Long-Term Outcome after Asphyxia and Therapeutic Hypothermia in Late Preterm Infants: A Pilot Study
Abstract
:1. Introduction
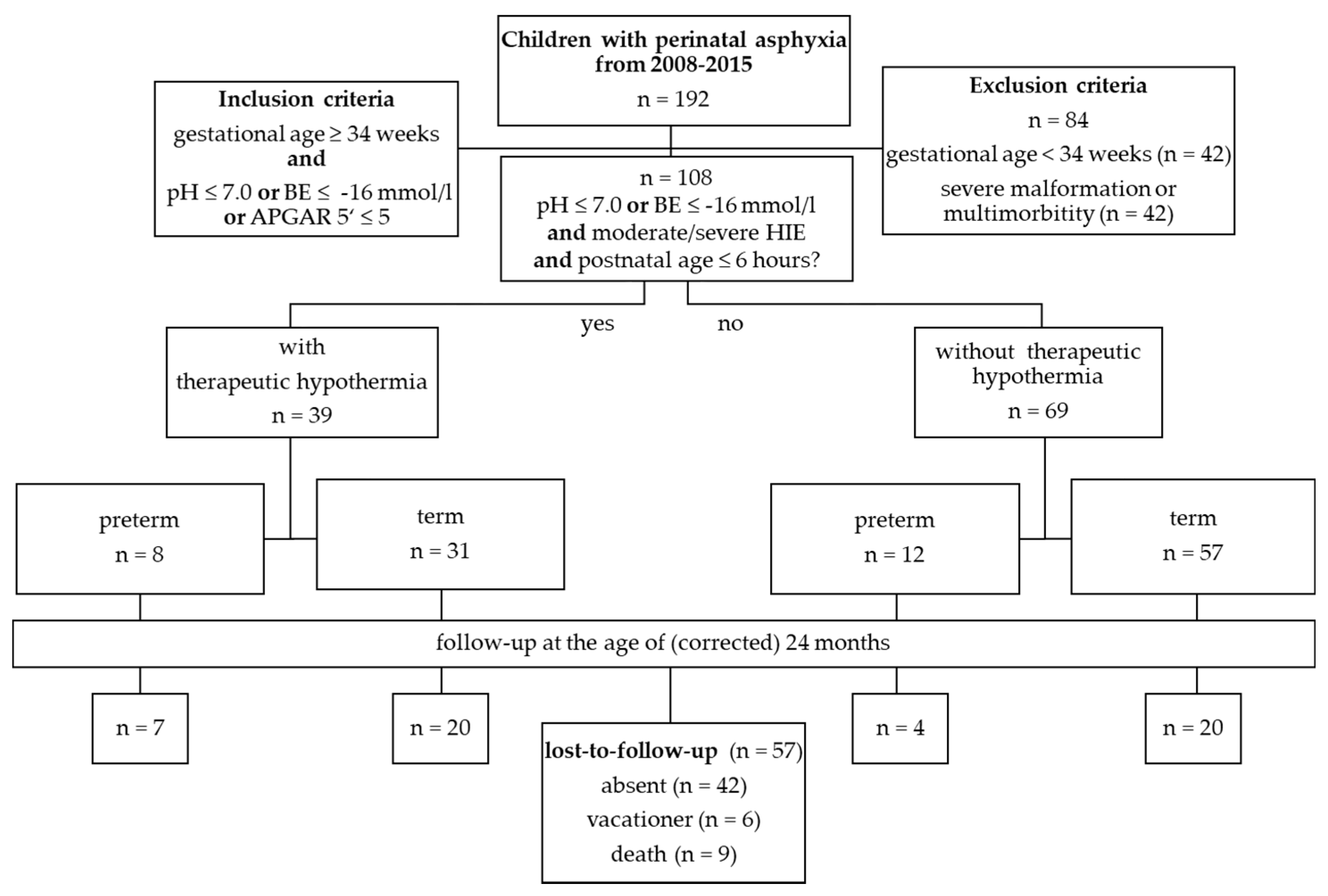
2. Materials and Methods
2.1. Perinatal Asphyxia
2.2. Hypoxic-Ischemic Encephalopathy
2.3. Therapeutic Hypothermia
- Severe acidosis (pH ≤ 7.0 or a base deficit ≤ −16 mmol/L) in umbilical cord blood or a blood sample from the first hour of life.
- Clinical signs of a moderate or severe encephalopathy (severity 2 or 3 according to Sarnat and Sarnat [31] or pathological aEEG).
- Postnatal age ≤6 h.
2.4. Outcome
2.5. Statistics
2.6. Ethics
3. Results
3.1. Perinatal Asphyxia
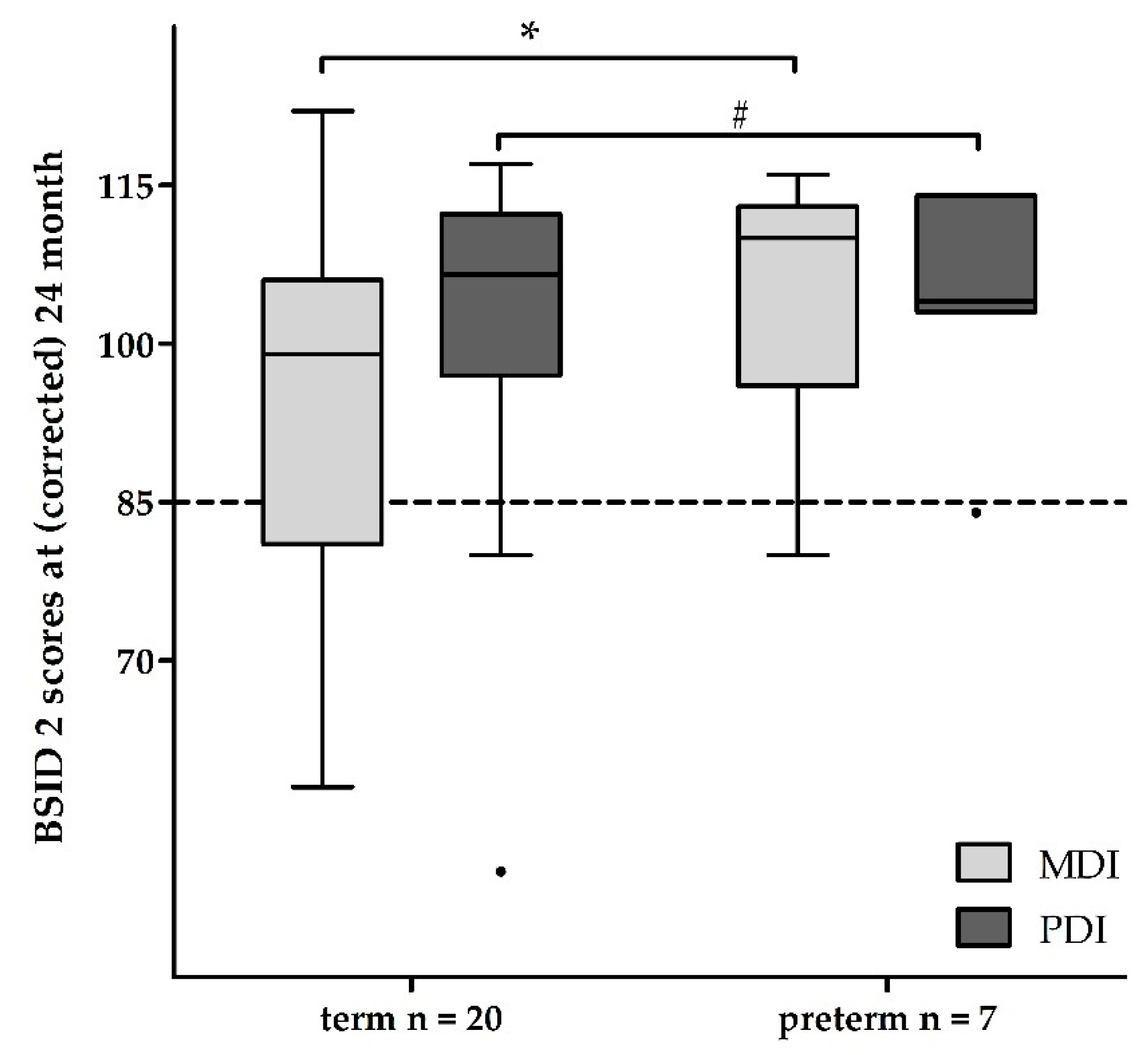
3.2. Follow-Up Examinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawn, J.E.; Cousens, S.; Zupan, J. 4 million neonatal deaths: When? Where? Why? Lancet 2005, 365, 891–900. [Google Scholar] [CrossRef]
- Flemmer, A.W.; Maier, R.F.; Hummler, H. Behandlung der neonatalen Asphyxie unter besonderer Berücksichtigung der therapeutischen Hypothermie. Klin. Pädiatrie 2014, 226, 29–37. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Pinal, C.S.; Gunn, A.J. Hypoxic-ischemic brain injury in the newborn: Pathophysiology and potential strategies for intervention. Semin. Neonatol. 2001, 6, 109–120. [Google Scholar] [CrossRef]
- Ikeda, T.; Choi, B.H.; Yee, S.; Murata, Y.; Quilligan, E.J. Oxidative stress, brain white matter damage and intrauterine asphyxia in fetal lambs. Int. J. Dev. Neurosci. 1999, 17, 1–14. [Google Scholar] [CrossRef]
- Seidl, R.; Stockler-Ipsiroglu, S.; Rolinski, B.; Kohlhauser, C.; Herkner, K.R.; Lubec, B.; Lubec, G. Energy metabolism in graded perinatal asphyxia of the rat. Life Sci. 2000, 67, 421–435. [Google Scholar] [CrossRef]
- Taylor, D.L.; Edwards, A.D.; Mehmet, H. Oxidative metabolism, apoptosis and perinatal brain injury. Brain Pathol. 1999, 9, 93–117. [Google Scholar] [CrossRef]
- Rocha-Ferreira, E.; Hristova, M. Plasticity in the Neonatal Brain following Hypoxic-Ischaemic Injury. Neural Plast. 2016, 2016, 4901014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousset, C.I.; Baburamani, A.A.; Thornton, C.; Hagberg, H. Mitochondria and perinatal brain injury. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. 1), 35–38. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, D.; Brocklehurst, P.; Edwards, D.; Halliday, H.; Levene, M.; Thoresen, M.; Whitelaw, A. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: A randomised controlled trial. BMC Pediatr. 2008, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gluckman, P.D.; Wyatt, J.S.; Azzopardi, D.; Ballard, R.; Edwards, A.D.; Ferriero, D.M.; Polin, R.A.; Robertson, C.M.; Thoresen, M.; Whitelaw, A.; et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet 2005, 365, 663–670. [Google Scholar] [CrossRef]
- Johnston, M.V.; Fatemi, A.; Wilson, M.A.; Northington, F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011, 10, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Bruno, V.M.; Goldberg, M.P.; Dugan, L.L.; Giffard, R.G.; Choi, D.W. Neuroprotective effect of hypothermia in cortical cultures exposed to oxygen-glucose deprivation or excitatory amino acids. J. Neurochem. 1994, 63, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Thoresen, M. Hypothermic neuroprotection. NeuroRx 2006, 3, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Polderman, K.H. Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med. 2009, 37, S186–S202. [Google Scholar] [CrossRef]
- Evans, D.J.; Levene, M.I.; Tsakmakis, M. Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia. Cochrane Database Syst. Rev. 2007, 3. [Google Scholar] [CrossRef]
- Thornberg, E.; Thiringer, K.; Odeback, A.; Milsom, I. Birth asphyxia: Incidence, clinical course and outcome in a Swedish population. Acta Paediatr. 1995, 84, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Kariholu, U.; Montaldo, P.; Markati, T.; Lally, P.J.; Pryce, R.; Teiserskas, J.; Liow, N.; Oliveira, V.; Soe, A.; Shankaran, S.; et al. Therapeutic hypothermia for mild neonatal encephalopathy: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2018. [Google Scholar] [CrossRef]
- Azzopardi, D.; Strohm, B.; Marlow, N.; Brocklehurst, P.; Deierl, A.; Eddama, O.; Goodwin, J.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 2014, 371, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Shankaran, S.; Pappas, A.; McDonald, S.A.; Vohr, B.R.; Hintz, S.R.; Yolton, K.; Gustafson, K.E.; Leach, T.M.; Green, C.; Bara, R.; et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 2012, 366, 2085–2092. [Google Scholar] [CrossRef]
- Shankaran, S.; Woldt, E.; Koepke, T.; Bedard, M.P.; Nandyal, R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum. Dev. 1991, 25, 135–148. [Google Scholar] [CrossRef]
- Azzopardi, D.V.; Strohm, B.; Edwards, A.D.; Dyet, L.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; Levene, M.; Marlow, N.; Porter, E.; et al. Moderate Hypothermia to Treat Perinatal Asphyxial Encephalopathy. N. Engl. J. Med. 2009, 361, 1349–1358. [Google Scholar] [CrossRef] [Green Version]
- Gunn, A.J.; Bennet, L. Brain cooling for preterm infants. Clin. Perinatol. 2008, 35, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Trivedi, S.; Vesoulis, Z.; Liao, S.M.; Smyser, C.D.; Mathur, A.M. Safety and Short-Term Outcomes of Therapeutic Hypothermia in Preterm Neonates 34-35 Weeks Gestational Age with Hypoxic-Ischemic Encephalopathy. J. Pediatr. 2017, 183, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Herrera, T.I.; Edwards, L.; Malcolm, W.F.; Smith, P.B.; Fisher, K.A.; Pizoli, C.; Gustafson, K.E.; Goldstein, R.F.; Cotten, C.M.; Goldberg, R.N.; et al. Outcomes of preterm infants treated with hypothermia for hypoxic-ischemic encephalopathy. Early Hum. Dev. 2018, 125, 1–7. [Google Scholar] [CrossRef]
- Eicher, D.J.; Wagner, C.L.; Katikaneni, L.P.; Hulsey, T.C.; Bass, W.T.; Kaufman, D.A.; Horgan, M.J.; Languani, S.; Bhatia, J.J.; Givelichian, L.M.; et al. Moderate hypothermia in neonatal encephalopathy: Efficacy outcomes. Pediatr. Neurol. 2005, 32, 11–17. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Morley, C.J.; Inder, T.E.; Stewart, M.J.; Smith, K.R.; McNamara, P.J.; Wright, I.M.R.; Kirpalani, H.M.; Darlow, B.A.; Doyle, L.W. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: A randomized controlled trial. Arch. Pediatr. Adolesc. Med. 2011, 165, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Bayley, N. Bayley Scales of Infant Development—Second Edition Manual; Harcourt Test Services: Frankfurt, Germany, 1993. [Google Scholar]
- Thoresen, M. Who should we cool after perinatal asphyxia? Semin. Fetal Neonatal Med. 2015, 20, 66–71. [Google Scholar] [CrossRef]
- Gancia, P.; Pomero, G. Therapeutic hypothermia in the prevention of hypoxic-ischaemic encephalopathy: New categories to be enrolled. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. 4), 94–96. [Google Scholar] [CrossRef]
- Burnsed, J.; Zanelli, S.A. Neonatal therapeutic hypothermia outside of standard guidelines: A survey of U.S. neonatologists. Acta Paediatr. 2017, 106, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, H.B.; Sarnat, M.S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 1976, 33, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Luttikhuizen dos Santos, E.S.; de Kieviet, J.F.; Königs, M.; van Elburg, R.M.; Oosterlaan, J. Predictive value of the Bayley scales of infant development on development of very preterm/very low birth weight children: A meta-analysis. Early Hum. Dev. 2013, 89, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, A.B.R.; de Mello, R.R.; Morsch, D.S.; Meio, M.D.B.B.; da Silva, K.S. Mental performance of very low birth weight preterm infants: Assessment of stability in the first two years of life and factors associated with mental performance. Rev. Bras. Epidemiol. 2012, 15, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, S.E.; Berg, M.; Hunt, R.; Tarnow-Mordi, W.O.; Inder, T.E.; Davis, P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, 1, CD003311. [Google Scholar] [CrossRef] [PubMed]
- NICHD Neonatal Research Network; Eunice Kennedy Shriver National Institute of Child Health and Human Development. A Randomized Trial of Targeted Temperature Management with Whole Body Hypothermia for Moderate and Severe Hypoxic-Ischemic Encephalopathy in Premature Infants 33–35 Weeks Gestational Age: NCT01793129, NICHD-NRN-0051. Available online: https://clinicaltrials.gov/ct2/show/NCT01793129 (accessed on 21 February 2021).
| Late Preterm Newborn | Term Newborn | ||||||
|---|---|---|---|---|---|---|---|
| with THT (n = 8) | without THT (n = 12) | p | with THT (n = 31) | without THT (n = 57) | p | ||
| female | N (%) | 4 (50) | 5 (42) | † b | 15 (48) | 19 (53) | † b |
| gestational age [weeks] | mv ± sd | 35 ± 0.8 | 35 ± 0.9 | † b | 39.5 ± 1.2 | 39.3 ± 1.4 | † b |
| birth weight [gram] | 2681 ± 522 | 2504 ± 432 | † b | 3599 ± 576 | 3394 ± 600 | † b | |
| head circumference [cm] | 34 ± 1 | 33 ± 2 | † b | 35 ± 2 | 35 ± 3 | † b | |
| max. base excess [mmol/L] | −23 ± 5 | −14 ± 4 | * b | −21 ± 5 | −17 ± 4 | * b | |
| umbilical cord pH | 6.9 ± 0.2 | 7.1 ± 0.2 | * b | 6.9 ± 0.2 | 7.0 ± 0.2 | * b | |
| Apgar score at 5′ | median ± IQR | 4 ± 3 | 5 ± 3 | † b | 4 ± 4 | 6 ± 3 | ** b |
| seizures | n (%) | 5 (63) | 0 | * a | 19 (61) | 4 (7) | * a,c |
| palsy | 3 (38) | 0 | * a | 10 (32) | 0 | * a,c | |
| coma | 1 (13) | 0 | † a | 9(29) | 0 | * a,c | |
| pathologic aEEG | 8 (100) | 2 (17) | * a | 27 (87) | 3 (5) | ** a | |
| HIE | ** a | ** a | |||||
| no | 0 | 9 (75) | 0 | 38 (67) | |||
| mild | 0 | 2 (17) | 0 | 5 (9) | |||
| moderate | 7 (88) | 0 | 14 (45) | 12 (21) | |||
| severe | 1 (13) | 1 (8) | 17 (55) | 2 (4) | |||
| death | 1(13) | 0 | † a | 6 (19) | 2 (4) | † a | |
| Late Preterm Newborn | Term Newborn | |||||
|---|---|---|---|---|---|---|
| without THT (n = 4) | with THT (n = 7) | p | with THT (n = 20) | p | ||
| female | n (%) | 1 (25) | 3 (43) | † a | 9 (45) | † a |
| age at birth [months] | mv ± sd | 35 ± 1 | 35 ± 1 | † a | 40 ± 1 | † a |
| weight [gram] | 11,435 ± 1198 | 12,208 ± 1116 | † a | 12,492 ± 1677 | * a | |
| head circumference [cm] | 47.8 ± 2.5 | 49.2 ± 1.0 | † a | 47.9 ± 1.7 | † a | |
| HIE | n (%) | |||||
| no | 2 (50) | 0 | 0 | |||
| mild | 0 | 0 | 0 | |||
| moderate | 2 (50) | 6 (86) | * b,d | 11 (55) | † b,d | |
| severe | 0 | 1 (14) | † b,d | 9 (45) | † b,d | |
| MDI | mv ± sd | 96 ± 20 | 105 ± 13 | † c | 93 ± 18 | * c |
| ≥115 | n (%) | 1 (25) | 1 (14) | 1 (5) | ||
| ≥85 | 1 (25) | 5 (72) | 13 (65) | |||
| <85 | 2 (50) | 1 (14) | 3 (15) | |||
| <70 | 0 | 0 | 3 (15) | |||
| PDI | mv ± sd | 105 ± 15 | 105 ± 11 | † c | 101 ± 16 | † c |
| ≥115 | n (%) | 1 (25) | 1 (14) | 1 (5) | ||
| ≥85 | 3 (75) | 5 (72) | 15 (75) | |||
| <85 | 0 | 1 (14) | 3 (15) | |||
| <70 | 0 | 0 | 1 (5) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lademann, H.; Abshagen, K.; Janning, A.; Däbritz, J.; Olbertz, D. Long-Term Outcome after Asphyxia and Therapeutic Hypothermia in Late Preterm Infants: A Pilot Study. Healthcare 2021, 9, 994. https://doi.org/10.3390/healthcare9080994
Lademann H, Abshagen K, Janning A, Däbritz J, Olbertz D. Long-Term Outcome after Asphyxia and Therapeutic Hypothermia in Late Preterm Infants: A Pilot Study. Healthcare. 2021; 9(8):994. https://doi.org/10.3390/healthcare9080994
Chicago/Turabian StyleLademann, Hanne, Karl Abshagen, Anna Janning, Jan Däbritz, and Dirk Olbertz. 2021. "Long-Term Outcome after Asphyxia and Therapeutic Hypothermia in Late Preterm Infants: A Pilot Study" Healthcare 9, no. 8: 994. https://doi.org/10.3390/healthcare9080994
APA StyleLademann, H., Abshagen, K., Janning, A., Däbritz, J., & Olbertz, D. (2021). Long-Term Outcome after Asphyxia and Therapeutic Hypothermia in Late Preterm Infants: A Pilot Study. Healthcare, 9(8), 994. https://doi.org/10.3390/healthcare9080994






