Novel Pillar[5]arenes Show High Cross-Sensitivity in PVC-Plasticized Membrane Potentiometric Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Pillar[5]arenes
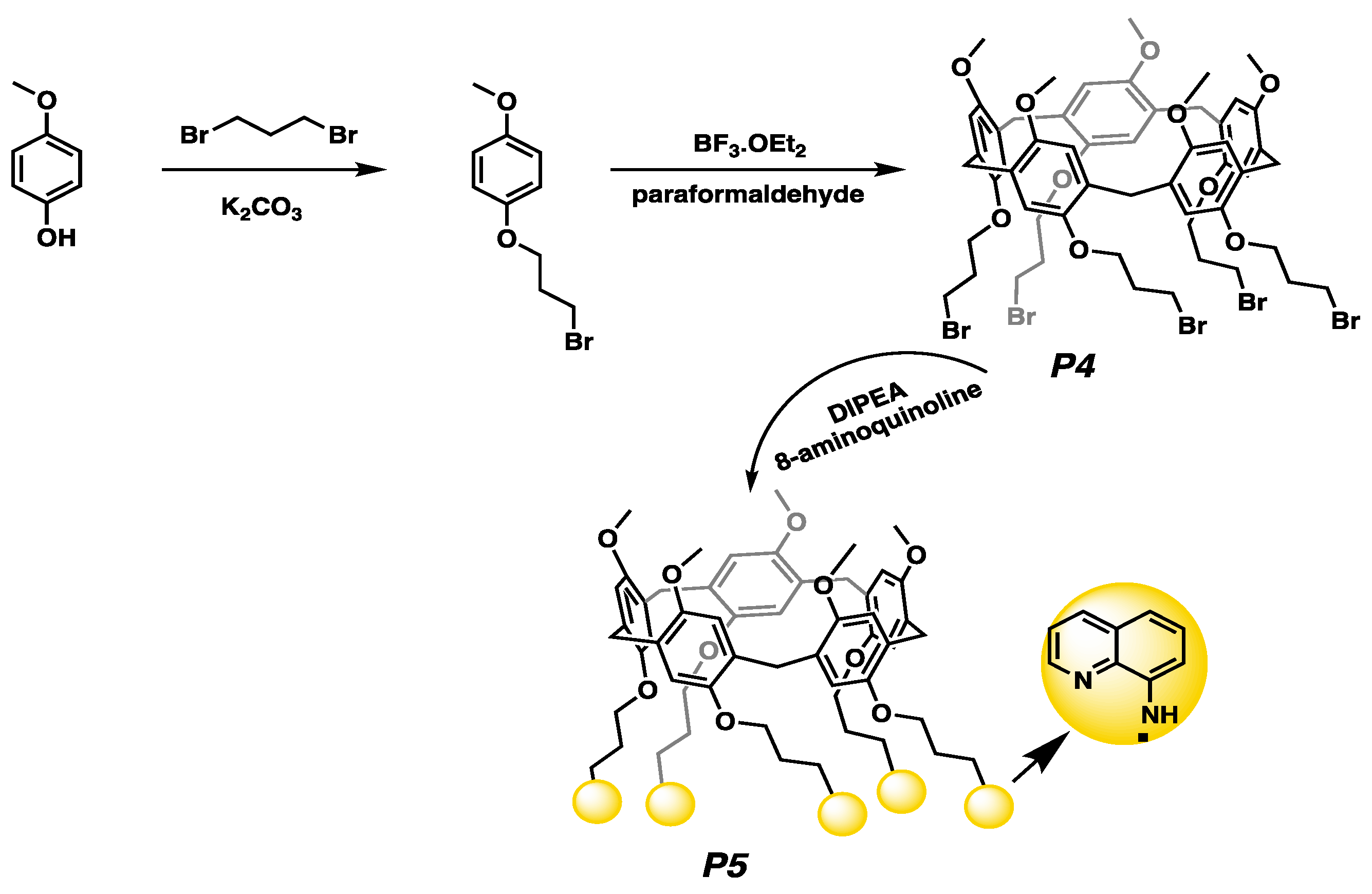
2.1.1. The Synthesis of 1-(3-Bromopropoxy)-4-methoxybenzene
2.1.2. The Synthesis of P2 Compound
2.1.3. The Synthesis of P4 Compound
2.1.4. The Synthesis of P5 Compound
2.2. Potentiometric Sensors Preparation
2.3. Potentiometric Measurements
2.4. Data Processing
3. Results and Discussion
3.1. Sensitivity of Potentiometric Sensors
3.2. Selectivity of Potentiometric Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.A.; Nakamoto, Y. Para-bridged symmetrical pillar[5]arenes: Their Lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T.A.; Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar[n]arenes: New key players for supramolecular chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef] [PubMed]
- Cragg, P.J.; Sharma, K. Pillar[5]arenes: Fascinating cyclophanes with a bright future. Chem. Soc. Rev. 2012, 41, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. [Google Scholar] [CrossRef]
- Holler, M.; Allenbach, N.; Sonet, J.; Nierengarten, J.F. The high yielding synthesis of pillar[5]arenes under Friedel-Crafts conditions explained by dynamic covalent bond formation. Chem. Commun. 2012, 48, 2576–2578. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Quan, K.; Yang, X.; Li, Z.; Zhao, L.; Qiu, H. Recent developments for the investigation of chiral properties and applications of pillar[5]arenes in analytical chemistry. TrAC- Trends Anal. Chem. 2020, 131, 116026. [Google Scholar] [CrossRef]
- Liu, X.; Jia, K.; Wang, Y.; Shao, W.; Yao, C.; Peng, L.; Zhang, D.; Hu, X.Y.; Wang, L. Dual-responsive bola-type supra-amphiphile constructed from water-soluble pillar[5]arene and naphthalimide-containing amphiphile for intracellular drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 4843–4850. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; He, Z.; Hao, M.; Zuo, M.; Xu, Z.; Hu, X.Y.; Zhu, J.J.; Wang, L. Dual acid-responsive bola-type supramolecular vesicles for efficient intracellular anticancer drug delivery. J. Mater. Chem. B 2019, 7, 3944–3949. [Google Scholar] [CrossRef]
- Wang, Y.; Du, J.; Wang, Y.; Jin, Q.; Ji, J. Pillar[5]arene based supramolecular prodrug micelles with pH induced aggregate behavior for intracellular drug delivery. Chem. Commun. 2015, 51, 2999–3002. [Google Scholar] [CrossRef]
- Yang, K.; Pei, Y.; Wen, J.; Pei, Z. Recent advances in pillar[n]arenes: Synthesis and applications based on host-guest interactions. Chem. Commun. 2016, 52, 9316–9326. [Google Scholar] [CrossRef] [PubMed]
- Cragg, P.J. Pillar[n]arenes at the chemistry-biology interface. Isr. J. Chem. 2018, 58, 1158–1172. [Google Scholar] [CrossRef]
- Dube, L.E.; Patel, B.A.; Fagan-murphy, A.; Kothur, R.R.; Cragg, P.J. Detection of clinically important cations by a pillar[5]arene-modified electrochemical sensor. Chem. Sens. 2013, 3, 1–6. [Google Scholar]
- Zhang, Y.M.; He, J.X.; Zhu, W.; Li, Y.F.; Fang, H.; Yao, H.; Wei, T.B.; Lin, Q. Novel pillar[5]arene-based supramolecular organic framework gel for ultrasensitive response Fe3+ and F− in water. Mater. Sci. Eng. C 2019, 100, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yuan, X.; Wu, L.; Peng, Z.; Feng, W.; Liu, N.; Xu, D.; Li, S.; Sengupta, A.; Mohapatra, P.K.; et al. Ditopic CMPO-pillar[5]arenes as unique receptors for efficient separation of americium(iii) and europium(iii). Chem. Commun. 2015, 51, 4263–4266. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Zhang, Y.M.; Ma, X.Q.; Dong, H.Q.; Zhang, Y.F.; Guan, W.L.; Yao, H.; Wei, T.B.; Lin, Q. A pillar[5]arene-based fluorescent sensor for sensitive detection of L-Met through a dual-site collaborative mechanism. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 240, 118569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Gong, G.F.; Zhang, Y.M.; Fan, Y.Q.; Guan, X.W.; Zhou, Q.; Yang, H.L.; Yao, H.; Wei, T.B.; Lin, Q. A novel pillar[5]arene-based chemosensor for dual-channel detecting L-Arg by multiple supramolecular interactions. Dye. Pigment. 2019, 171, 107706. [Google Scholar] [CrossRef]
- Wu, L.; Fang, Y.; Jia, Y.; Yang, Y.; Liao, J.; Liu, N.; Yang, X.; Feng, W.; Ming, J.; Yuan, L. Pillar[5]arene-based diglycolamides for highly efficient separation of americium(III) and europium(III). Dalt. Trans. 2014, 43, 3835–3838. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, L.; Chen, L.; Yuan, X.; Cai, Y.; Feng, W.; Liu, N.; Ren, Y.; Sengupta, A.; Murali, M.S.; et al. Highly efficient extraction of actinides with pillar[5]arene-derived diglycolamides in ionic liquids via a unique mechanism involving competitive host-guest interactions. Dalt. Trans. 2016, 45, 19299–19310. [Google Scholar] [CrossRef]
- Yuan, X.; Cai, Y.; Chen, L.; Lu, S.; Xiao, X.; Yuan, L.; Feng, W. Phosphine oxides functionalized pillar[5]arenes for uranyl extraction: Solvent effect and thermodynamics. Sep. Purif. Technol. 2020, 230, 11584. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Shurpik, D.N.; Makhmutova, A.R.; Stoikov, I.I. Pillar[5]arenes bearing amide and carboxylic groups as synthetic receptors for alkali metal ions. Macroheterocycles 2017, 10, 226–232. [Google Scholar] [CrossRef]
- Lin, Q.; Zheng, F.; Liu, L.; Mao, P.P.; Zhang, Y.M.; Yao, H.; Wei, T.B. Efficient sensing of fluoride ions in water using a novel water soluble self-assembled supramolecular sensor based on pillar[5]arene. RSC Adv. 2016, 6, 111928–111933. [Google Scholar] [CrossRef]
- Wei, T.B.; Cheng, X.B.; Li, H.; Zheng, F.; Lin, Q.; Yao, H.; Zhang, Y.M. A Novel functionalized pillar[5]arene: Synthesis, assembly and application in sequential fluorescent sensing for Fe3+ and F− in aqueous media. RSC Adv. 2016, 6, 20987–20993. [Google Scholar] [CrossRef]
- Ma, X.Q.; Wang, Y.; Wei, T.B.; Qi, L.H.; Jiang, X.M.; Ding, J.D.; Zhu, W.B.; Yao, H.; Zhang, Y.M.; Lin, Q. A novel AIE chemosensor based on quinoline functionalized Pillar[5]arene for highly selective and sensitive sequential detection of toxic Hg2+ and CN−. Dye. Pigment. 2019, 164, 279–286. [Google Scholar] [CrossRef]
- Shamagsumova, R.V.; Shurpik, D.N.; Padnya, P.L.; Stoikov, I.I.; Evtugyn, G.A. Acetylcholinesterase biosensor for inhibitor measurements based on glassy carbon electrode modified with carbon black and pillar[5]arene. Talanta 2015, 144, 559–568. [Google Scholar] [CrossRef]
- Smolko, V.A.; Shurpik, D.N.; Shamagsumova, R.V.; Porfireva, A.V.; Evtugyn, V.G.; Yakimova, L.S.; Stoikov, I.I.; Evtugyn, G.A. Electrochemical behavior of pillar[5]arene on glassy carbon electrode and its interaction with Cu2+ and Ag+ ions. Electrochim. Acta 2014, 147, 726–734. [Google Scholar] [CrossRef]
- Sun, J.; Guo, F.; Shi, Q.; Wu, H.; Sun, Y.; Chen, M.; Diao, G. Electrochemical detection of paraquat based on silver nanoparticles/water-soluble pillar[5]arene functionalized graphene oxide modified glassy carbon electrode. J. Electroanal. Chem. 2019, 847, 113221. [Google Scholar] [CrossRef]
- Stoikova, E.E.; Sorvin, M.I.; Shurpik, D.N.; Budnikov, H.C.; Stoikov, I.I.; Evtugyn, G.A. Solid-contact potentiometric sensor based on polyaniline and unsubstituted pillar[5]arene. Electroanalysis 2015, 27, 440–449. [Google Scholar] [CrossRef]
- Kothur, R.R.; Hall, J.; Patel, B.A.; Leong, C.L.; Boutelle, M.G.; Cragg, P.J. A low pH sensor from an esterified pillar[5]arene. Chem. Commun. 2014, 50, 852–854. [Google Scholar] [CrossRef]
- Acikbas, Y.; Aksoy, M.; Aksoy, M.; Karaagac, D.; Bastug, E.; Kursunlu, A.N.; Erdogan, M.; Capan, R.; Ozmen, M.; Ersoz, M. Recent progress in pillar[n]arene-based thin films on chemical sensor applications. J. Incl. Phenom. Macrocycl. Chem. 2021, 100, 39–54. [Google Scholar] [CrossRef]
- Zinin, A.I.; Stepanova, E.V.; Jost, U.; Kondakov, N.N.; Shpirt, A.M.; Chizhov, A.O.; Torgov, V.I.; Kononov, L.O. An efficient multigram-scale synthesis of 4-(ω-chloroalkoxy)phenols. Russ. Chem. Bull. 2017, 66, 304–312. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Acikbas, Y.; Ozmen, M.; Erdogan, M.; Capan, R. Fabrication of LB thin film of pillar[5]arene-2-amino-3-hydroxypyridine for the sensing of vapors. Mater. Lett. 2020, 267, 127538. [Google Scholar] [CrossRef]
- Bastug, E.; Kursunlu, A.N.; Guler, E. A fluorescent clever macrocycle: Deca-bodipy bearing a pillar [5]arene and its selective binding of asparagine in half-aqueous medium. J. Lumin. 2020, 225, 117343. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef] [Green Version]
- Kursunlu, A.; Baslak, C. A Bodipy-bearing pillar[5]arene for mimicking photosynthesis: Multi-fluorophoric light harvesting system. Tetrahed. Lett. 2018, 59, 1958–1962. [Google Scholar] [CrossRef]
- Kursunlu, A.; Acikbas, Y.; Ozmen, M.; Erdogan, M.; Capan, R. Haloalkanes and aromatic hydrocarbons sensing using Langmuir–Blodgett thin film of pillar[5]arene-biphenylcarboxylic acid. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 108–117. [Google Scholar] [CrossRef]
- Ciosek, P.; Wróblewski, W. Sensor arrays for liquid sensing—Electronic tongue systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef]
- Bratov, A.; Abramova, N.; Ipatov, A. Recent trends in potentiometric sensor arrays-A review. Anal. Chim. Acta 2010, 678, 149–159. [Google Scholar] [CrossRef]
- Mimendia, A.; Gutiérrez, J.M.; Leija, L.; Hernández, P.R.; Favari, L.; Muñoz, R.; del Valle, M. A review of the use of the potentiometric electronic tongue in the monitoring of environmental systems. Environ. Model. Software 2010, 25, 1023–1030. [Google Scholar] [CrossRef]
- Geană, E.I.; Ciucure, C.T.; Apetrei, C. Electrochemical sensors coupled with multivariate statistical analysis as screening tools for wine authentication issues: A review. Chemosensors 2020, 8, 59. [Google Scholar] [CrossRef]
- Parshina, A.; Kolganova, T.; Safronova, E.; Osipov, A.; Lapshina, E.; Yelnikova, A.; Bobreshova, O.; Yaroslavtsev, A. Perfluorosulfonic acid membranes thermally treated and modified by dopants with proton-acceptor properties for asparaginate and potassium ions determination in pharmaceuticals. Membranes 2019, 9, 142. [Google Scholar] [CrossRef]
- Khaydukova, M.; Militsyn, D.; Karnaukh, M.; Grüner, B.; Selucký, P.; Babain, V.; Wilden, A.; Kirsanov, D.; Legin, A. Modified diamide and phosphine oxide extracting compounds as membrane components for cross-sensitive chemical sensors. Chemosensors 2019, 7, 41. [Google Scholar] [CrossRef]
- Yaroshenko, I.; Kirsanov, D.; Kartsova, L.; Sidorova, A.; Borisova, I.; Legin, A. Determination of urine ionic composition with potentiometric multisensor system. Talanta 2015, 131, 556–561. [Google Scholar] [CrossRef] [PubMed]





| Sensor | Ligand | Polymer Matrix | Plasticizer | Cation Exchanger | Ionophore | |
|---|---|---|---|---|---|---|
| PVC (wt.%) | NPOE (wt.%) | DOS (wt.%) | NaTFPB (wt.%) | Ligand (wt.%) | ||
| L1 | P1 | 33 | 55.64 | - | 0.88 | 10.45 |
| L2 | P1 | 33 | - | 55.64 | 0.88 | 10.45 |
| L3 | P1 | 33 | 56.52 | - | - | 10.45 |
| L4 | P2 | 33 | 54.55 | - | 0.88 | 11.55 |
| L5 | P2 | 33 | - | 54.55 | 0.88 | 11.55 |
| L6 | P2 | 33 | 55.42 | - | - | 11.56 |
| L7 | P3 | 33 | 57.70 | - | 0.88 | 8.40 |
| L8 | P3 | 33 | - | 57.70 | 0.88 | 8.39 |
| L9 | P3 | 33 | 58.58 | - | - | 8.39 |
| L10 | P4 | 33 | 59.67 | - | 0.88 | 6.42 |
| L11 | P4 | 33 | - | 59.67 | 0.88 | 6.43 |
| L12 | P4 | 33 | 60.55 | - | - | 6.42 |
| L13 | P5 | 33 | 58.45 | - | 0.88 | 7.64 |
| L14 | P5 | 33 | - | 58.45 | 0.88 | 7.64 |
| L15 | P5 | 33 | 59.33 | - | - | 7.64 |
| L16 | - | 33 | 66.09 | - | 0.88 | - |
| L17 | - | 33 | - | 66.09 | 0.88 | - |
| L18 | - | 33 | 67.00 | - | - | - |
| L19 | - | 33 | - | 67.00 | - | - |
| Sensor Number | L1 | L2 | L3 | L4 | L5 | L6 | L7 | L8 | L9 | L10 | L11 | L12 | L13 | L14 | L15 | L16 | L17 | L18 | L19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NH4+ | 32 | 47 | 22 | 0 | 16 | 0 | 48 | 54 | 50 | 52 | 56 | 26 | 50 | 52 | 20 | 24 | 22 | 14 | 13 |
| Li+ | 6 | 8 | 8 | 6 | 8 | 5 | 4 | 27 | 13 | 17 | 55 | 8 | 11 | 40 | 11 | 9 | 8 | 7 | 9 |
| Na+ | 16 | 16 | 14 | 15 | 15 | 13 | 13 | 46 | 21 | 32 | 54 | 5 | 21 | 46 | 8 | 16 | 17 | 15 | 15 |
| K+ | 24 | 33 | 17 | 8 | 9 | 8 | 34 | 41 | 36 | 45 | 47 | 11 | 38 | 43 | 11 | 10 | 9 | 9 | 10 |
| Rb+ | 28 | 32 | 7 | 5 | 6 | 0 | 37 | 43 | 37 | 54 | 48 | 6 | 50 | 45 | 9 | 14 | 11 | 9 | 12 |
| Cs+ | 43 | 40 | 17 | 0 | 0 | 0 | 49 | 44 | 37 | 59 | 52 | 7 | 50 | 48 | 11 | 36 | 37 | 9 | 6 |
| Mg2+ | 22 | 26 | 18 | 0 | 21 | 0 | 6 | 10 | 15 | 15 | 15 | 11 | 13 | 13 | 7 | 26 | 0 | 16 | 16 |
| Ca2+ | 10 | 0 | 13 | 0 | 0 | 0 | 11 | 15 | 22 | 18 | 18 | 5 | 17 | 17 | 4 | 18 | 10 | 9 | 13 |
| Sr2+ | 30 | 28 | 26 | 27 | 32 | 30 | 10 | 12 | 16 | 17 | 16 | 4 | 16 | 15 | 5 | 30 | 28 | 30 | 34 |
| Ba2+ | 21 | 18 | 9 | 0 | 2 | 0 | 12 | 15 | 23 | 21 | 16 | 9 | 20 | 16 | 9 | 14 | 19 | 14 | 7 |
| Co2+ | 24 | 21 | 14 | 0 | 19 | 0 | 9 | 12 | 27 | 18 | 30 | 9 | 12 | 15 | 12 | 9 | 13 | 13 | 14 |
| Ni2+ | 11 | 23 | 8 | 0 | 20 | 0 | 10 | 15 | 15 | 22 | 33 | 11 | 17 | 21 | 16 | 0 | 8 | 12 | 14 |
| Cu2+ | 26 | 11 | 29 | 22 | 35 | 0 | 9 | 9 | 28 | 14 | 17 | 13 | 9 | 30 | 6 | 0 | 21 | 16 | 14 |
| Zn2+ | 18 | 14 | 9 | 0 | 24 | 0 | 17 | 15 | 26 | 16 | 19 | 15 | 17 | 15 | 15 | 0 | 14 | 0 | 22 |
| Cd2+ | 15 | 10 | 10 | 0 | 19 | 0 | 12 | 16 | 24 | 16 | 18 | 9 | 15 | 17 | 11 | 12 | 14 | 16 | 16 |
| Pb2+ | 46 | 32 | 25 | 0 | 31 | 0 | 17 | 18 | 29 | 24 | 19 | 25 | 24 | 21 | 23 | 25 | 25 | 25 | 23 |
| La3+ | 4 | 3 | 2 | 0 | 0 | 2 | 0 | 0 | 8 | 0 | 6 | 7 | 1 | 0 | 0 | 4 | 3 | 0 | 3 |
| Sm3+ | 4 | 6 | 10 | 5 | 6 | 7 | 0 | 0 | 12 | 2 | 0 | 12 | 3 | 0 | 5 | 0 | 6 | 9 | 10 |
| Lu3+ | 9 | 11 | 15 | 11 | 11 | 12 | 0 | 0 | 16 | 2 | 0 | 16 | 1 | 0 | 6 | 4 | 10 | 14 | 14 |
| Sensor Number | L1 | L2 | L7 | L8 | L10 | L11 | L13 | L14 |
|---|---|---|---|---|---|---|---|---|
| NH4+/Na+ | −0.4 | 0.0 | 0.8 | −0.1 | 0.7 | 0.4 | 1.1 | 0.3 |
| Li+/Na+ | 0.5 | 0.6 | −3.3 | −1.4 | −0.7 | −0.1 | 0.6 | 0.8 |
| K+/Na+ | 1.1 | 0.7 | 2.4 | 0.0 | 1.2 | 0.4 | 1.8 | 0.6 |
| Rb+/Na+ | 1.4 | 0.7 | 1.6 | 0.1 | 1.6 | 0.3 | 2.0 | 0.6 |
| Cs+/Na+ | 1.4 | 0.3 | 1.9 | 0.2 | 2.1 | 0.5 | 2.6 | 0.7 |
| Mg2+/Ca2+ | −0.1 | −0.9 | −0.1 | −0.8 | −0.7 | −0.9 | −0.9 | −0.7 |
| Sr2+/Ca2+ | −1.5 | −2.0 | 0.8 | 0.3 | 0.3 | 0.1 | 0.4 | 0.9 |
| Ba2+/Ca2+ | −0.6 | −0.6 | 0.8 | −0.1 | 0.9 | −0.1 | 0.9 | 0.1 |
| Co2+/Cu2+ | 0.2 | −0.2 | 0.6 | −0.6 | 0.0 | 0.1 | 0.4 | −0.9 |
| Ni2+/Cu2+ | 0.1 | −0.1 | 0.3 | −0.4 | 0.1 | 0.2 | 0.1 | −0.5 |
| Zn2+/Cu2+ | 0.6 | −0.3 | −0.1 | −0.9 | −0.3 | 0.1 | −0.1 | −1.2 |
| Cd2+/Cu2+ | 1.5 | 1.2 | 0.7 | −0.3 | 0.2 | 0.2 | 0.8 | −0.7 |
| Pb2+/Cu2+ | 1.7 | 1.7 | 3.4 | 1.1 | 1.4 | 0.8 | 2.1 | 0.7 |
| Mg2+/Cu2+ | 1.1 | 0.6 | 3.9 | −0.8 | 0.2 | −0.9 | 0.7 | −1.7 |
| Ca2+/Cu2+ | 2.8 | 2.3 | 2.3 | −0.1 | 0.9 | 0.4 | 1.2 | −0.5 |
| Sr2+/Cu2+ | −0.5 | −1.0 | 3.3 | 0.2 | 0.8 | 0.2 | 1.7 | −0.6 |
| Ba2+/Cu2+ | 0.7 | 0.8 | 3.7 | −0.2 | 1.6 | 0.4 | 1.9 | −0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehabadi, M.; Yemisci, E.; Kursunlu, A.N.; Kirsanov, D. Novel Pillar[5]arenes Show High Cross-Sensitivity in PVC-Plasticized Membrane Potentiometric Sensors. Chemosensors 2022, 10, 420. https://doi.org/10.3390/chemosensors10100420
Dehabadi M, Yemisci E, Kursunlu AN, Kirsanov D. Novel Pillar[5]arenes Show High Cross-Sensitivity in PVC-Plasticized Membrane Potentiometric Sensors. Chemosensors. 2022; 10(10):420. https://doi.org/10.3390/chemosensors10100420
Chicago/Turabian StyleDehabadi, Monireh, Elif Yemisci, Ahmed Nuri Kursunlu, and Dmitry Kirsanov. 2022. "Novel Pillar[5]arenes Show High Cross-Sensitivity in PVC-Plasticized Membrane Potentiometric Sensors" Chemosensors 10, no. 10: 420. https://doi.org/10.3390/chemosensors10100420
APA StyleDehabadi, M., Yemisci, E., Kursunlu, A. N., & Kirsanov, D. (2022). Novel Pillar[5]arenes Show High Cross-Sensitivity in PVC-Plasticized Membrane Potentiometric Sensors. Chemosensors, 10(10), 420. https://doi.org/10.3390/chemosensors10100420





