Validity of Pneumonia Severity Assessment Scores in Africa and South Asia: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Sources
2.2. Study Selection
2.2.1. Eligibility Criteria
2.2.2. Screening
2.3. Data Extraction and Quality Assessment
2.4. Data Analysis
3. Results
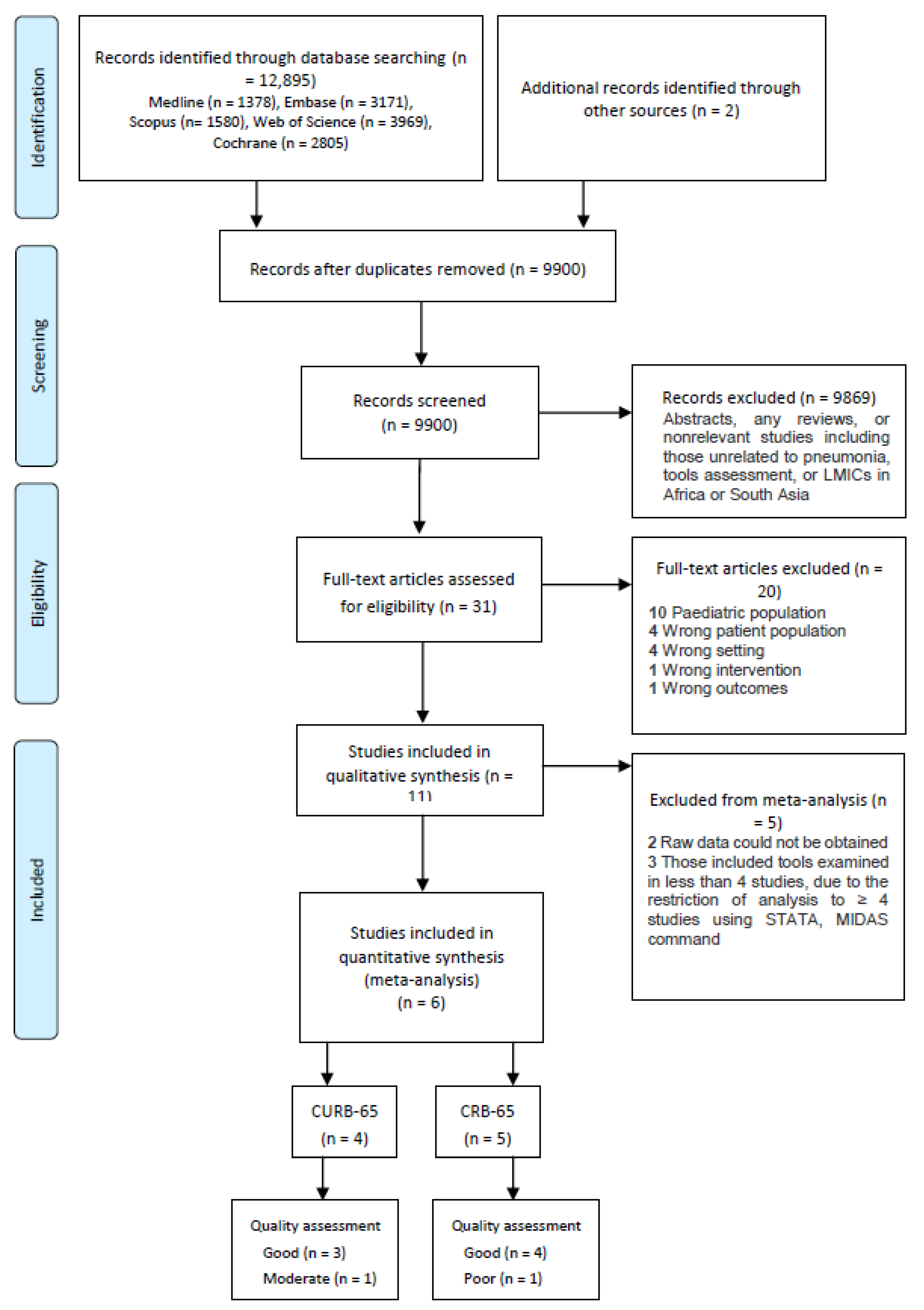
3.1. Search Results
3.2. Study Characteristics
3.3. Methodological Quality
3.4. Study Outcome
3.5. Analysis of the Outcome
3.5.1. Association between CURB-65/CRB-65 and Mortality
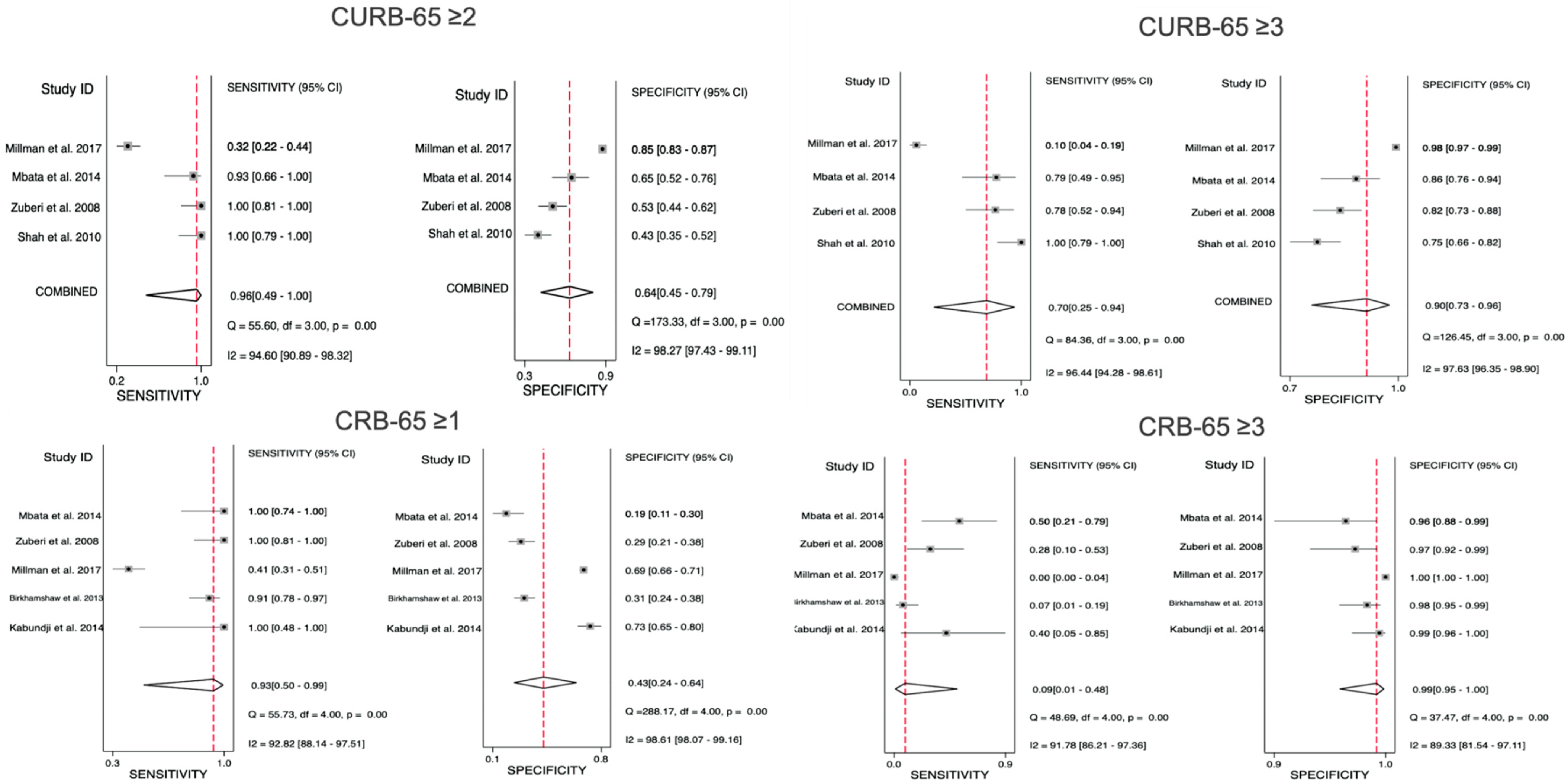
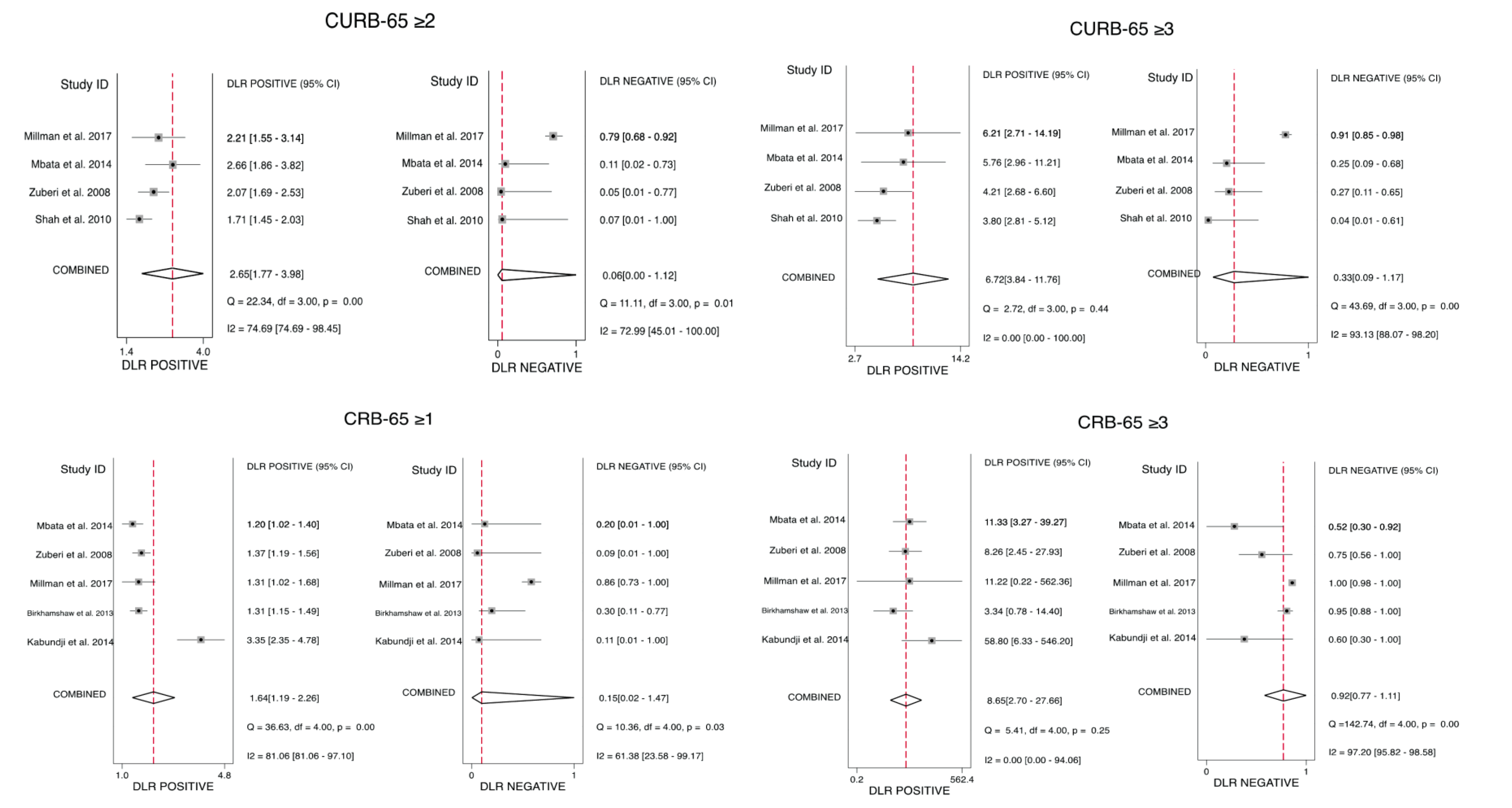
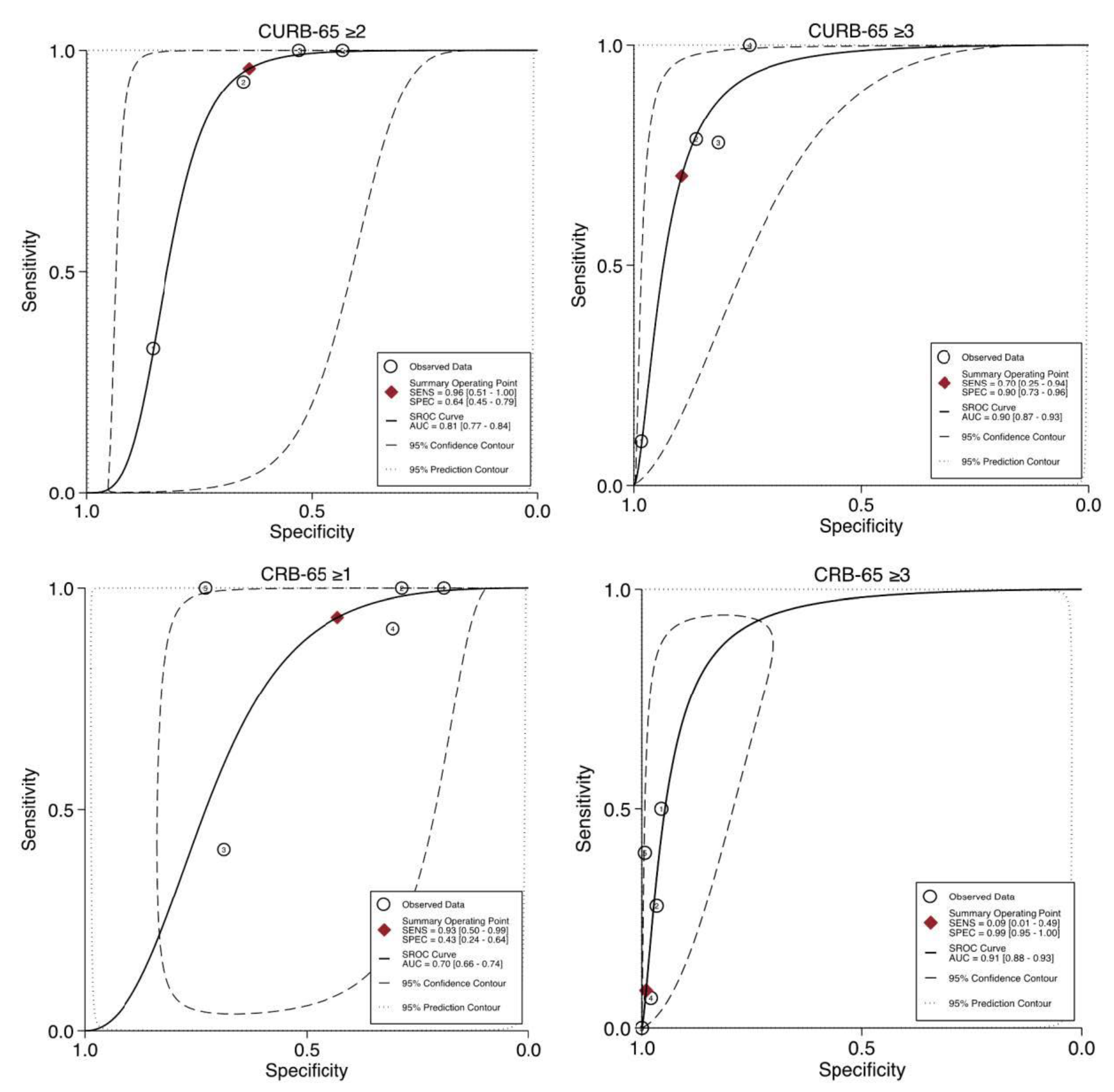
3.5.2. CURB-65 Predictive Performance for Mortality
3.5.3. CRB-65 Predictive Performance for Mortality
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Swartz, S.; Fullman, N.; Mosser, J.; Thompson, R.L.; Reiner, R.C.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1133–1161. [Google Scholar] [CrossRef] [Green Version]
- Zar, H.J.; Madhi, S.A.; Aston, S.J.; Gordon, S.B. Pneumonia in low and middle income countries: Progress and challenges. Thorax 2013, 68, 1052–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.M.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [Green Version]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aston, S.J. Pneumonia in the developing world: Characteristic features and approach to management. Respirology 2017, 22, 1276–1287. [Google Scholar] [CrossRef] [Green Version]
- Tornheim, J.A.; Manya, A.S.; Oyando, N.; Kabaka, S.; Breiman, R.F.; Feikin, D.R. The epidemiology of hospitalized pneumonia in rural Kenya: The potential of surveillance data in setting public health priorities. Int. J. Infect. Dis. 2007, 11, 536–543. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, J.D.; Singanayagam, A.; Akram, A.; Mandal, P.; Short, P.M.; Choudhury, G.; Wood, V.; Hill, A.T. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 2010, 65, 878–883. [Google Scholar] [CrossRef] [Green Version]
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A prediction rule to identify low- risk patients with community- acquired pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef]
- Lim, W.S.; Van Der Eerden, M.M.; Laing, R.; Boersma, W.G.; Karalus, N.; Town, G.; Lewis, S.; Macfarlane, J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef] [Green Version]
- National Institute for Health Care Excellence. Pneumonia (Community-Acquired): Antimicrobial Prescribing: NICE Guideline [NG138]. 2019. Available online: https://www.nice.org.uk/guidance/ng138 (accessed on 26 July 2020).
- Ogunleye, O.O.; Basu, D.; Mueller, D.; Sneddon, J.; Seaton, R.A.; Yinka-Ogunleye, A.F.; Wamboga, J.; Miljković, N.; Mwita, J.C.; Rwegerera, G.M.; et al. Response to the Novel Corona Virus (COVID-19) Pandemic Across Africa: Successes, Challenges, and Implications for the Future. Front. Pharmacol. 2020, 11, 1205. [Google Scholar] [CrossRef]
- World Health Organization. More than One in Three Low- and Middle-Income Countries Face Both Extremes of Malnutrition; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/news/item/16-12-2019-more-than-one-in-three-low--and-middle-income-countries-face-both-extremes-of-malnutrition (accessed on 22 December 2020).
- UNAIDS. UNAIDS Data 2020. Available online: https://www.unaids.org/en/resources/documents/2020/unaids-data (accessed on 22 December 2020).
- World Health Organization. Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 22 December 2020).
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges; WHO: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/337660 (accessed on 22 December 2020).
- Koss, C.A.; Jarlsberg, L.G.; Boon, S.D.; Cattamanchi, A.; Davis, J.L.; Worodria, W.; Ayakaka, I.; Sanyu, I.; Huang, L.; International HIV-associated Opportunistic Pneumonias (IHOP) Study. A clinical predictor score for 30-day mortality among HIV-infected adults hospitalized with pneumonia in Uganda. PLoS ONE 2015, 10, e0126591. [Google Scholar] [CrossRef] [Green Version]
- Buss, I.M.; Birkhamshaw, E.; Innes, M.A.; Magadoro, I.; Waitt, P.I.; Rylance, J. Validating a novel index (SWAT-Bp) to predictmortality risk of community-acquired pneumonia in Malawi. Malawi Med. J. 2018, 30, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Birkhamshaw, E.; Waitt, C.J.; Innes, M.; Waitt, P.I. Severity assessment of lower Respiratory tract infection in malawi: Derivation of a novel index (SWAT-Bp) which outperforms CRB-65. PLoS ONE 2013, 8, e82178. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The World Bank. World Bank Country and Lending Groups: Country Classification. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 26 July 2020).
- Godman, B.; Egwuenu, A.; Haque, M.; Malande, O.; Schellack, N.; Kumar, S.; Saleem, Z.; Sneddon, J.; Hoxha, I.; Islam, S.; et al. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life 2021, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Methods Prognosis Group Prognosis Tools. Available online: https://methods.cochrane.org/prognosis/tools (accessed on 26 July 2020).
- Marti, C.; Garin, N.; Grosgurin, O.; Poncet, A.; Combescure, C.; Carballo, S.; Perrier, A. Prediction of severe community-acquired pneumonia: A systematic review and meta-analysis. Crit. Care 2012, 16, R141. [Google Scholar] [CrossRef] [Green Version]
- Dwamena, B. MIDAS: Stata Module for Meta-Analytical Integration of Diagnostic Test Accuracy Studies. Boston College Department of Economics. Statistical Software Components. 2007. Available online: https://ideas.repec.org/c/boc/bocode/s456880.html (accessed on 22 December 2020).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- Kabundji, D.M.; Musekiwa, A.; Mukansi, M.; Feldman, C. Determining need for hospitalisation: Evaluation of the utility of the CRB-65 score in patients with community-acquired pneumonia presenting to an emergency department. S. Afr. Med. J. 2014, 104, 769–772. [Google Scholar] [CrossRef] [Green Version]
- Mbata, G.C.; Chukwuka, C.J.; Onyedum, C.C.; Onwubere, B.J.C.; Aguwa, E.N. Comparison of two predictive rules for assessing severity of Community acquired pneumonia. Afr. J. Respir. Med. 2014, 10, 10–14. [Google Scholar]
- Shah, B.A.; Ahmed, W.; Dhobi, G.N.; Shah, N.N.; Khursheed, S.Q.; Haq, I. Validity of pneumonia severity index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J. Chest Dis. Allied Sci. 2010, 52, 9–17. [Google Scholar]
- Zuberi, F.F.; Khan, J.A. Prospective comparison of prediction rules of mortality risk for CAP in a developing country. Int. J. Tuberc. Lung Dis. 2008, 12, 447–452. [Google Scholar] [PubMed]
- Millman, A.J.; Greenbaum, A.; Walaza, S.; Cohen, A.L.; Groome, M.J.; Reed, C.; McMorrow, M.; Tempia, S.; Venter, M.; Treurnicht, F.K.; et al. Development of a respiratory severity score for hospitalized adults in a high HIV-prevalence setting-South Africa, 2010–2011. BMC Pulm. Med. 2017, 17, 28. [Google Scholar] [CrossRef] [Green Version]
- Aston, S.J.; Ho, A.; Jary, H.; Huwa, J.; Mitchell, T.; Ibitoye, S.; Greenwood, S.; Joekes, E.; Daire, A.; Mallewa, J.; et al. Etiology and Risk Factors for Mortality in an Adult Community-acquired Pneumonia Cohort in Malawi. Am. J. Respir. Crit. Care Med. 2019, 200, 359–369. [Google Scholar] [CrossRef]
- Abd-El-Gawad, W.M.; Adly, N.N.; Salem, H.M. Diagnostic accuracy of activities of daily living in prediction of community-acquired pneumonia outcomes in elderly patients admitted to intensive care units. J. Clin. Gerontol. Geriatr. 2013, 4, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Rajarajan, J.; Chellappa, D. the Clinical Profile and Severity of Community Acquired Pneumonia in Special Reference with Pneumonia Severity Index. J. Evol. Med. Dent. Sci. 2017, 6, 6480–6485. [Google Scholar] [CrossRef]
- Loke, Y.K.; Kwok, C.S.; Niruban, A.; Myint, P.K. Value of severity scales in predicting mortality from community-acquired pneumonia: Systematic review and meta-analysis. Thorax 2010, 65, 884–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [Green Version]
- Ebell, M.H.; Walsh, M.E.; Fahey, T.; Kearney, M.; Marchello, C. Meta-analysis of Calibration, Discrimination, and Stratum-Specific Likelihood Ratios for the CRB-65 Score. J. Gen. Intern. Med. 2019, 34, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, J.J.; Altman, D.G. Diagnostic tests 4: Likelihood ratios. BMJ 2004, 329, 168–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinnes, J.; Deeks, J.; Kirby, J.; Roderick, P. A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol. Assess 2005, 9, 1–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Author. (Year) | Country | Study Settings | Study Design | Age in Years | Male n (%) | Sample Size | Assessed Score(s) | Outcome(s) | Mortality Definition | Mortality Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abd-El-Gawad (2013) [35] | Egypt | Ain Shams University Hospitals | Prospective cohort | 69.9 (±11.4) | 42 (60) | 65 | CURB-65, SCAP and ADL | Mortality and MV | 30-day mortality | 40 |
| Aston (2019) [34] | Malawi | Queen Elizabeth Central Hospital | Prospective observational | 34.7 (29.4–41.9) a | 285 (62.1) | 459 | CURB-65, CRB-65, SMRT-CO, SWAT-Bp and Modified IDSA/ATS | Mortality | 30-day mortality | 14.6 b |
| Birkhamshaw (2013) [19] | Malawi | Medical admission ward of Queen Elizabeth Central Hospital | Retrospective | 37 (29–48) a | 116 (48.3) | 240 | SWAT-Bp and CRB-65 | Mortality | In-hospital mortality | 18.3 |
| Buss (2018) [18] | Malawi | Medical admission ward of Queen Elizabeth Central Hospital | Prospective cohort | 35 (16–79) | 90 (41.7) | 216 | SWAT-Bp | Mortality | In-hospital mortality | 12.5 |
| Kabundji (2014) [29] | South Africa | ED at Helen Joseph Hospital | Prospective observational | 36.5 (20–87) | 73 (48.0) | 152 | CRB-65 | Mortality, hospital admission and time to clinical stability | During hospitalisation or 2 weeks after ED visit | 3.3 |
| Koss (2015) [17] | Uganda | Mulago Hospital | Prospective cohort | Mean: 34 | 389 (46.6) | 835 | Koss et al. new score | Mortality | 30-day mortality | 18.2 |
| Mbata (2014) [30] | Nigeria | The Accident and Emergency, medical outpatients and medical wards of the University of Nigeria Teaching Hospital | Prospective observational | 56 (±18) | 39 (48.8) | 80 | CURB-65 and CRB-65 | Mortality and ICU admission | 30-day mortality | 15 |
| Millman (2017) [33] | South Africa | Tshepong Hospital, Chris Hani Baragwanath Academic Hospital, and Selby Hospital | Retrospective chart review | NR | 2780 (38.6) | 1356 | CURB-65, CRB-65, CTA, CURB-45 and ACHU | Mortality | In-hospital mortality | 7.4 |
| Rajarajan (2017) [36] | India | A tertiary care hospital | Prospective observational | 43.38 ± 16.43 | 29 (58) | 50 | PSI | Mortality | In-hospital or within 30 days of discharge | 2 |
| Shah (2010) [31] | India | Out- and in-patient departments of Sher-i-Kashmir Institute of Medical Sciences | Prospective study | 60.8 (±13.6) | 89 (59.3) | 150 | CURB-65 and PSI | Mortality and ICU admission | In-hospital or within 30 days of discharge | 10.7 |
| Zuberi (2008) [32] | Pakistan | Aga Khan University Hospital, | Longitudinal observational cohort | 60.4 (±18.5) | 65 (47.7) | 137 | CURB-65 and CRB-65 | Mortality | 30-day mortality | 13.1 |
| High-Risk Cut-Offs | Intermediate-Risk Cut-Offs | |||
|---|---|---|---|---|
| CURB-65 ≥ 3 | CRB-65 ≥ 3 | CURB-65 ≥ 2 | CRB-65 ≥ 1 | |
| Pooled Estimate | Summary Statistic | Summary Statistic | Summary Statistic | Summary Statistic |
| Sensitivity (95% CI) | 0.70 (0.25–0.94) | 0.09 (0.01–0.48) | 0.96 (0.49–1.00) | 0.93 (0.50–0.99) |
| Specificity (95% CI) | 0.90 (0.73–0.96) | 0.99 (0.95–1.00) | 0.64 (0.45–0.79) | 0.43 (0.24–0.64) |
| PLR (95% CI) | 6.72 (3.84–11.76) | 8.65 (2.70–27.66) | 2.65 (1.77–3.98) | 1.64 (1.19–2.26) |
| NLR (95% CI) | 0.33 (0.09–1.17) | 0.92 (0.77–1.11) | 0.06 (0.00–1.12) | 0.15 (0.02–1.47) |
| DOR (95% CI) | 20.19 (7.32–55.63) | 9.36 (2.57–34.03) | 41.02 (2.87–586.97) | 10.70 (1.04–109.87) |
| AUROC (95% CI) | 0.90 (0.87–0.93) | 0.91 (0.88–0.93) | 0.81 (0.77–0.84) | 0.70 (0.66–0.74) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hussain, S.K.; Kurdi, A.; Abutheraa, N.; AlDawsari, A.; Sneddon, J.; Godman, B.; Seaton, R.A. Validity of Pneumonia Severity Assessment Scores in Africa and South Asia: A Systematic Review and Meta-Analysis. Healthcare 2021, 9, 1202. https://doi.org/10.3390/healthcare9091202
Al Hussain SK, Kurdi A, Abutheraa N, AlDawsari A, Sneddon J, Godman B, Seaton RA. Validity of Pneumonia Severity Assessment Scores in Africa and South Asia: A Systematic Review and Meta-Analysis. Healthcare. 2021; 9(9):1202. https://doi.org/10.3390/healthcare9091202
Chicago/Turabian StyleAl Hussain, Sarah Khalid, Amanj Kurdi, Nouf Abutheraa, Asma AlDawsari, Jacqueline Sneddon, Brian Godman, and Ronald Andrew Seaton. 2021. "Validity of Pneumonia Severity Assessment Scores in Africa and South Asia: A Systematic Review and Meta-Analysis" Healthcare 9, no. 9: 1202. https://doi.org/10.3390/healthcare9091202
APA StyleAl Hussain, S. K., Kurdi, A., Abutheraa, N., AlDawsari, A., Sneddon, J., Godman, B., & Seaton, R. A. (2021). Validity of Pneumonia Severity Assessment Scores in Africa and South Asia: A Systematic Review and Meta-Analysis. Healthcare, 9(9), 1202. https://doi.org/10.3390/healthcare9091202








