshi, N.; Cui, Y.; Wang, X.; You, Z.; Lin, L. AC phase sensing of graphene FETs for chemical vapors with fast recovery and minimal baseline drift. Sens. Actuators B Chem. 2018, 263, 94–102. [Google Scholar] [CrossRef]
- Tang, X.; Lahem, D.; Raskin, J.-P.; Gerard, P.; Geng, X.; Andre, N.; Debliquy, M. A Fast and Room-Temperature Operation Ammonia Sensor Based on Compound of Graphene With Polypyrrole. IEEE Sens. J. 2018, 18, 9088–9096. [Google Scholar] [CrossRef]
- Tang, X.; Raskin, J.-P.; Kryvutsa, N.; Hermans, S.; Slobodian, O.; Nazarov, A.N.; Debliquy, M. An ammonia sensor composed of polypyrrole synthesized on reduced graphene oxide by electropolymerization. Sens. Actuators B Chem. 2020, 305, 127423. [Google Scholar] [CrossRef]
- Decroly, A.; Krumpmann, A.; Debliquy, M.; Lahem, D. Nanostructured TiO2 Layers for Photovoltaic and Gas Sensing Applications. In Green Nanotechnology—Overview and Further Prospects; Larramendy, M.L., Soloneski, S., Eds.; InTech: Rijeka, Croatia, 2016; Available online: http://www.intechopen.com/books/green-nanotechnology-overview-and-further-prospects/nanostructured-tio2-layers-for-photovoltaic-and-gas-sensing-applications (accessed on 28 July 2016).
- Antonov, R.D.; Johnson, A.T. Subband Population in a Single-Wall Carbon Nanotube Diode. Phys. Rev. Lett. 1999, 83, 3274–3276. [Google Scholar] [CrossRef]
- Cristoloveanu, S.; Lee, K.H.; Park, H.; Parihar, M.S. The concept of electrostatic doping and related devices. Solid-State Electron. 2019, 155, 32–43. [Google Scholar] [CrossRef]
- Colinge, J.-P. Conduction Mechanisms in Thin-Film Accumulation-Mode SO1 p-Channel MOSFET’s. IEEE Trans. Electron Devices 1990, 37, 718–723. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Ragupathi, V.; Raman, S.; Panigrahi, P.; Nagarajan, G.S. Study of optical and electrical property of NaI-doped PPy thin film with excellent photocatalytic property at visible light. Polym. Bull. 2019, 76, 5213–5231. [Google Scholar] [CrossRef]
- Irfan, M.; Shakoor, A. Structural, Electrical and Dielectric Properties of Dodecylbenzene Sulphonic Acid Doped Polypyrrole/Nano-Y2O3 Composites. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1287–1292. [Google Scholar] [CrossRef]
- Flandre, D.; Teraot, A. Extended Theoretical Analysis of the Steady-State Linear Behavior of Accumulation-Mode, Long-Channel p-MOSFETs on SOI Substrates. Solid-State Electron. 1992, 35, 1085–1092. [Google Scholar] [CrossRef]

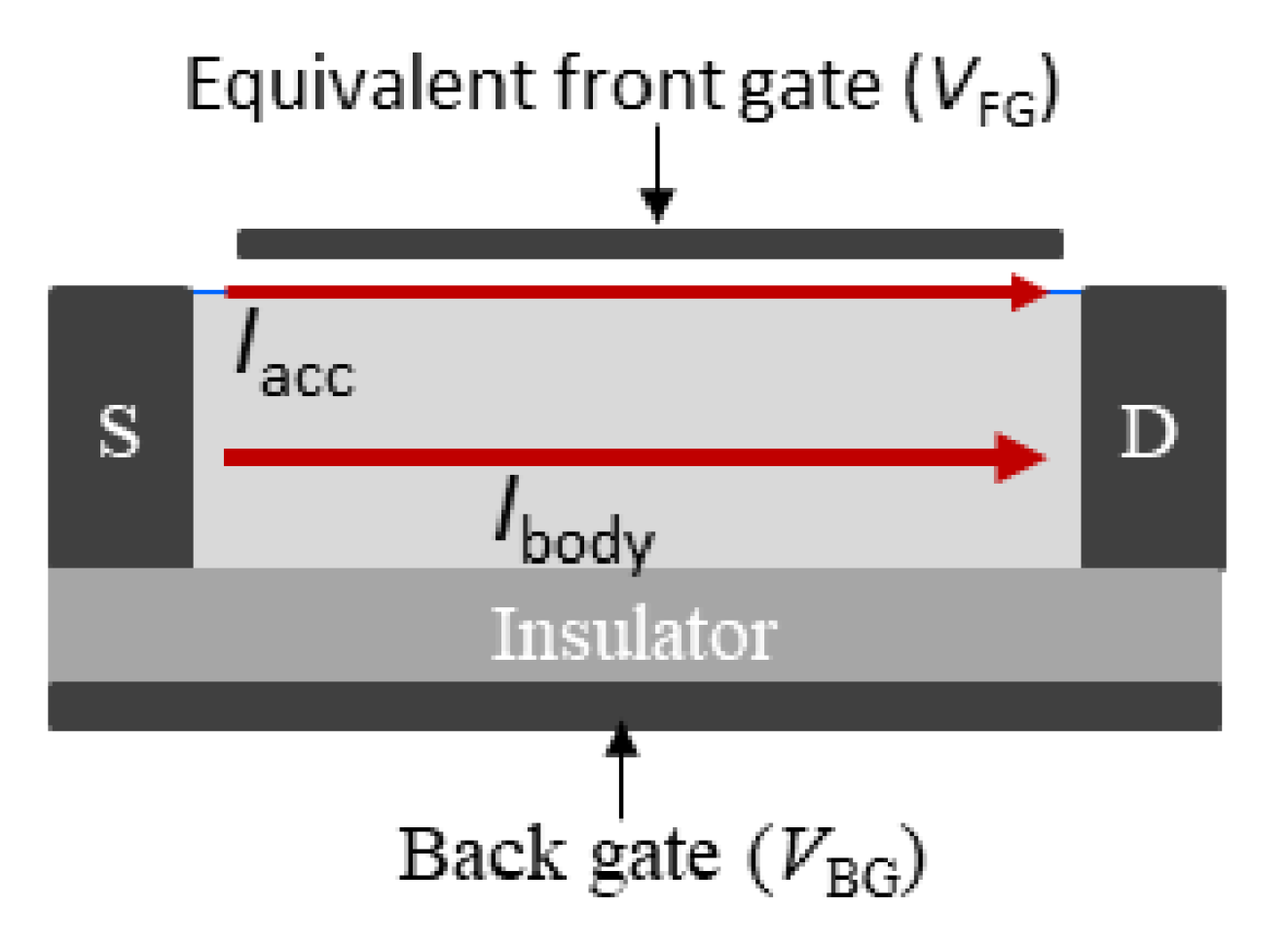
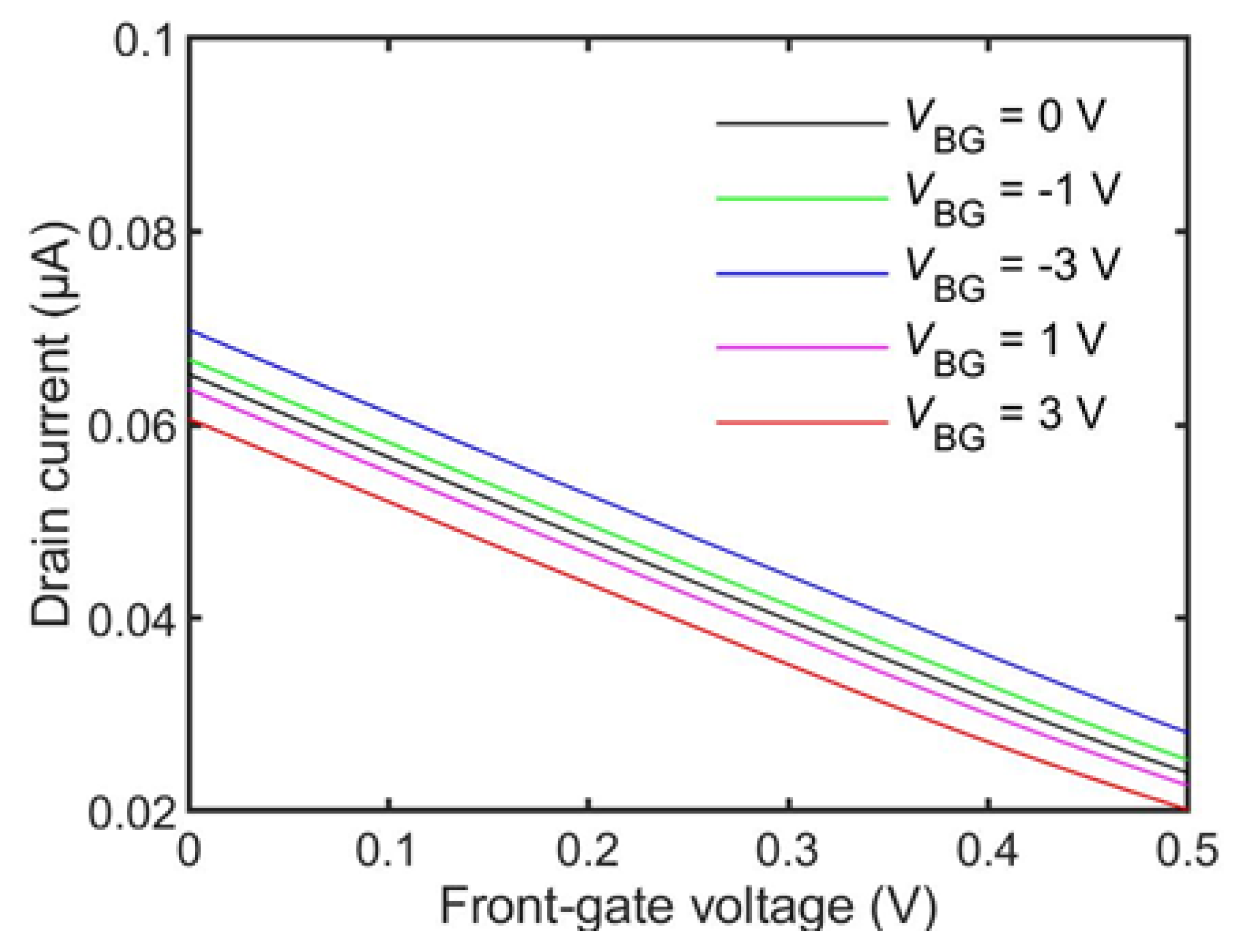
| Parameter Name | Property | Value Range |
|---|---|---|
| Front-gate voltage (VFG) | Variable | 0 to 0.1 V |
| Back-gate voltage (VBG) | Variable | −3 to 3 V |
| Front-gate oxide (SiO2) | Constant | 20 nm |
| Back-gate oxide (SiO2) | Constant | 300 nm |
| Channel thickness | Constant | 100 nm |
| Channel length | Constant | 2 µm |
| Front-gate length | Constant | 2 µm |
| Back-gate length | Constant | 3 µm |
| Polymer doping concentration | Constant | 4 × 1016 cm−3 |
| Drain voltage (VDS) | Constant | −0.8 V |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Raskin, J.-P.; Reckinger, N.; Yan, Y.; André, N.; Lahem, D.; Debliquy, M. Enhanced Gas Detection by Altering Gate Voltage Polarity of Polypyrrole/Graphene Field-Effect Transistor Sensor. Chemosensors 2022, 10, 467. https://doi.org/10.3390/chemosensors10110467
Tang X, Raskin J-P, Reckinger N, Yan Y, André N, Lahem D, Debliquy M. Enhanced Gas Detection by Altering Gate Voltage Polarity of Polypyrrole/Graphene Field-Effect Transistor Sensor. Chemosensors. 2022; 10(11):467. https://doi.org/10.3390/chemosensors10110467
Chicago/Turabian StyleTang, Xiaohui, Jean-Pierre Raskin, Nicolas Reckinger, Yiyi Yan, Nicolas André, Driss Lahem, and Marc Debliquy. 2022. "Enhanced Gas Detection by Altering Gate Voltage Polarity of Polypyrrole/Graphene Field-Effect Transistor Sensor" Chemosensors 10, no. 11: 467. https://doi.org/10.3390/chemosensors10110467
APA StyleTang, X., Raskin, J.-P., Reckinger, N., Yan, Y., André, N., Lahem, D., & Debliquy, M. (2022). Enhanced Gas Detection by Altering Gate Voltage Polarity of Polypyrrole/Graphene Field-Effect Transistor Sensor. Chemosensors, 10(11), 467. https://doi.org/10.3390/chemosensors10110467







