MoS2/MWCNT-COOH-Modified Glassy Carbon Electrode for Nitrite Detection in Water Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Preparation of MoS2/MWCNT-COOH/GCE and Electrochemical Measurements
2.4. Real Samples Preparation
3. Results and Discussions
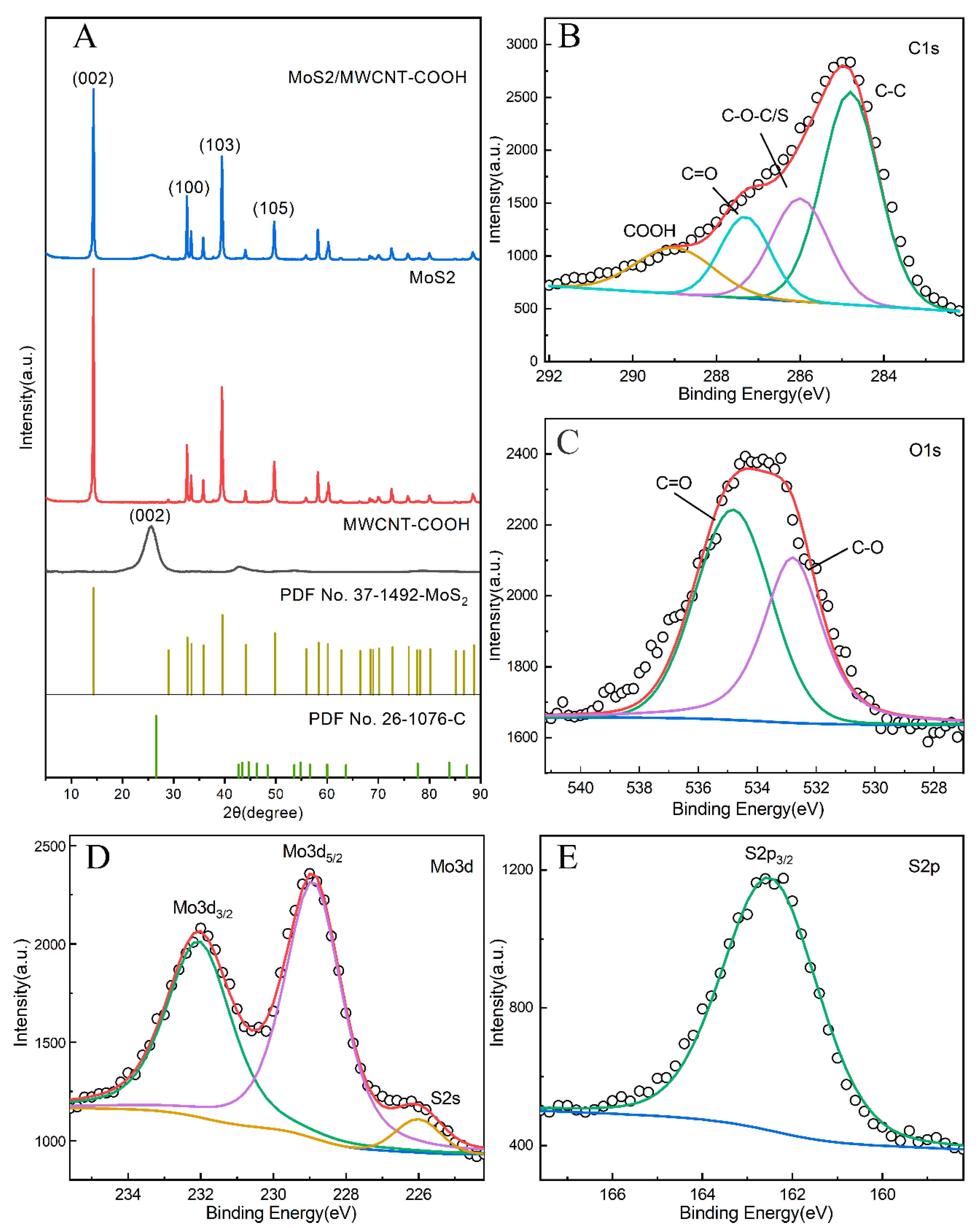
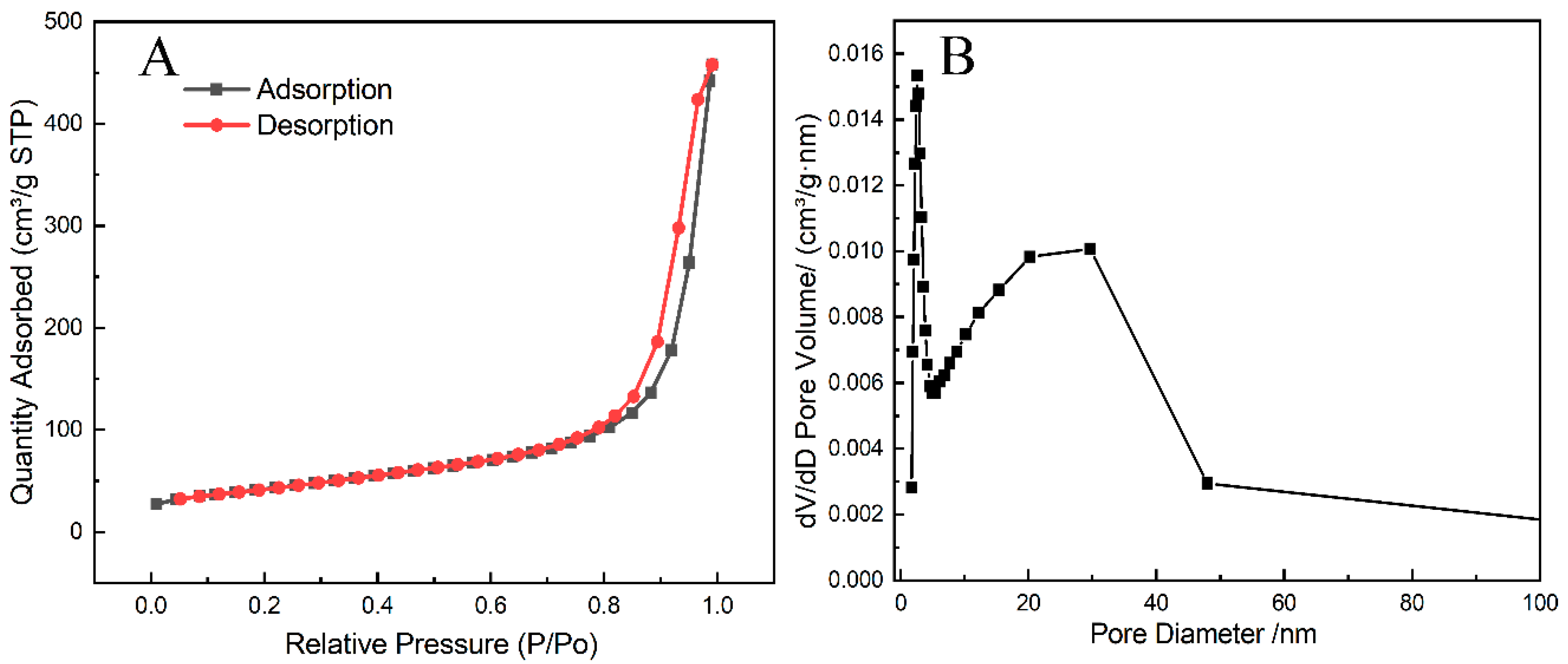
3.1. Structural and Morphology Characterization of MoS2/MWCNT-COOH Hybrid
3.2. Electrochemical Properties of MoS2/ MWCNT-COOH Hybrid
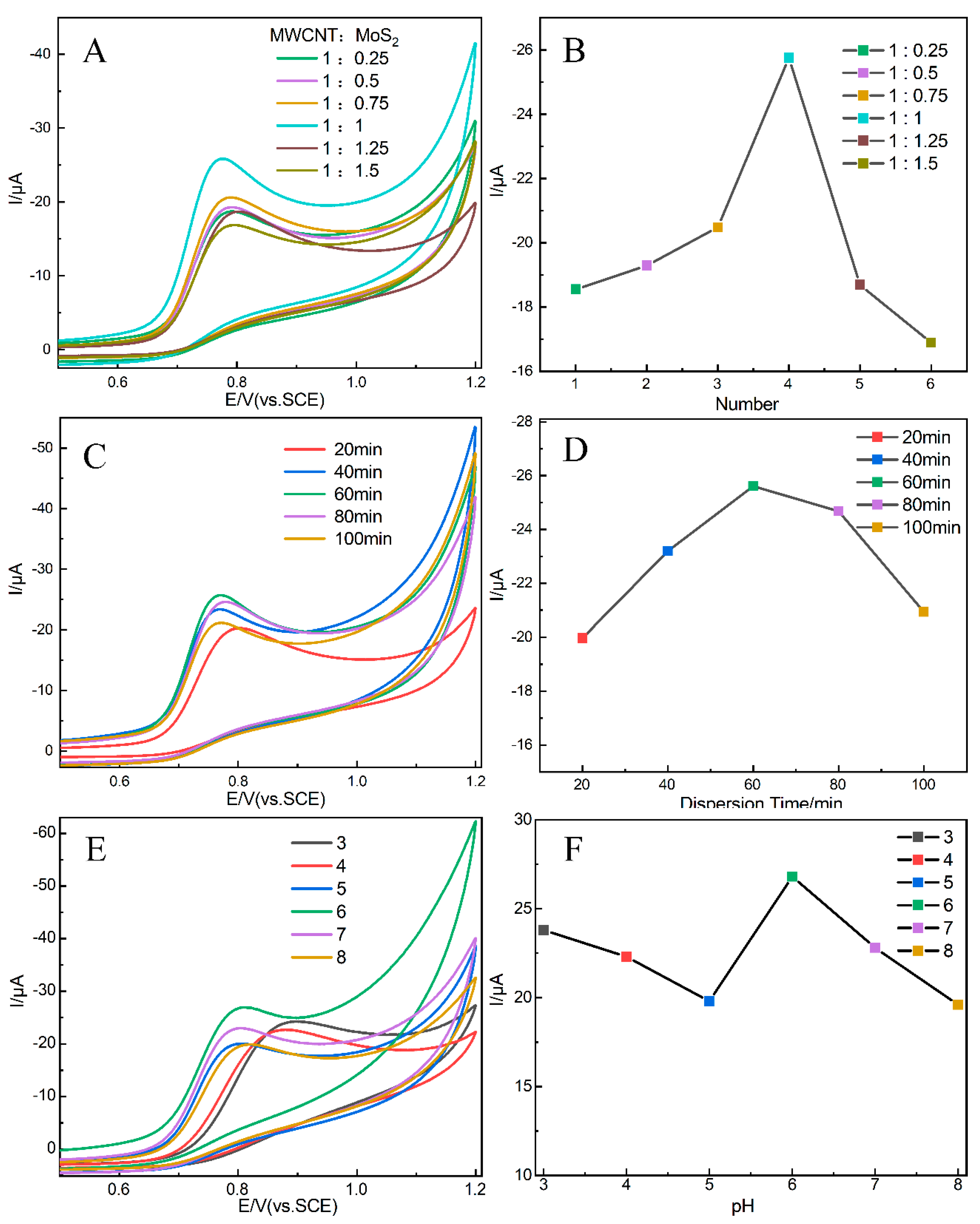
3.3. Condition Optimization
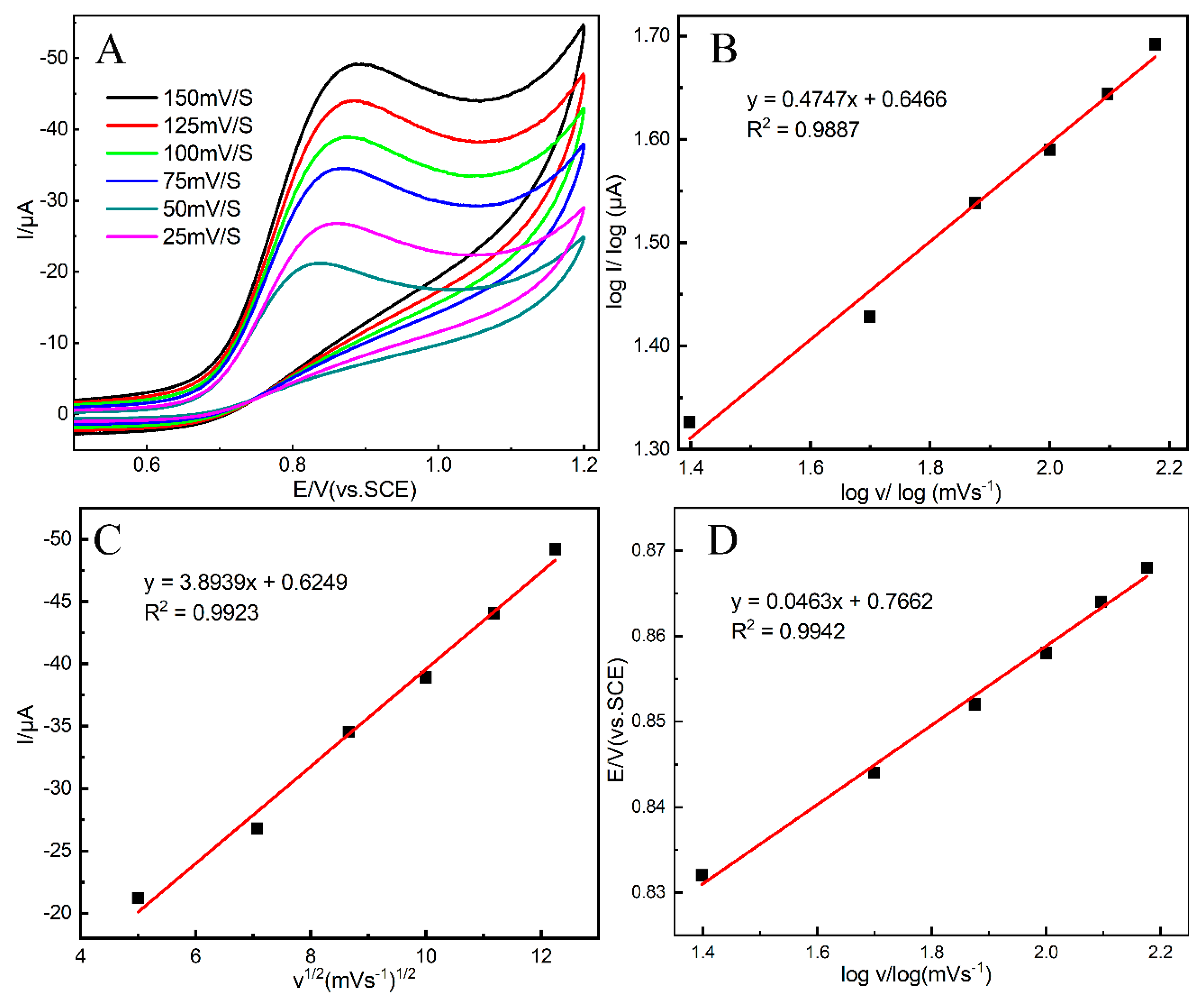
3.4. Scan Rate Analysis and Reaction Mechanism of Nitrite
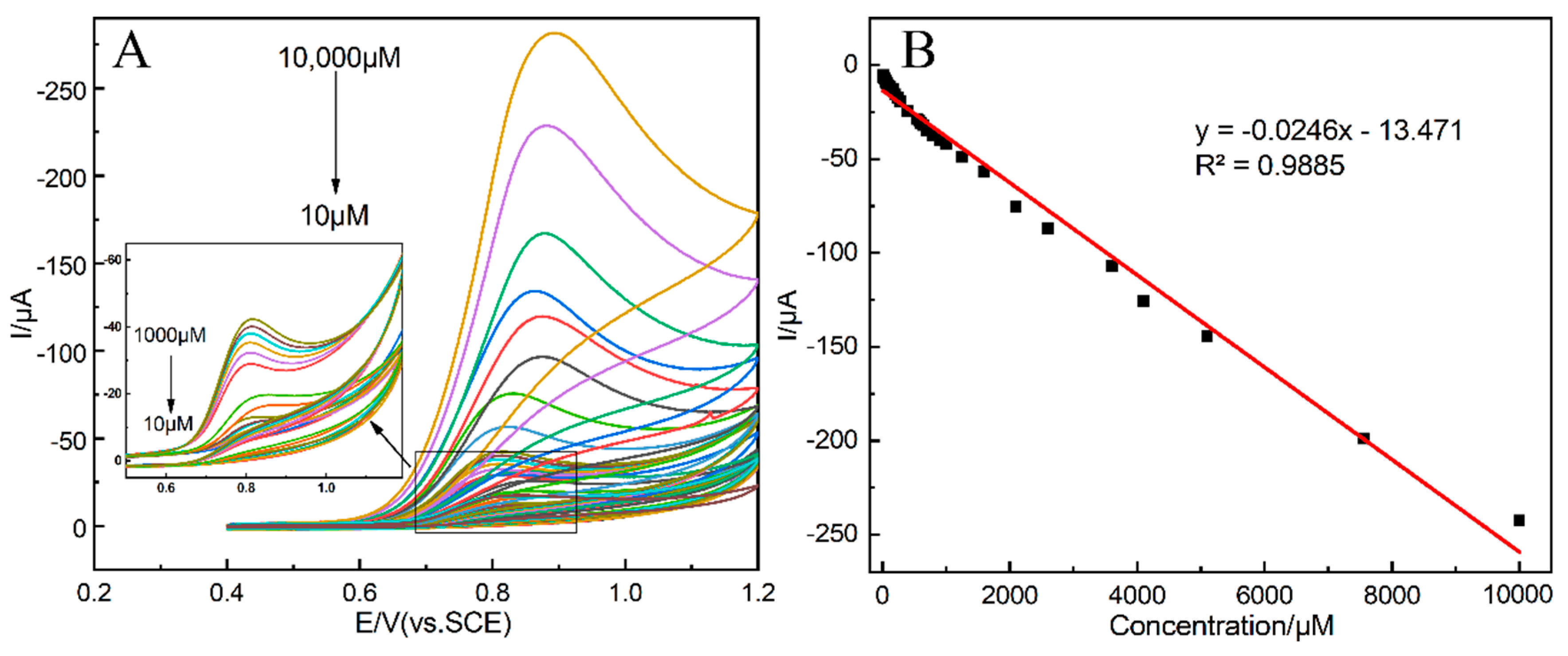
3.5. CV Determination of Nitrite
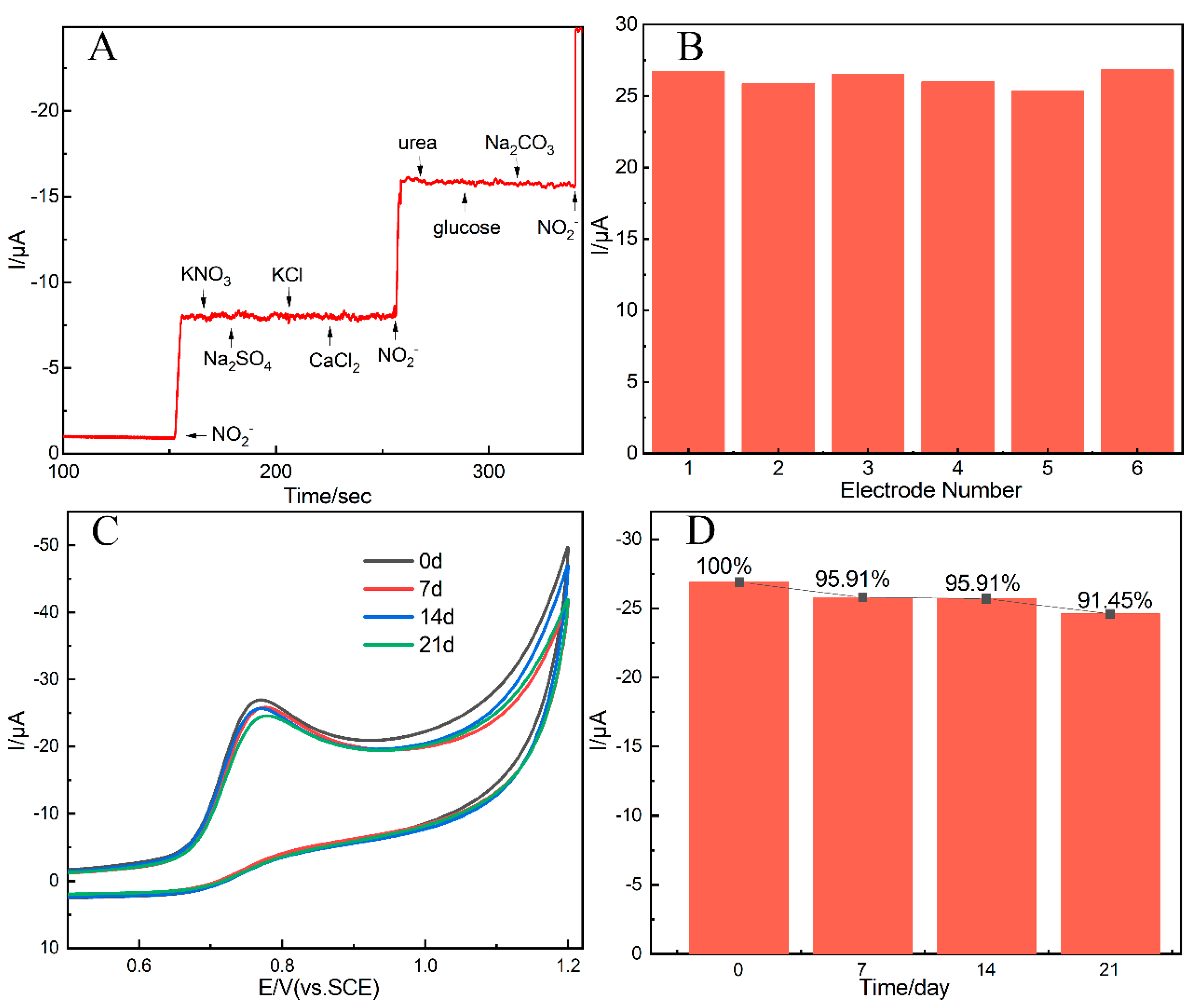
3.6. The Selectivity, Reproducibility, and Stability Analysis
3.7. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Ping, J.; Ying, Y. Recent Developments in Carbon Nanomaterial-Enabled Electrochemical Sensors for Nitrite Detection. TrAC Trends Anal. Chem. 2019, 113, 1–12. [Google Scholar] [CrossRef]
- Stamm, P.; Oelze, M.; Steven, S.; Kröller-Schön, S.; Kvandova, M.; Kalinovic, S.; Jasztal, A.; Kij, A.; Kuntic, M.; Bayo Jimenez, M.T.; et al. Direct Comparison of Inorganic Nitrite and Nitrate on Vascular Dysfunction and Oxidative Damage in Experimental Arterial Hypertension. Nitric Oxide 2021, 113–114, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chuang, C.Y.; Malle, E.; Gamon, L.F.; Hawkins, C.L.; Davies, M.J. Influence of Plasma Halide, Pseudohalide and Nitrite Ions on Myeloperoxidase-Mediated Protein and Extracellular Matrix Damage. Free Radic. Biol. Med. 2022, 188, 162–174. [Google Scholar] [CrossRef]
- Han, S.; Chen, X. Copper Nanoclusters-Enhanced Chemiluminescence for Folic Acid and Nitrite Detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 315–320. [Google Scholar] [CrossRef]
- Lin, K.; Xu, J.; Dong, X.; Huo, Y.; Yuan, D.; Lin, H.; Zhang, Y. An Automated Spectrophotometric Method for the Direct Determination of Nitrite and Nitrate in Seawater: Nitrite Removal with Sulfamic Acid before Nitrate Reduction Using the Vanadium Reduction Method. Microchem. J. 2020, 158, 105272. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Campillo-Brocal, J.C.; Jiménez-Quero, A.; Crespo, G.A.; Cuartero, M. Electrochemical Biosensor for Glycine Detection in Biological Fluids. Biosens. Bioelectron. 2021, 182, 113154. [Google Scholar] [CrossRef]
- Promsuwan, K.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. Flow Injection Amperometric Nitrite Sensor Based on Silver Microcubics-Poly (Acrylic Acid)/Poly (Vinyl Alcohol) Modified Screen Printed Carbon Electrode. Electrochim. Acta 2017, 232, 357–369. [Google Scholar] [CrossRef]
- Rashed, M.A.; Faisal, M.; Alsaiari, M.; Alsareii, S.A.; Harraz, F.A. MWCNT-Doped Polypyrrole-Carbon Black Modified Glassy Carbon Electrode for Efficient Electrochemical Sensing of Nitrite Ions. Electrocatalysis 2021, 12, 650–666. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Hu, S.; Xia, J.; Anderson, T.; Dinh, X.-Q.; Meng, X.-M.; Coquet, P.; Yong, K.-T. Graphene-MoS2 Hybrid Nanostructures Enhanced Surface Plasmon Resonance Biosensors. Sens. Actuators B Chem. 2015, 207, 801–810. [Google Scholar] [CrossRef]
- Kıranşan, K.D.; Topçu, E. Free-Standing and Flexible MoS2/RGO Paper Electrode for Amperometric Detection of Folic Acid. Electroanalysis 2018, 30, 810–818. [Google Scholar] [CrossRef]
- Devi, R.; Gogoi, S.; Barua, S.; Sankar Dutta, H.; Bordoloi, M.; Khan, R. Electrochemical Detection of Monosodium Glutamate in Foodstuffs Based on Au@MoS2/Chitosan Modified Glassy Carbon Electrode. Food Chem. 2019, 276, 350–357. [Google Scholar] [CrossRef]
- Kumar, M.; Wang, M.; Kumara Swamy, B.E.; Praveen, M.; Zhao, W. Poly (Alanine)/NaOH/ MoS2/MWCNTs Modified Carbon Paste Electrode for Simultaneous Detection of Dopamine, Ascorbic Acid, Serotonin and Guanine. Colloids Surf. B Biointerfaces 2020, 196, 111299. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Chakraborty, A.K.; Bera, S.; Krishnamurthy, S. Novel Hydrothermal Synthesis of CoS2/MWCNT Nanohybrid Electrode for Supercapacitor: A Systematic Investigation on the Influence of MWCNT. J. Phys. Chem. C 2018, 122, 18237–18246. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Z.; Chen, Y.; Zou, M.; Yousaf, M.; Yang, Y.; Yang, L.; Cao, A.; Han, R.P.S. Controlled Synthesis of Core–Shell Carbon@MoS2 Nanotube Sponges as High-Performance Battery Electrodes. Adv. Mater. 2016, 28, 10175–10181. [Google Scholar] [CrossRef] [PubMed]
- Motaghedifard, M.H.; Pourmortazavi, S.M.; Alibolandi, M.; Mirsadeghi, S. Au-Modified Organic/Inorganic MWCNT/Cu/PANI Hybrid Nanocomposite Electrode for Electrochemical Determination of Nitrate Ions. Microchim. Acta 2021, 188, 99. [Google Scholar] [CrossRef]
- Chakraborty, I.; Chakrabarty, N.; Senapati, A.; Chakraborty, A.K. CuO@NiO/Polyaniline/MWCNT Nanocomposite as High-Performance Electrode for Supercapacitor. J. Phys. Chem. C 2018, 122, 27180–27190. [Google Scholar] [CrossRef]
- Qin, J.; Huang, F.; Li, X.; Deng, L.; Kang, T.; Markov, A.; Yue, F.; Chen, Y.; Wen, X.; Liu, S.; et al. Enhanced Second Harmonic Generation from Ferroelectric HfO2-Based Hybrid Metasurfaces. ACS Nano 2019, 13, 1213–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, S.; Tian, F.; Li, M.; Liu, Y.; Sheng, J.; Yang, W.; Yu, Y.; Hou, Y. Activating Interfacial S Sites of MoS2 Boosts Hydrogen Evolution Electrocatalysis. Nano Res. 2022, 15, 1809–1816. [Google Scholar] [CrossRef]
- Yang, W.; Guo, H.; Xue, R.; Zhao, X.; Guan, Q.; Fan, T.; Zhang, L.; Yang, F.; Yang, W. 0.2CNT/NiSex Composite Derived from CNT/MOF-74 as Electrode Material for Electrochemical Capacitor and Electrochemical Sensor. Microchem. J. 2021, 168, 106519. [Google Scholar] [CrossRef]
- Yang, M.; Guo, H.; Sun, L.; Wu, N.; Wang, M.; Yang, F.; Zhang, T.; Zhang, J.; Pan, Z.; Yang, W. Simultaneous Electrochemical Detection of Hydroquinone and Catechol Using MWCNT-COOH/CTF-1 Composite Modified Electrode. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126917. [Google Scholar] [CrossRef]
- Karade, S.S.; Dubal, D.P.; Sankapal, B.R. Decoration of Ultrathin MoS2 Nanoflakes over MWCNTs: Enhanced Supercapacitive Performance through Electrode to Symmetric All-Solid-State Device. ChemistrySelect 2017, 2, 10405–10412. [Google Scholar] [CrossRef]
- Chen, B.; Lu, H.; Zhou, J.; Ye, C.; Shi, C.; Zhao, N.; Qiao, S.-Z. Porous MoS2/Carbon Spheres Anchored on 3D Interconnected Multiwall Carbon Nanotube Networks for Ultrafast Na Storage. Adv. Energy Mater. 2018, 8, 1702909. [Google Scholar] [CrossRef]
- Prasad, J.; Singh, A.K.; Yadav, A.N.; Kumar, A.; Tomar, M.; Srivastava, A.; Kumar, P.; Gupta, V.; Singh, K. Molybdenum Disulfide-Wrapped Carbon Nanotube-Reduced Graphene Oxide (CNT/MoS2-RGO) Nanohybrids for Excellent and Fast Removal of Electromagnetic Interference Pollution. ACS Appl. Mater. Interfaces 2020, 12, 40828–40837. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Ding, P.; Wang, Y.; Wu, Y.; Deng, J.; Jia, L.; Li, Y. Interlayer-Expanded MoS2 Assemblies for Enhanced Electrochemical Storage of Potassium Ions. Nano Res. 2020, 13, 225–230. [Google Scholar] [CrossRef]
- Arunbalaji, S.; Vasudevan, R.; Arivanandhan, M.; Alsalme, A.; Alghamdi, A.; Jayavel, R. CuO/MoS2 Nanocomposites for Rapid and High Sensitive Non-Enzymatic Glucose Sensors. Ceram. Int. 2020, 46, 16879–16885. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Rouquerol, F.; Rouquerol, J. 5-Classical Interpretation of Physisorption Isotherms at the Gas-Solid Interface. In Adsorption by Powders and Porous Solids, 2nd ed.; Rouquerol, F., Rouquerol, J., Sing, K.S.W., Llewellyn, P., Maurin, G., Eds.; Academic Press: Oxford, UK, 2014; pp. 159–189. ISBN 978-0-08-097035-6. [Google Scholar]
- Ahammad, A.J.S.; Pal, P.R.; Shah, S.S.; Islam, T.; Hasan, M.; Qasem, M.A.A.; Odhikari, N.; Sarker, S.; Kim, D.M.; Aziz, A. Activated Jute Carbon Paste Screen-Printed FTO Electrodes for Nonenzymatic Amperometric Determination of Nitrite. J. Electroanal. Chem. 2019, 832, 368–379. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, P.; Ge, Y.; Wu, R.; Xue, T.; Sheng, Y.; Ai, S.; Tang, K.; Wen, Y. MoS2/MWCNTs Porous Nanohybrid Network with Oxidase-like Characteristic as Electrochemical Nanozyme Sensor Coupled with Machine Learning for Intelligent Analysis of Carbendazim. J. Electroanal. Chem. 2020, 862, 113940. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhao, X.; Cui, K.; Shen, Q.; Li, P.; Qu, X.; Jiao, L. Molecular Engineering on MoS2 Enables Large Interlayers and Unlocked Basal Planes for High-Performance Aqueous Zn-Ion Storage. Angew. Chem. 2021, 133, 20448–20455. [Google Scholar] [CrossRef]
- Lu, X.; Liu, G.; Di, P.; Li, Y.; Xue, T.; Duan, X.; Wen, Y.; Zhu, Y.; Cai, Y.; Xu, Q.; et al. Electrochemical Nanozyme Sensor Based on MoS2-COOH-MWCNT Nanohybrid for a New Plant Growth Regulator 5-Nitroguaiacol. Food Anal. Methods 2020, 13, 2028–2038. [Google Scholar] [CrossRef]
- Chen, Y.; Waterhouse, G.I.N.; Qiao, X.; Sun, Y.; Xu, Z. Sensitive Analytical Detection of Nitrite Using an Electrochemical Sensor with STAB-Functionalized Nb2C@MWCNTs for Signal Amplification. Food Chem. 2022, 372, 131356. [Google Scholar] [CrossRef] [PubMed]
- Retter, U.; Lohse, H. Electrochemical Impedance Spectroscopy. In Electroanalytical Methods: Guide to Experiments and Applications; Scholz, F., Bond, A.M., Compton, R.G., Fiedler, D.A., Inzelt, G., Kahlert, H., Komorsky-Lovrić, Š., Lohse, H., Lovrić, M., Marken, F., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 159–177. ISBN 978-3-642-02915-8. [Google Scholar]
- Zhang, Y.; Wang, B.; Lv, S.; Wu, Y.; Jiang, L.; Wang, J.; Liu, X.; Yan, X.; Wang, C.; Sun, P.; et al. Introduction of MWCNT for Enhancing Sensitivity of Room-Temperature Mixed-Potential Type NO Sensor Attached with Ni-MOF Sensing Electrode. Sens. Actuators B Chem. 2022, 361, 131736. [Google Scholar] [CrossRef]
- Abrego-Martinez, J.C.; Jafari, M.; Chergui, S.; Pavel, C.; Che, D.; Siaj, M. Aptamer-Based Electrochemical Biosensor for Rapid Detection of SARS-CoV-2: Nanoscale Electrode-Aptamer-SARS-CoV-2 Imaging by Photo-Induced Force Microscopy. Biosens. Bioelectron. 2022, 195, 113595. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Ahmadi, S.A.; Askari, M.B.; Di Bartolomeo, A. Screen-Printed Electrode Surface Modification with NiCo2O4/RGO Nanocomposite for Hydroxylamine Detection. Nanomaterials 2021, 11, 3208. [Google Scholar] [CrossRef]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Li, Y.W.; Shan, Q.; Wu, W. Ni Nanosheets Evenly Distributed on MoS2 for Selective Electrochemical Detection of Nitrite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126865. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Anurag, A.; Teixeira, P.R.; Ameur, S.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Investigation of Laser Induced Graphene Electrodes Modified by MWNT/AuNPs for Detection of Nitrite. In Proceedings of the 2019 16th International Multi-Conference on Systems, Signals & Devices (SSD), Istanbul, Turkey, 21–24 March 2019; Volume 3, pp. 615–620. [Google Scholar]
- Sha, R.; Gopalakrishnan, A.; Sreenivasulu, K.V.; Srikanth, V.V.; Badhulika, S. Template-Cum-Catalysis Free Synthesis of α-MnO2 Nanorods-Hierarchical MoS2 Microspheres Composite for Ultra-Sensitive and Selective Determination of Nitrite. J. Alloy. Compd. 2019, 794, 26–34. [Google Scholar] [CrossRef]
- Wang, H.; Chen, P.; Wen, F.; Zhu, Y.; Zhang, Y. Flower-like Fe2O3@MoS2 Nanocomposite Decorated Glassy Carbon Electrode for the Determination of Nitrite. Sens. Actuators B Chem. 2015, 220, 749–754. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Y.; Chen, Y.; Zheng, J. Synthesis of Gold Nanoparticles Coated on Flower-like MoS2 Microsphere and Their Application for Electrochemical Nitrite Sensing. J. Electroanal. Chem. 2019, 839, 195–201. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chen, Y.C.; Chuang, W.S.; Li, J.H.; Wang, Y.S.; Chuang, C.H.; Chen, C.Y.; Kung, C.-W. Pore-Confined Silver Nanoparticles in a Porphyrinic Metal–Organic Framework for Electrochemical Nitrite Detection. ACS Appl. Nano Mater. 2020, 3, 9440–9448. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Huang, H.-P.; Xu, L. A Novel Electrochemical Sensor Based on Au-Dy2(WO4)3 Nanocomposites for Simultaneous Determination of Uric Acid and Nitrite. Chin. J. Anal. Chem. 2020, 48, e20032–e20037. [Google Scholar] [CrossRef]
- Menart, E.; Jovanovski, V.; Hočevar, S.B. Silver Particle-Decorated Carbon Paste Electrode Based on Ionic Liquid for Improved Determination of Nitrite. Electrochem. Commun. 2015, 52, 45–48. [Google Scholar] [CrossRef]
- Norouzi, B.; Rajabi, M. Fabrication of Poly(4-Aminobenzoic Acid/o-Toluidine) Modified Carbon Paste Electrode and Its Electrocatalytic Property to the Oxidation of Nitrite. J. Anal. Chem. 2017, 72, 897–903. [Google Scholar] [CrossRef]


| Material | Method | Sensitivity (μA μM−1 cm−2) | Linear Range | Detection Limit (μM) | Refs. |
|---|---|---|---|---|---|
| Ni/MoS2/GCE | DPV a* | 0.01509 | 20–1000 | 2.74 | [37] |
| i-t | 0.00512 | 5–800 | 2.48 | ||
| Fe2O3/MoS2/GCE | i-t | - | 2–6730 | 1 | [41] |
| AuNPs/MoS2/GCE | i-t | 0.01239 | 5–27,800 | 1.67 | [42] |
| α-MnO2-MoS2/GCE | i-t | 0.516 | 100–800 | 16 | [40] |
| MWCNTs/PPy-C/GCE b* | LSV c* | 0.1558 | 500–10,500 | 2.3 | [8] |
| i-t | 0.1171 | 5–9500 | 3.06 | ||
| LIG/MWCNT/AuNPs d* | CV | - | 10–90 | 6.75 | [39] |
| Ag/Zr-MOF/FTO e* | i-t | - | 40–2000 | 9.1 | [43] |
| AgMCs-PAA/PVA/SPCE f* | i-t | 0.0596 | 2–800 | 4.5 | [7] |
| Au-Dy2(WO4)3/GCE g* | DPV | 0.0815 | 10–1000 | 3.5 | [44] |
| AgPs-IL-CPE h* | SWV | - | 50–1000 | 3 | [45] |
| Poly (4- AB/OT) /CPE i* | i-t | 0.187 | 6–600 | 3.5 | [46] |
| MoS2/MWCNT-COOH/GCE | CV | 0.35 | 10–10,000 | 3.6 | This work |
| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%) n = 3 |
|---|---|---|---|---|
| Tap water | 5 | 5.23 | 104.6 | 2.8 |
| 25 | 25.32 | 101.3 | 2.5 | |
| 50 | 48.60 | 97.2 | 3.6 | |
| 250 | 246.25 | 98.5 | 1.4 | |
| 500 | 496.50 | 99.3 | 0.7 | |
| River water | 5 | 5.35 | 107 | 2.3 |
| 25 | 25.50 | 102 | 3.1 | |
| 50 | 50.95 | 101.9 | 1.9 | |
| 250 | 250.25 | 100.1 | 1 | |
| 500 | 497.50 | 99.5 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Zhang, Y.; Qin, R.; Xu, H.; Ye, M.; Nie, P. MoS2/MWCNT-COOH-Modified Glassy Carbon Electrode for Nitrite Detection in Water Environment. Chemosensors 2022, 10, 419. https://doi.org/10.3390/chemosensors10100419
Ren S, Zhang Y, Qin R, Xu H, Ye M, Nie P. MoS2/MWCNT-COOH-Modified Glassy Carbon Electrode for Nitrite Detection in Water Environment. Chemosensors. 2022; 10(10):419. https://doi.org/10.3390/chemosensors10100419
Chicago/Turabian StyleRen, Shijie, Yahui Zhang, Ruimiao Qin, Honggang Xu, Minger Ye, and Pengcheng Nie. 2022. "MoS2/MWCNT-COOH-Modified Glassy Carbon Electrode for Nitrite Detection in Water Environment" Chemosensors 10, no. 10: 419. https://doi.org/10.3390/chemosensors10100419





