Retrospective Analysis of Clinicopathological Features and Familial Cancer History of Synchronous Bilateral Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindor, N.M.; Johnson, K.J.; Harvey, H.; Shane Pankratz, V.; Domchek, S.M.; Hunt, K.; Wilson, M.; Cathie Smith, M.; Couch, F. Predicting BRCA1 and BRCA2 gene mutation carriers: Comparison of PENN II model to previous study. Fam. Cancer 2010, 9, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzola, E.; Blackford, A.; Parmigiani, G.; Biswas, S. Recent Enhancements to the Genetic Risk Prediction Model BRCAPRO. Cancer Inform. 2015, 14, 147–157. [Google Scholar] [CrossRef] [Green Version]
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (accessed on 4 December 2019).
- Chen, Y.; Thompson, W.; Semenciw, R.; Mao, Y. Epidemiology of contralateral breast cancer. Cancer Epidemiol. Biomark. Prev. 1999, 8, 855–861. [Google Scholar]
- Narod, S.A. Bilateral breast cancers. Nat. Rev. Clin. Oncol. 2014, 11, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Seong, M.W.; Park, S.K.; Lee, J.W.; Lee, J.; Kim, L.S.; Lee, J.E.; Kim, S.Y.; Jeong, J.; Han, S.A.; et al. The prevalence and spectrum of BRCA1 and BRCA2 mutations in Korean population: Recent update of the Korean Hereditary Breast Cancer (KOHBRA) study. Breast Cancer Res. Treat. 2015, 151, 157–168. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Q.; Lang, G.T.; Cao, A.Y.; Shao, Z.M. Concordance of Hormone Receptor Status and BRCA1/2 Mutation Among Women With Synchronous Bilateral Breast Cancer. Front. Oncol. 2020, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Rogozinska-Szczepka, J.; Utracka-Hutka, B.; Grzybowska, E.; Maka, B.; Nowicka, E.; Smok-Ragankiewicz, A.; Zientek, H.; Steffen, J.; Wojciechowska-Lacka, A. BRCA1 and BRCA2 mutations as prognostic factors in bilateral breast cancer patients. Ann. Oncol. 2004, 15, 1373–1376. [Google Scholar] [CrossRef]
- Hartman, M.; Czene, K.; Reilly, M.; Adolfsson, J.; Bergh, J.; Adami, H.O.; Dickman, P.W.; Hall, P. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4210–4216. [Google Scholar] [CrossRef]
- Kuo, W.H.; Yen, A.M.; Lee, P.H.; Chen, K.M.; Wang, J.; Chang, K.J.; Chen, T.H.; Tsau, H.S. Cumulative survival in early-onset unilateral and bilateral breast cancer: An analysis of 1907 Taiwanese women. Br. J. Cancer 2009, 100, 563–570. [Google Scholar] [CrossRef]
- Jobsen, J.J.; van der Palen, J.; Ong, F.; Riemersma, S.; Struikmans, H. Bilateral breast cancer, synchronous and metachronous; differences and outcome. Breast Cancer Res. Treat. 2015, 153, 277–283. [Google Scholar] [CrossRef]
- McCart Reed, A.E.; Kutasovic, J.R.; Lakhani, S.R.; Simpson, P.T. Invasive lobular carcinoma of the breast: Morphology, biomarkers and ’omics. Breast Cancer Res. 2015, 17, 12. [Google Scholar] [CrossRef]
- Health Promotion Administration, Ministry of Health and Welfare. Available online: https://www.hpa.gov.tw/Home/Index.aspx (accessed on 24 July 2021).
- Lewis, T.R.; Casey, J.; Buerk, C.A.; Cammack, K.V. Incidence of lobular carcinoma in bilateral breast cancer. Am. J. Surg. 1982, 144, 635–638. [Google Scholar] [CrossRef]
- Rahman, N. Realizing the promise of cancer predisposition genes. Nature 2014, 505, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Al Ajmi, K.; Lophatananon, A.; Mekli, K.; Ollier, W.; Muir, K.R. Association of Nongenetic Factors With Breast Cancer Risk in Genetically Predisposed Groups of Women in the UK Biobank Cohort. JAMA Netw. Open 2020, 3, e203760. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, J.; Ronneberg, J.A.; Tost, J.; Kristensen, V. The epigenetics of breast cancer. Mol. Oncol. 2010, 4, 242–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; You, R.; Wang, X.; Liu, C.; Xu, Z.; Zhou, J.; Yu, B.; Xu, T.; Cai, H.; Zou, Q. Effectiveness of Prophylactic Surgeries in BRCA1 or BRCA2 Mutation Carriers: A Meta-analysis and Systematic Review. Clin. Cancer Res. 2016, 22, 3971–3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleje, G.U.; Eke, A.C.; Ezebialu, I.U.; Ikechebelu, J.I.; Ugwu, E.O.; Okonkwo, O.O. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst. Rev. 2018, 8, CD012464. [Google Scholar] [CrossRef]
- King, M.C.; Marks, J.H.; Mandell, J.B. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.H.; Lin, P.H.; Huang, A.C.; Chien, Y.H.; Liu, T.P.; Lu, Y.S.; Bai, L.Y.; Sargeant, A.M.; Lin, C.H.; Cheng, A.L.; et al. Multimodel assessment of BRCA1 mutations in Taiwanese (ethnic Chinese) women with early-onset, bilateral or familial breast cancer. J. Hum. Genet. 2012, 57, 130–138. [Google Scholar] [CrossRef]
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Barcenas, C.H.; Valero, V.; et al. Neoadjuvant Talazoparib for Patients With Operable Breast Cancer With a Germline BRCA Pathogenic Variant. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 388–394. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmana, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Goncalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer. Version 5. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 1 July 2021).

| Characteristic | Median (Range) or n (%) | |
|---|---|---|
| Age at time of diagnosis | Median, years | 52 (31–83) |
| <50 years old | 134 (43.6%) | |
| ≥50 years old | 173 (56.3%) | |
| Age of menarche | Median, years | 14 (11–19) |
| Menopausal Status | Premenopausal | 103 (33.6%) |
| Postmenopausal | 144 (46.9%) | |
| Perimenopausal/unknown | 60 (19.5%) | |
| Childbearing | Parous | 184 (60.0%) |
| Nulliparous | 44 (14.3%) | |
| Unknown | 79 (25.7%) | |
| Initial symptomatic breast | Right | 68 (22.1%) |
| Left | 54 (17.4%) | |
| Bilateral | 44 (14.3%) | |
| Asymptomatic | 88 (28.6%) | |
| Unknown | 53 (17.4%) | |
| Pathological diagnosis | Bilaterally concordant | 177 (57.7%) |
| IDC | 42 (46.2%) | |
| ILC | 11 (3.6%) | |
| DCIS | 23 (7.5%) | |
| Mucinous | 1 (0.3%) | |
| Bilaterally disconcordant | 129 (42.0%) | |
| IDC-DCIS | 95 (30.9%) | |
| ILC-DCIS | 6 (2.0%) | |
| IDC-ILC | 13 (4.2%) | |
| DCIS-LCIS | 2 (0.7%) | |
| IDC-LCIS | 1 (0.3%) | |
| IDC-other 1 | 9 (2.9%) | |
| ILC-other 2 | 3 (1.0%) | |
| Unknown | 1 (0.3%) | |
| Tumor grade | Bilateral grade 3 | 29 (9.4%) |
| Unilateral grade 3 | 56 (18.2%) | |
| Bilateral grade 1/2 | 183 (59.6%) | |
| Unknown/Other | 39 (12.7%) | |
| Tumor stage 3 | Stage 0 | 19 (6.2%) |
| Stage 1 | 81 (26.4%) | |
| Stage 2 | 100 (32.6%) | |
| Stage 3 | 69 (22.5%) | |
| Stage 4 | 37 (12.1%) | |
| Unknown | 1 (0.3%) | |
| Axillary lymph node metastasis | Bilateral | 44 (14.3%) |
| Unilateral | 113 (36.8%) | |
| None | 150 (48.9%) |
| Right Breast | |||||
|---|---|---|---|---|---|
| Mastectomy | BCS | Non-Surgical | Total | ||
| Left Breast | Mastectomy | 159 (51.8%) | 19 (6.1%) | 1 (0.3%) | 179 (58.3%) |
| BCS | 24 (7.8%) | 71 (23.1%) | 0 (0%) | 95 (31.0%) | |
| Non-Surgical | 1 (0.3%) | 0 (0%) | 32 (10.4%) | 33 (10.7%) | |
| Total | 184 (60.0%) | 90 (29.3%) | 33 (11.0%) | 307 (100%) | |
| Characteristic | Description | n (%) |
|---|---|---|
| IHC by the number of the affected breasts | Unknown | 75 (12.2%) |
| IHC available | 539 (87.8%) | |
| HR+ HER2− | 379 (70.3%) 1 | |
| HR+ HER2+ | 66 (12.2%) 1 | |
| HR− HER2+ | 42 (7.8%) 1 | |
| HR− HER2− | 52 (9.6%) 1 | |
| Total | 614 (100%) | |
| Bilaterally concordant expression | ||
| IHC by the number of the affected patients | HR+ HER2− | 143 (82.2%) 2 |
| HR+ HER2+ | 13 (7.5%) 2 | |
| HR− HER2+ | 7 (4.0%) 2 | |
| HR− HER2− | 11 (6.3%) 2 | |
| Total concordant cases | 174 (56.7%) | |
| Bilaterally disconcordant expression | ||
| HR+ HER2−/HR+ HER2+ | 24 (34.3%) 3 | |
| HR+ HER2−/HR− HER2+ | 15 (21.4%) 3 | |
| HR+ HER2−/HR− HER2− | 20 (28.6%) 3 | |
| HR+ HER2+/HR− HER2+ | 3 (4.3%) 3 | |
| HR+ HER2+/HR− HER2− | 5 (7.1%) 3 | |
| HR− HER2+/HR− HER2− | 3 (4.3%) 3 | |
| Total discordant cases | 70 (22.8%) | |
| Unknown4 | 63 (20.5%) |
| Cancer History | n (%) | |
|---|---|---|
| Personal | Known | 302 (98.3%) |
| With cancer history | 13 (4.2%) | |
| Without cancer history | 289 (94.1%) | |
| Unknown | 5 (1.6%) | |
| Familial | Known | 285 (92.8%) |
| With cancer history | 70 (22.8%) | |
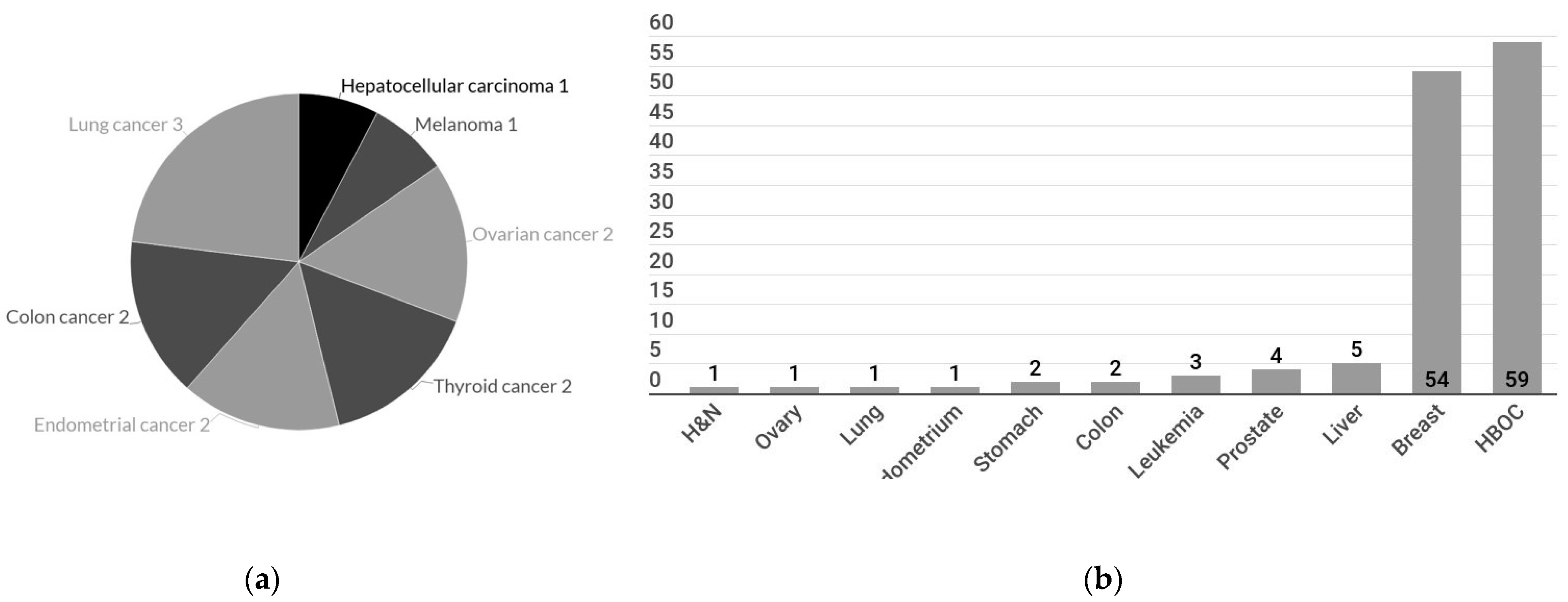
| Breast, ovary, or prostate cancer | 59 (19.2%) | |
| Other cancer types | 11 (3.5%) | |
| Without cancer history | 215 (70.0%) | |
| Unknown | 22 (7.2%) |
| Survival | Stage 1 | Event (%) | Mean Survival (Months, 95% CI) | 5-Year Survival (%) | p-Value |
|---|---|---|---|---|---|
| Disease-free survival | Stage 0 | 3/19 (15.7%) | 150.809 (109.560–192.057) | 85.3% | 0.124 |
| Stage 1 | 8/81 (9.9%) | 208.520 (168.813–248.228) | 88.4% | ||
| Stage 2 | 16/100 (16%) | 221.792 (190.726–252.858) | 85.6% | ||
| Stage 3 | 17/51 (33.3%) | 175.703 (148.917–202.489) | 71.8% | ||
| Overall Survival | Stage 0 | 1/19 (5.2%) | 235.539 (235.529–235.529) | 100% | <0.001 |
| Stage 1 | 3/81 (3.7%) | 232.416 (194.108–270.724) | 97.7% | ||
| Stage 2 | 5/100 (5%) | 263.129 (242.890–283.368) | 97.1% | ||
| Stage 3 | 10/69 (14.5%) | 199.920 (176.751–223.643) | 81.2% | ||
| Stage 4 | 10/38 (26.3%) | 121.101 (86.273–155.929) | 55.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.-L.; Liu, Y.-L.; Hsu, Y.-Y.; Kuo, W.-L. Retrospective Analysis of Clinicopathological Features and Familial Cancer History of Synchronous Bilateral Breast Cancer. Healthcare 2021, 9, 1203. https://doi.org/10.3390/healthcare9091203
Huang K-L, Liu Y-L, Hsu Y-Y, Kuo W-L. Retrospective Analysis of Clinicopathological Features and Familial Cancer History of Synchronous Bilateral Breast Cancer. Healthcare. 2021; 9(9):1203. https://doi.org/10.3390/healthcare9091203
Chicago/Turabian StyleHuang, Kai-Ling, Yu-Ling Liu, Ya-Ying Hsu, and Wen-Ling Kuo. 2021. "Retrospective Analysis of Clinicopathological Features and Familial Cancer History of Synchronous Bilateral Breast Cancer" Healthcare 9, no. 9: 1203. https://doi.org/10.3390/healthcare9091203







