Determination of Pb(II) Ions in Water by Fluorescence Spectroscopy Based on Silver Nanoclusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Pb(II) Ions Sensor (AgNCs-PMAA)
2.3. Optical Characterization
2.4. Selectivity and Interferogram in Presence of Different Metal Ions
2.5. System Calibration, Test Solutions, and Tests in Real Waters
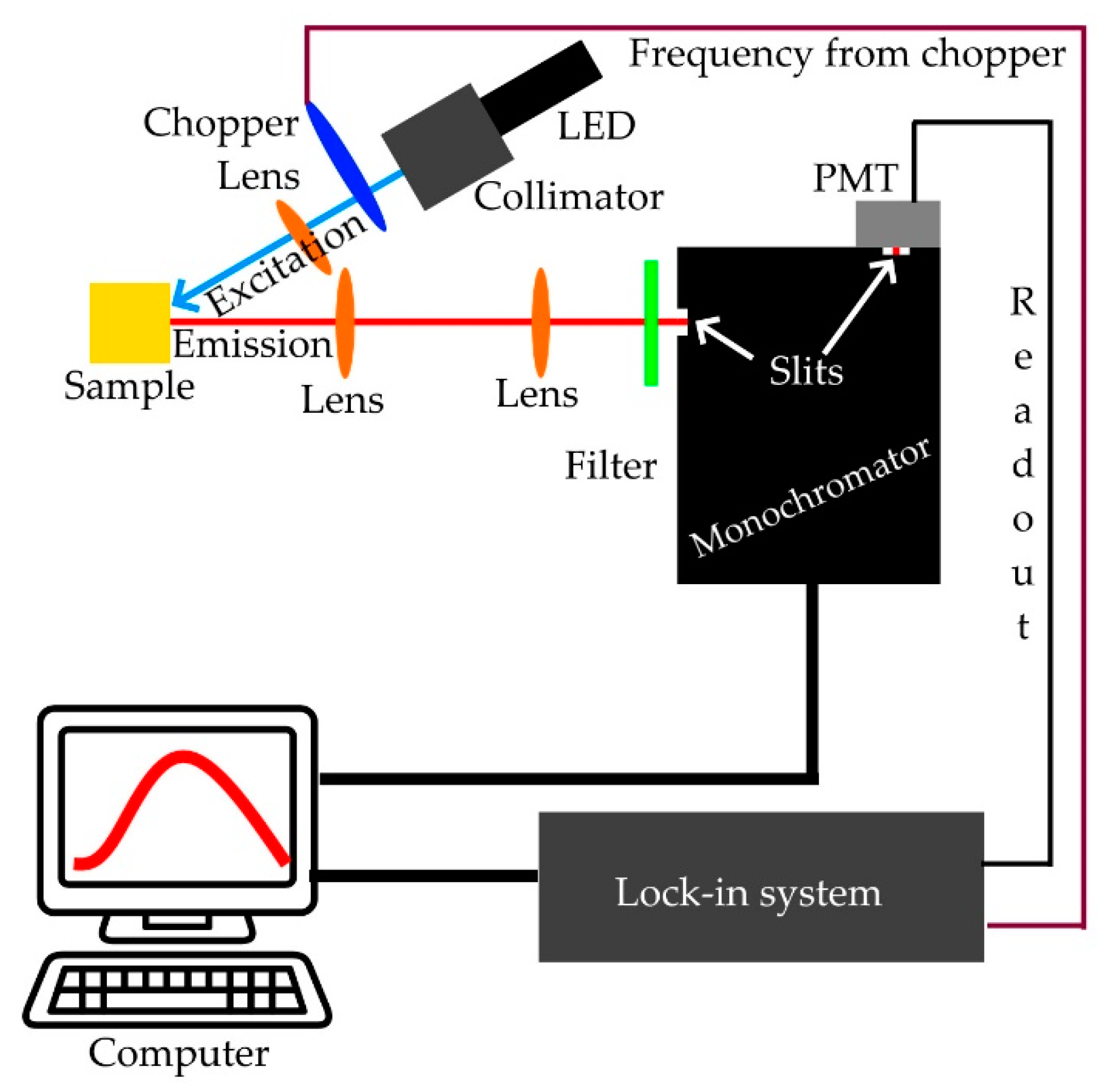
2.6. Instrumentation
3. Results and Discussion
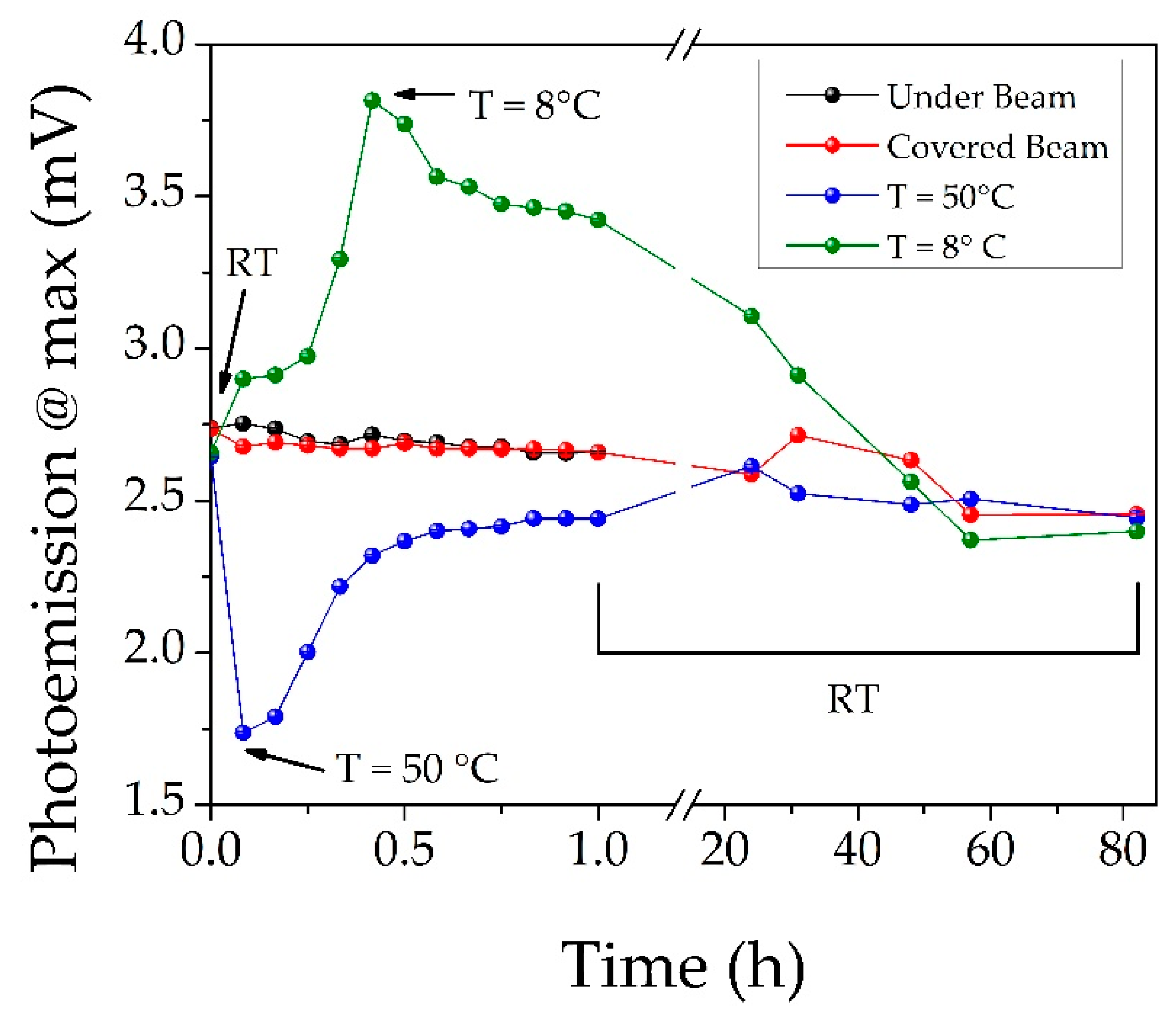
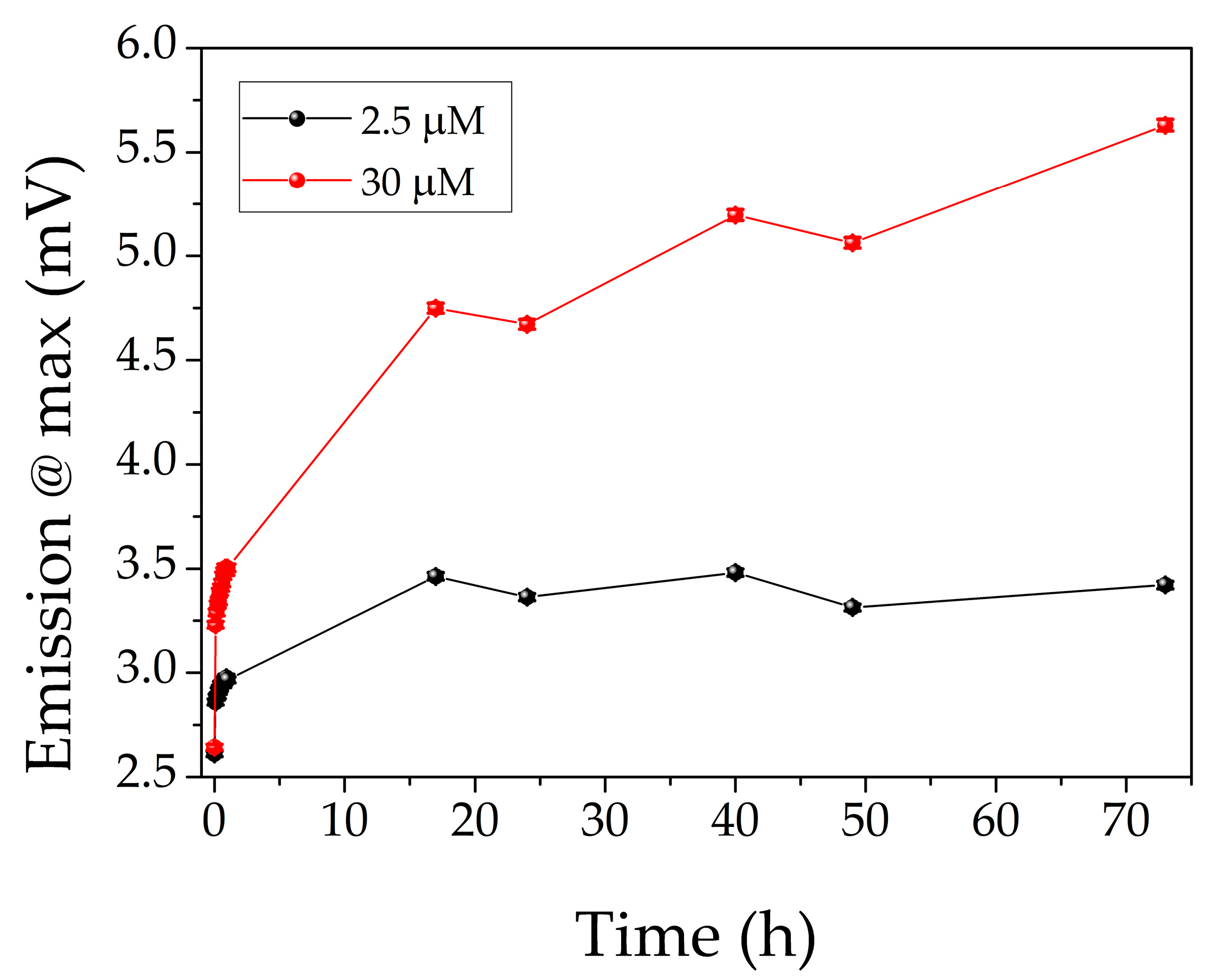
3.1. Evaluation of AgNCs Stability
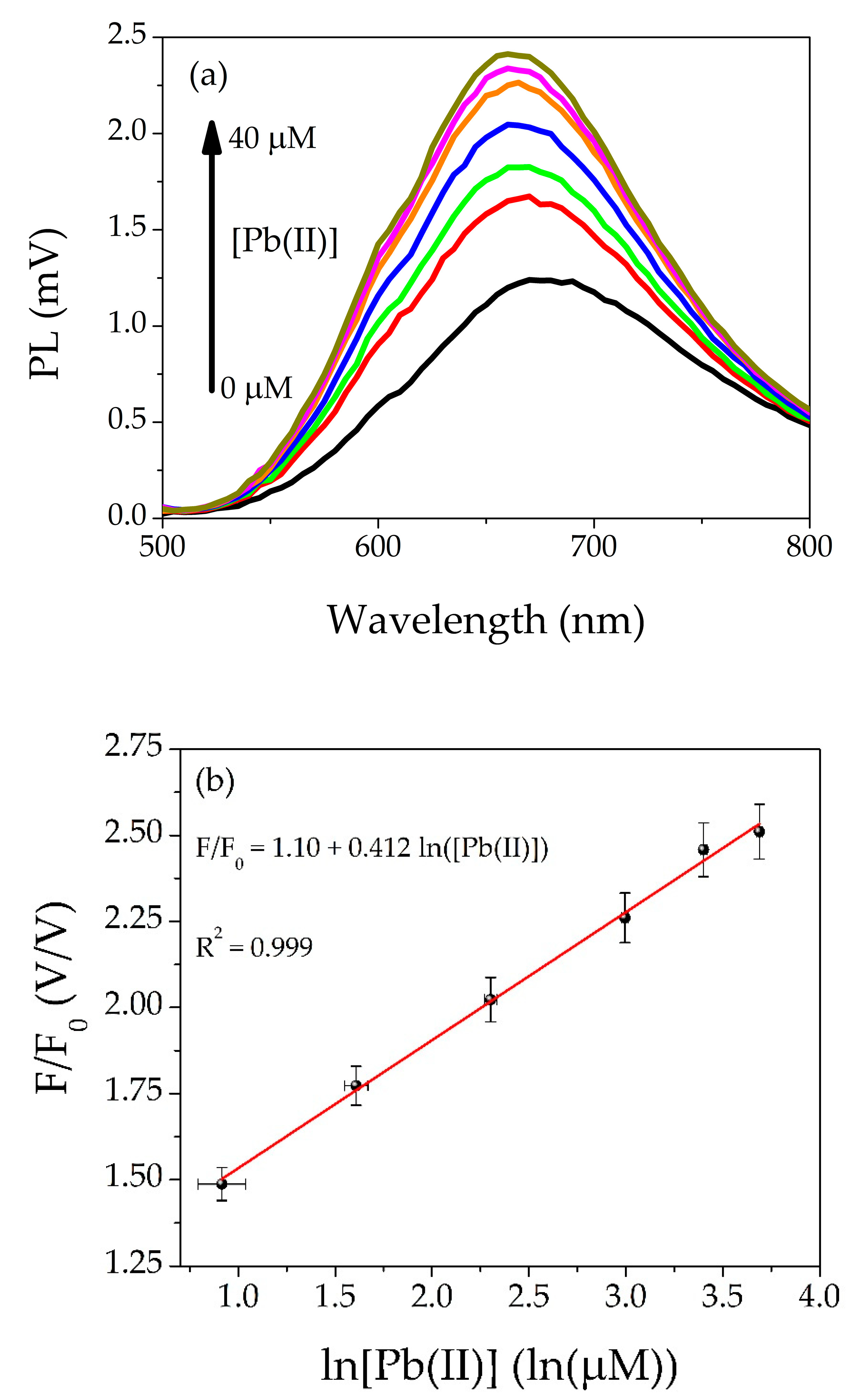
3.2. Calibration Curve
3.3. Test Solutions
3.4. Real Water Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramamurthy, A.S.; Memarian, R. Phytoremediation of Mixed Soil Contaminants. Water Air Soil Pollut. 2012, 223, 511–518. [Google Scholar] [CrossRef]
- Hansda, A.; Kumar, V.; Usmani, Z. Phytoremediation of heavy metals contaminated soil using plant growth promoting rhizobacteria (PGPR): A current perspective. Recent Res. Sci. Technol. 2014, 6, 131–134. [Google Scholar]
- Govind, P.; Madhuri, S. Heavy metals causing toxicity in humans, animals and environment. Res. J. Anim. Vet. Fish. Sci. 2014, 2, 17–23. [Google Scholar]
- Raj, D.; Maiti, S.K. Sources, bioaccumulation, health risks and remediation of potentially toxic metal(loid)s (As, Cd, Cr, Pb and Hg): An epitomised review. Environ. Monit. Assess. 2020, 192, 108–208. [Google Scholar] [CrossRef]
- Islam, A.; Zaidi, N.; Ahmad, H.; Kumar, S. Functionalized carbon nanotubes for dispersive solid-phase extraction and atomic absorption spectroscopic determination of toxic metals ions. Int. J. Environ. Sci. Technol. 2019, 16, 707–718. [Google Scholar] [CrossRef]
- Vojoudi, H.; Badiei, A.; Amiri, A.; Banaei, A.; Ziarani, G.M.; Schenk-Joß, K. Pre-concentration of Zn(II) ions from aqueous solutions using meso-porous pyridine-enrobed magnetite nanostructures. Food Chem. 2018, 257, 189–195. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Huang, A. EDTA-Functionalized Covalent Organic Framework for the Removal of Heavy-Metal Ions. ACS Appl. Mater. Interfaces 2019, 11, 32186–32191. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R. H2O2 treatment enhanced the heavy metals removal by manure biochar in aqueous solutions. Sci. Total Environ. 2018, 629, 1139–1148. [Google Scholar] [CrossRef]
- Thirumalai, M.; Kumar, S.N.; Prabhakaran, D.; Sivaraman, N.; Maheswari, M.A. Dynamically modified C18 silica monolithic column for the rapid determinations of lead, cadmium and mercury ions by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2018, 1569, 62–69. [Google Scholar] [CrossRef]
- Michalski, R. Ion Chromatography Applications in Wastewater Analysis. Separations 2018, 5, 16. [Google Scholar] [CrossRef]
- Fan, H.; Ma, X.; Zhou, S.; Huang, J.; Liu, Y.; Liu, Y. Highly efficient removal of heavy metal ions by carboxymethyl cellulose-immobilized Fe3O4 nanoparticles prepared via high-gravity technology. Carbohydr. Polym. 2019, 213, 39–49. [Google Scholar] [CrossRef]
- Xu, J.; Liu, C.; Hsu, P.C.; Zhao, J.; Wu, T.; Tang, J.; Liu, K.; Cui, Y. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat. Commun. 2019, 10, 2440. [Google Scholar] [CrossRef] [Green Version]
- Farrukh, M.A. Atomic Absorption Spectroscopy, 1st ed.; Akhyar Farrukh, M., Ed.; InTech: London, UK, 2012; ISBN 978-953-307-817-5. [Google Scholar]
- Zhou, F.; Li, C.; Zhu, H.; Li, Y. A novel method for simultaneous determination of zinc, nickel, cobalt and copper based on UV–vis spectrometry. Optik 2019, 182, 58–64. [Google Scholar] [CrossRef]
- Alorabi, A.Q.; Abdelbaset, M.; Zabin, S.A. Colorimetric Detection of Multiple Metal Ions Using Schiff Base 1-(2-Thiophenylimino)-4-(N-dimethyl)benzene. Chemosensors 2020, 8, 1. [Google Scholar] [CrossRef]
- Burratti, L.; Ciotta, E.; De Matteis, F.; Prosposito, P. Metal Nanostructures for Environmental Pollutant Detection Based on Fluorescence. Nanomaterials 2021, 11, 276. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, X.; Zou, T.; Yang, Y.; Xing, X.; Zhao, R.; Wang, Z.; Wang, Y. Citric Acid Capped CdS Quantum Dots for Fluorescence Detection of Copper Ions (II) in Aqueous Solution. Nanomaterials 2018, 9, 32. [Google Scholar] [CrossRef]
- Bi, J.; Li, T.; Ren, H.; Ling, R.; Wu, Z.; Qin, W. Capillary electrophoretic determination of heavy-metal ions using 11-mercaptoundecanoic acid and 6-mercapto-1-hexanol co-functionalized gold nanoparticle as colorimetric probe. J. Chromatogr. A 2019, 1594, 208–215. [Google Scholar] [CrossRef]
- Oluwafemi, O.S.; Anyik, J.L.; Zikalala, N.E.; Sakho, E.H.M. Biosynthesis of silver nanoparticles from water hyacinth plant leaves extract for colourimetric sensing of heavy metals. Nano-Struct. Nano-Objects 2019, 20, 100387–100391. [Google Scholar] [CrossRef]
- Xiao, N.; Dong, J.X.; Liu, S.G.; Li, N.; Fan, Y.Z.; Ju, Y.J.; Li, N.B.; Luo, H.Q. Multifunctional fluorescent sensors for independent detection of multiple metal ions based on Ag nanoclusters. Sens. Actuators B Chem. 2018, 264, 184–192. [Google Scholar] [CrossRef]
- Gao, P.; Wu, S.; Chang, X.; Liu, F.; Zhang, T.; Wang, B.; Zhang, K.-Q. Aprotinin Encapsulated Gold Nanoclusters: A Fluorescent Bioprobe with Dynamic Nuclear Targeting and Selective Detection of Trypsin and Heavy Metal. Bioconjug. Chem. 2018, 29, 4140–4148. [Google Scholar] [CrossRef] [PubMed]
- Bolli, E.; Mezzi, A.; Burratti, L.; Prosposito, P.; Casciardi, S.; Kaciulis, S. X-ray and UV photoelectron spectroscopy of Ag nanoclusters. Surf. Interface Anal. 2020, 52, 1017–1022. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, S.; Lee, J.; Mehta, A.; Kumar, S.; Kim, K.-H.; Basu, S.; Rawat, M. Highly fluorescent carbon dots derived from Mangifera indica leaves for selective detection of metal ions. Sci. Total Environ. 2020, 720, 137604–137612. [Google Scholar] [CrossRef]
- Yarur, F.; Macairan, J.-R.; Naccache, R. Ratiometric detection of heavy metal ions using fluorescent carbon dots. Environ. Sci. Nano 2019, 6, 1121–1130. [Google Scholar] [CrossRef]
- Burratti, L.; Ciotta, E.; Bolli, E.; Kaciulis, S.; Casalboni, M.; De Matteis, F.; Garzón-Manjón, A.; Scheu, C.; Pizzoferrato, R.; Prosposito, P. Fluorescence enhancement induced by the interaction of silver nanoclusters with lead ions in water. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 579, 123634–123642. [Google Scholar] [CrossRef]
- Burratti, L.; Ciotta, E.; Bolli, E.; Casalboni, M.; De Matteis, F.; Francini, R.; Casciardi, S.; Prosposito, P. Synthesis of fluorescent silver nanoclusters with potential application for heavy metal ions detection in water. AIP Conf. Proc. 2019, 2145, 020007–020012. [Google Scholar]
- Shang, L.; Dong, S. Facile preparation of water-soluble fluorescent silver nanoclusters using a polyelectrolyte template. Chem. Commun. 2008, 9, 1088–1090. [Google Scholar] [CrossRef]
- Ruiz-Pérez, L.; Pryke, A.; Sommer, M.; Battaglia, G.; Soutar, I.; Swanson, L.; Geoghegan, M. Conformation of poiy(methacrylic acid) chains in dilute aqueous solution. Macromolecules 2008, 41, 2203–2211. [Google Scholar] [CrossRef]
- de Groot, G.W.; Santonicola, M.G.; Sugihara, K.; Zambelli, T.; Reimhult, E.; Vörös, J.; Vancso, G.J. Switching Transport through Nanopores with pH-Responsive Polymer Brushes for Controlled Ion Permeability. ACS Appl. Mater. Interfaces 2013, 5, 1400–1407. [Google Scholar] [CrossRef]
- Wang, X.; Ye, X.; Zhang, G. Investigation of pH-induced conformational change and hydration of poly(methacrylic acid) by analytical ultracentrifugation. Soft Matter 2015, 11, 5381–5388. [Google Scholar] [CrossRef]
- Weber, G.; Teale, F.W.J. Determination of the absolute quantum yield of fluorescent solutions. Trans. Faraday Soc. 1957, 53, 646–651. [Google Scholar] [CrossRef]
- Casalboni, M.; De Matteis, F.; Prosposito, P.; Quatela, A.; Sarcinelli, F. Fluorescence efficiency of four infrared polymethine dyes. Chem. Phys. Lett. 2003, 373, 372–378. [Google Scholar] [CrossRef]
- Taylor, J.R. An Introduction to Error Analysis. The Study of Uncertainties in Physical Measurements, 2nd ed.; University Science Books: Sausalito, CA, USA, 1982; ISBN 9780935702750. [Google Scholar]
- WHO. Chemical aspects. In Guideline for Drinking Water, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; pp. 155–201. [Google Scholar]
- Jiang, X.; Zhang, H.; Yang, C.; Xia, J.; Liu, G.; Luo, X. A novel electrostatic drive strategy to prepare glutathione-capped gold nanoclusters embedded quaternized cellulose membranes fluorescent colorimetric sensor for Pb(II) and Hg(II) ions detection. Sensors Actuators B Chem. 2022, 368, 132046. [Google Scholar] [CrossRef]
- Li, S.; Li, G.; Shi, H.; Yang, M.; Tan, W.; Wang, H.; Yang, W. A fluorescent probe based on tryptophan-coated silver nanoclusters for copper (II) ions detection and bioimaging in cells. Microchem. J. 2022, 175, 107222. [Google Scholar] [CrossRef]
- Mao, A.; Wei, C. Cytosine-rich ssDNA-templated fluorescent silver and copper/silver nanoclusters: Optical properties and sensitive detection for mercury(II). Microchim. Acta 2019, 186, 541. [Google Scholar] [CrossRef]
- Wang, L.; Cao, H.-X.; He, Y.-S.; Pan, C.-G.; Sun, T.-K.; Zhang, X.-Y.; Wang, C.-Y.; Liang, G.-X. Facile preparation of amino-carbon dots/gold nanoclusters FRET ratiometric fluorescent probe for sensing of Pb2+/Cu2+. Sens. Actuators B Chem. 2019, 282, 78–84. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhamore, J.R.; Malek, N.I.; Murthy, Z.V.P.; Kailasa, S.K. Trypsin mediated one-pot reaction for the synthesis of red fluorescent gold nanoclusters: Sensing of multiple analytes (carbidopa, dopamine, Cu2+, Co2+ and Hg2+ ions). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 209–217. [Google Scholar] [CrossRef]
- Liu, J.; Xue, H.; Liu, Y.; Bu, T.; Jia, P.; Shui, Y.; Wang, L. Visual and fluorescent detection of mercury ions using a dual-emission ratiometric fluorescence nanomixture of carbon dots cooperating with gold nanoclusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117364. [Google Scholar] [CrossRef]
- Yonesato, K.; Ito, H.; Itakura, H.; Yokogawa, D.; Kikuchi, T.; Mizuno, N.; Yamaguchi, K.; Suzuki, K. Controlled Assembly Synthesis of Atomically Precise Ultrastable Silver Nanoclusters with Polyoxometalates. J. Am. Chem. Soc. 2019, 141, 19550–19554. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, M.; Gao, P.; Zhang, G.; Shi, L.; Yuan, M.; Shuang, S. The synthesis of high bright silver nanoclusters with aggregation-induced emission for detection of tetracycline. Sens. Actuators B Chem. 2021, 326, 129009. [Google Scholar] [CrossRef]
- Shah, P.; Nagda, R.; Jung, I.L.; Bhang, Y.J.; Jeon, S.-W.; Lee, C.S.; Do, C.; Nam, K.; Kim, Y.M.; Park, S.; et al. Noncanonical Head-to-Head Hairpin DNA Dimerization Is Essential for the Synthesis of Orange Emissive Silver Nanoclusters. ACS Nano 2020, 14, 8697–8706. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Pang, L.; Fu, S.; Qin, X.; Chen, Q.; Man, C.; Jiang, Y. A novel fluorescent platform of DNA-stabilized silver nanoclusters based on exonuclease III amplification-assisted detection of Salmonella Typhimurium. Anal. Chim. Acta 2021, 1181, 338903. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-G.; Gao, X.-M.; Liu, T.; Hu, M.-L.; Jiang, D. All-Carboxylate-Protected Superatomic Silver Nanocluster with an Unprecedented Rhombohedral Ag 8 Core. J. Am. Chem. Soc. 2020, 142, 16905–16909. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Lin, S.-W.; Wu, Y.-H.; Hsieh, Y.-Z. Combining DNA-stabilized silver nanocluster synthesis with exonuclease III amplification allows label-free detection of coralyne. Anal. Chim. Acta 2018, 1042, 86–92. [Google Scholar] [CrossRef] [PubMed]

| Metal Precursor | Ion in Solution | Metal Precursor | Ion in Solution |
|---|---|---|---|
| AsO2Na | As(III) | Hg(NO3)2 | Hg(II) |
| HAsO4Na2 | As(V) | KClO4 | K(I) |
| Ca(ClO4)2*4H2O | Ca(II) | Mg(ClO4)2 | Mg(II) |
| Cd(NO3)2*4H2O | Cd(II) | NaNO2 | Na(I) |
| CoCl2*6H2O | Co(II) | NiCl2*6H2O | Ni(II) |
| CrCl3*6H2O | Cr(III) | Pb(NO3)2 | Pb(II) |
| Cu(NO3)2*H2O | Cu(II) | Zn(NO3)2*6H2O | Zn(II) |
| FeCl3*6H2O | Fe(III) |
| Concentration of Pb(II) in Cuvette (μM) | Volume from Reference Solution (μL) |
|---|---|
| 2.5 ± 0.3 | 1.25 ± 0.14 |
| 5.0 ± 0.3 | 2.50 ± 0.14 |
| 10.0 ± 0.4 | 5.00 ± 0.14 |
| 20.0 ± 0.4 | 10.00 ± 0.14 |
| 30.0 ± 0.5 | 15.00 ± 0.20 |
| 40.0 ± 0.6 | 20.00 ± 0.20 |
| Sample | By Synthesis (mM) | Fluorescence Method (mM) | ICP-OES (mM) | RSD |
|---|---|---|---|---|
| A | 13.7 ± 0.1 | 12.7 ± 2.7 | 14.6 ± 0.3 | 21% |
| B | 8.27 ± 0.08 | 7.4 ± 1.4 | 8.670 ± 0.005 | 19% |
| C | 3.72 ± 0.06 | 3.9 ± 0.6 | 3.890 ± 0.005 | 15% |
| D | 0.57 ± 0.05 | 0.50 ± 0.05 | 0.535 ± 0.002 | 10% |
| Sample | By Synthesis (mM) | Fluorescence Method (mM) | RSD |
|---|---|---|---|
| W1-A | 13.7 ± 0.1 | 11.2 ± 2.7 | 24% |
| W1-B | 8.27 ± 0.08 | 7.0 ± 1.5 | 21% |
| W1-C | 3.72 ± 0.06 | 3.0 ± 0.6 | 20% |
| W1-D | 0.57 ± 0.05 | 0.52 ± 0.07 | 13% |
| W2-A | 13.7 ± 0.1 | 13.0 ± 3.0 | 23% |
| W2-B | 8.27 ± 0.08 | 8.0 ± 1.7 | 21% |
| W2-C | 3.72 ± 0.06 | 3.0 ± 0.6 | 20% |
| W2-D | 0.57 ± 0.05 | 0.50 ± 0.07 | 14% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burratti, L.; Maranges, V.; Sisani, M.; Naryyev, E.; De Matteis, F.; Francini, R.; Prosposito, P. Determination of Pb(II) Ions in Water by Fluorescence Spectroscopy Based on Silver Nanoclusters. Chemosensors 2022, 10, 385. https://doi.org/10.3390/chemosensors10100385
Burratti L, Maranges V, Sisani M, Naryyev E, De Matteis F, Francini R, Prosposito P. Determination of Pb(II) Ions in Water by Fluorescence Spectroscopy Based on Silver Nanoclusters. Chemosensors. 2022; 10(10):385. https://doi.org/10.3390/chemosensors10100385
Chicago/Turabian StyleBurratti, Luca, Valentin Maranges, Michele Sisani, Eziz Naryyev, Fabio De Matteis, Roberto Francini, and Paolo Prosposito. 2022. "Determination of Pb(II) Ions in Water by Fluorescence Spectroscopy Based on Silver Nanoclusters" Chemosensors 10, no. 10: 385. https://doi.org/10.3390/chemosensors10100385
APA StyleBurratti, L., Maranges, V., Sisani, M., Naryyev, E., De Matteis, F., Francini, R., & Prosposito, P. (2022). Determination of Pb(II) Ions in Water by Fluorescence Spectroscopy Based on Silver Nanoclusters. Chemosensors, 10(10), 385. https://doi.org/10.3390/chemosensors10100385








