Nutritional Intervention Improves Nutrition Outcomes in Stomach and Colon Cancer Patients Receiving Chemotherapy: Finding from a Quasi-Experiment in Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
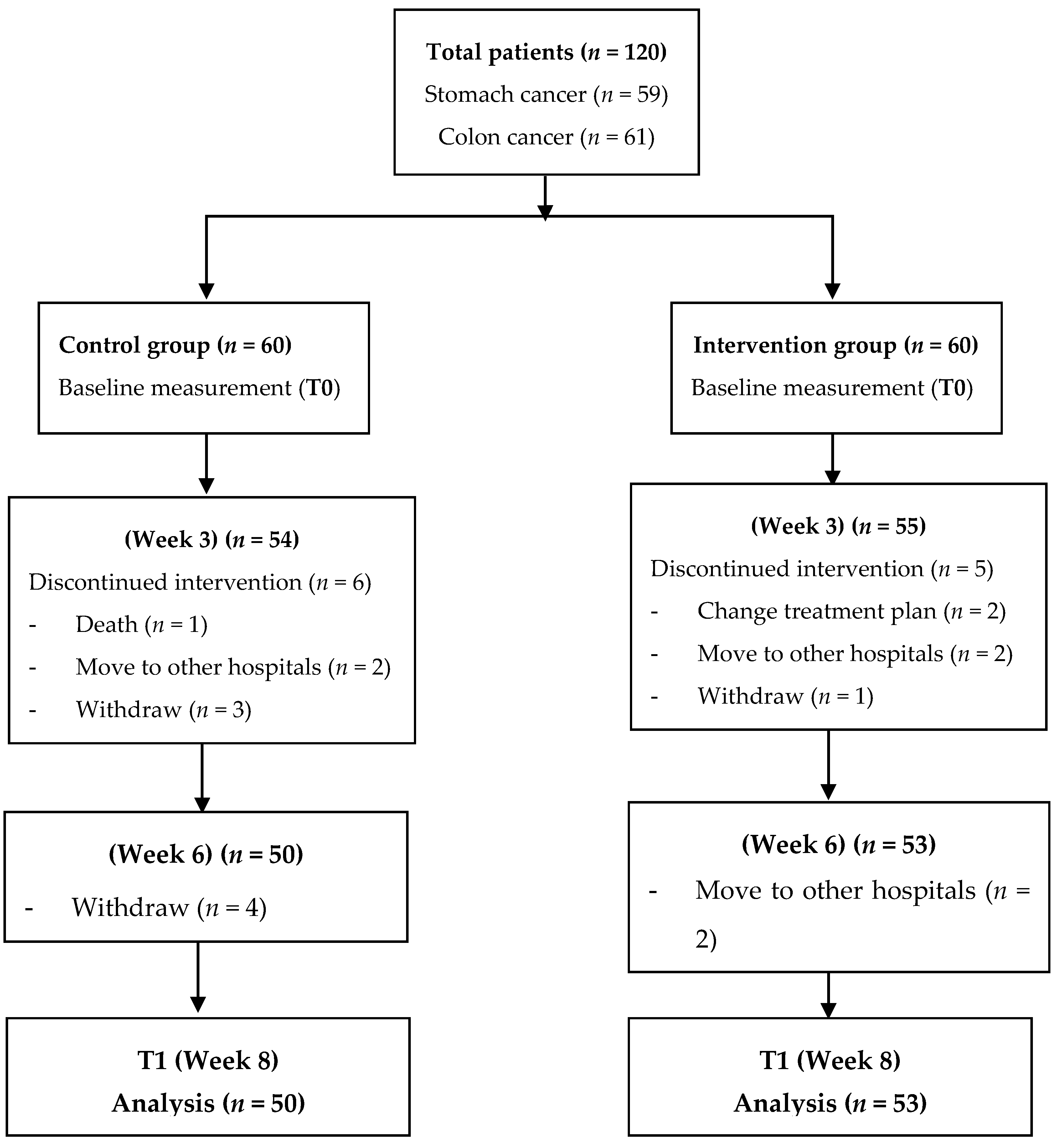
2.2. Participants
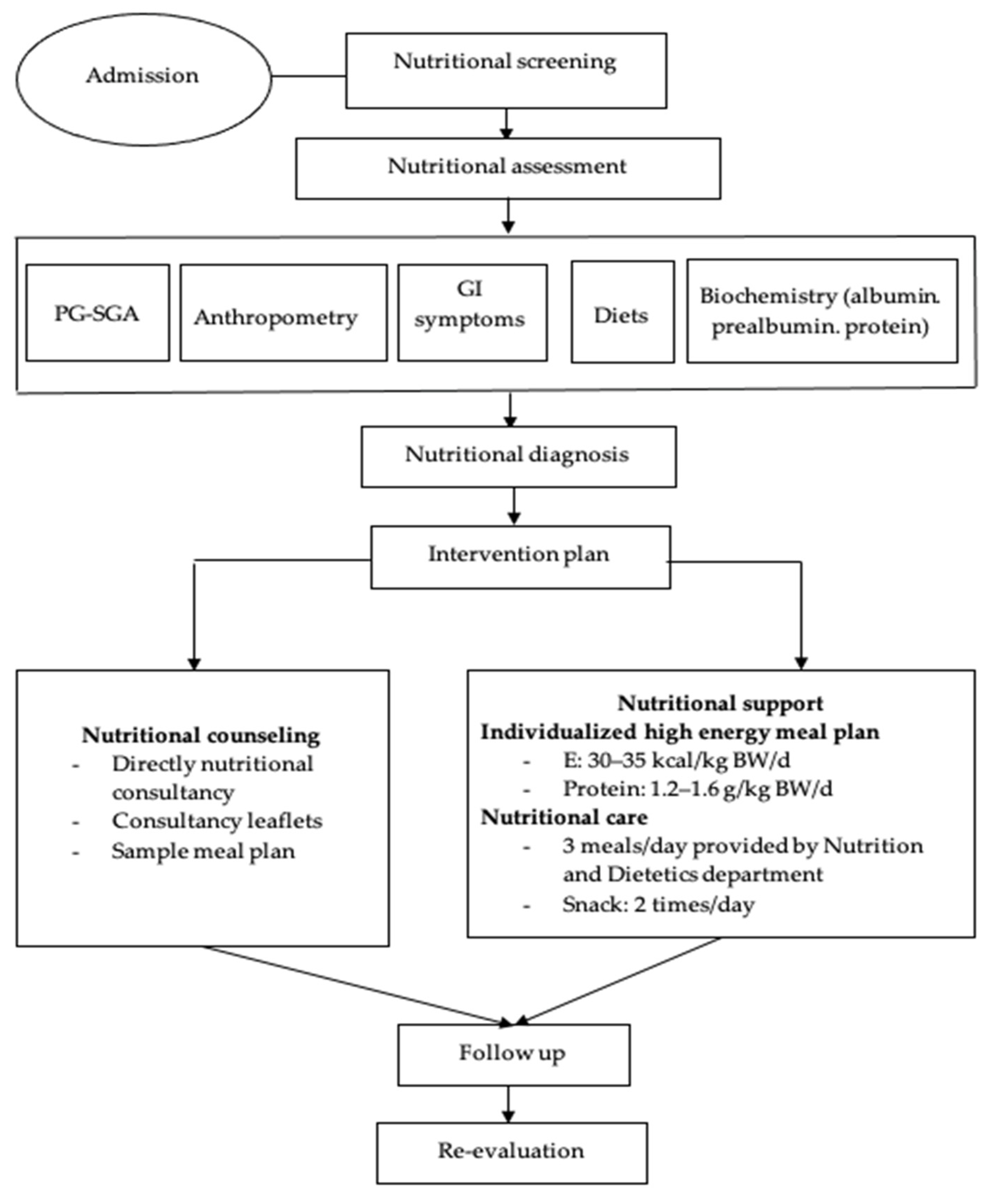
2.3. Developing the Intervention Protocol
- Consultancy leaflets.
- Menus based on energy levels following the demand recommendations by the European Society for Clinical Nutrition and Metabolism (ESPEN), with total energy being 30 kcal/kg of body weight/day, protein from 1.2 to 1.6 g/kg of body weight/day and 2 g EPA/day [5]. Depending on the weight and digestive condition of each patient, we classified the menus into different energy levels, which consisted of 1500–1600 kcal/day, 1700–1800 kcal/day, or 1900–2000 kcal/day. Patients were recommended to eat small and frequent meals 5–6 times a day to increase their overall intake. If the supplements were feasible and sensible, patients consumed two snacks with 200–400 mL ONS/day (Appendix A).
- Menus and high-energy soups were prepared, and these menus were evaluated based on suitability and acceptance of the patients.
- A handbook was prepared for processing high-energy soup preparations from common foods for cancer patients.
- Nutritional screening according to the MST (malnutrition screening tool) by dietitians within the first 24 h after the hospital admission took place at two time points (baseline—T0 and 2 months after chemotherapy—T1).
- Nutritional status was assessed according to anthropometric measurements (weight, BMI, percentage of weight loss, MUAC, muscle mass, fat mass); the PG-SGA; albumin, prealbumin and total protein at two time points (baseline—T0 and 2 months after chemotherapy—T1).
- Nutritional diagnosis.
- The control group received nutritional status screening and assessment, and nutritional counselling. They could freely select their own diet. In contrast, patients in the intervention group were treated with the intervention regimen, which consisted of: (1) nutritional counseling; (2) hospital meals: each patient was assigned a specific menu prepared by research members during the time of staying at the hospital; (3) instruction on food preparation at home: before discharge, patients were instructed on preparing their diet at home with high energy and protein intake as recommended and given formula milk (6 teaspoons, equivalent to 54 g of powdered milk with 180 mL warm water per time) within two months [20].
- To monitor the intervention process, the dieticians continuously evaluated the daily diet of each patient during hospitalization and tele-counseling every two weeks for weight monitoring and nutritional support advice after discharge. Patients were re-evaluated in terms of their nutritional status at every hospital admission for chemotherapy.
- The target of the nutritional intervention was to ensure the ability to meet the recommended energy and protein of patients. The criteria for making adjustments to the intervention was practicable and implemented by the patient. In addition, an appropriate treatment tailored to the patients’ eating preferences and digestive and absorptive capacity was taken into consideration.
2.4. Nutritional Evaluation
2.5. Data Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Effectiveness of Nutritional Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Nutritional Supplements for Snacks
| Energy | Kcal | 506 |
|---|---|---|
| Protein | g | 20 |
| Leuxin | g | 2.9 |
| Arginin | g | 1.4 |
| Glutamic | g | 6.3 |
| Fats | g | 26 |
| MUFA | g | 4.1 |
| PUFA | g | 1.3 |
| Omega 3 | mg | 30 |
| Omega 6 | mg | 120 |
| Glucose | g | 48 |
| FOS | g | 3.5 |
| Curcumin | mg | 80 |
| Nucleotieds | mg | 25 |
| Vitamin A | mcg | 550.0 |
| Vitamin D3 | mcg | 12.0 |
| Vitamin E | mg | 11.8 |
| Vitamin K | mcg | 33.0 |
| Vitamin C | mg | 126.0 |
| Vitamin B1 | mg | 1.0 |
| Vitamin B2 | mg | 0.9 |
| Niacin (Vitamin B3) | mg | 16.8 |
| Axit Pantothenic (Vitamin B5) | mg | 5.2 |
| Vitamin B6 (Pyridosin) | mg | 1.0 |
| Folic acid B9 | mcg | 112.0 |
| Vitamin B12 (Cobalamin | mcg | 7.1 |
| Biotin | mcg | 25.2 |
| Na (Sodium) | mg | 287 |
| Kali (chloride) | mg | 450 |
| Clo (Chloride) | mg | 350 |
| Canxi (Calcium) | mg | 450 |
| Photpho (Phosphorus) | mg | 350 |
| Mg (Magnesium) | mg | 84.4 |
| Fe (Iron) | mg | 3.8 |
| Zn (Zinc) | mg | 11.2 |
| Mn (Manganege) | mg | 1.4 |
| Cu (Copper) | mcg | 260.0 |
| Iod (Iodine) | mcg | 65.0 |
| Selen (Selenium) | mcg | 31.2 |
| Crom (Chromium) | mcg | 28.6 |
| Molypden (Molypdenum) | mcg | 31.2 |
References
- Mattox, T.W. Cancer Cachexia: Cause, Diagnosis, and Treatment. Nutr. Clin. Pract. 2017, 32, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Contaldo, F.; Pasanisi, F. Nutritional screening and early treatment of malnutrition in cancer patients. J. Cachexia Sarcopenia Muscle 2011, 2, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.; Deutz, N.; Erickson, N.; Laviano, A.; Lisanti, M.; Lobo, D.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.I.S.D.S.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2016, 36, 11–48. [Google Scholar] [CrossRef]
- Yang, Y.H.; Lee, D.S. The Relationship of Anorexia, Nausea, Vomiting, Oral Intake and Nutritional Status in Patients Receiving Chemotherapy. J. Korean Acad. Nurs. 2000, 30, 720–730. [Google Scholar] [CrossRef]
- Garla, P.; Waitzberg, D.L.; Tesser, A. Nutritional Therapy in Gastrointestinal Cancers. Gastroenterol. Clin. N. Am. 2018, 47, 231–242. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Escott-Stump, S. Section 13. Cancer. In Nutrition and Diagnosis-Related Care; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 672–688. [Google Scholar]
- Kiss, N.K.; Krishnasamy, M.; Isenring, E. The Effect of Nutrition Intervention in Lung Cancer Patients Undergoing Chemotherapy and/or Radiotherapy: A Systematic Review. Nutr. Cancer 2014, 66, 47–56. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M.; Jeung, H.C.; Lee, I.J.; Park, J.S.; Song, M.; Lee, D.K.; Lee, S.-M. The Effect of Nutrition Intervention with Oral Nutritional Supplements on Pancreatic and Bile Duct Cancer Patients Undergoing Chemotherapy. Nutrients 2019, 11, 1145. [Google Scholar] [CrossRef]
- Mislang, A.R.; Di Donato, S.; Hubbard, J.; Krishna, L.; Mottino, G.; Bozzetti, F.; Biganzoli, L. Nutritional management of older adults with gastrointestinal cancers: An International Society of Geriatric Oncology (SIOG) review paper. J. Geriatr. Oncol. 2018, 9, 382–392. [Google Scholar] [CrossRef]
- Choi, W.J.; Kim, J. Nutritional Care of Gastric Cancer Patients with Clinical Outcomes and Complications: A Review. Clin. Nutr. Res. 2016, 5, 65–78. [Google Scholar] [CrossRef]
- Jankowski, M.; Las-Jankowska, M.; Sousak, M.; Zegarski, W. Contemporary enteral and parenteral nutrition before surgery for gastrointestinal cancers: A literature review. World J. Surg. Oncol. 2018, 16, 94. [Google Scholar] [CrossRef]
- Du, H.; Liu, B.; Xie, Y.; Liu, J.; Wei, Y.; Hu, H.; Luo, B.; Li, Z. Comparison of different methods for nutrition assessment in patients with tumors. Oncol. Lett. 2017, 14, 165–170. [Google Scholar] [CrossRef]
- Blackwood, H.A.; Hall, C.C.; Balstad, T.R.; Solheim, T.S.; Fallon, M.; Haraldsdottir, E.; Laird, B.J. A systematic review examining nutrition support interventions in patients with incurable cancer. Support. Care Cancer 2020, 28, 1877–1889. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Garth, A.K.; Newsome, C.M.; Simmance, N.; Crowe, T.C. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J. Hum. Nutr. Diet 2010, 23, 393–401. [Google Scholar] [CrossRef]
- Zhong, B. How to Calculate Sample Size in Randomized Controlled Trial? J. Thorac. Dis. 2009, 1, 51–54. [Google Scholar] [PubMed]
- Nguyen, L.T.; Dang, A.K.; Duong, P.T.; Phan, H.B.T.; Pham, C.T.T.; Le Nguyen, A.T.; Le, H.T. Nutrition intervention is beneficial to the quality of life of patients with gastrointestinal cancer undergoing chemotherapy in Vietnam. Cancer Med. 2021, 10, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bharadwaj, S.; Ginoya, S.; Tandon, P.; Gohel, T.D.; Guirguis, J.; Vallabh, H.; Jevenn, A.; Hanouneh, I. Malnutrition: Laboratory markers vs nutritional assessment. Gastroenterol. Rep. 2016, 4, 272–280. [Google Scholar] [CrossRef]
- Cotogni, R.; Pedraxxoli, P.; Waele, E.D.; Aprile, G.; Farina, G.; Stragliotto, S.; De Lorenzo, F.; Caccialanza, R. Nutritional Therapy in Cancer Patients Receiving Chemoradiotherapy: Should We Need Stronger Recommendations to Act for Improving Outcomes? J. Cancer 2019, 10, 4318–4324. [Google Scholar] [CrossRef]
- Caccialanza, R.; Cereda, E.; Pinto, C.; Cotogni, P.; Farina, G.; Gavazzi, C.; Gandini, C.; Nardi, M.; Zagonel, V.; Pedrazzoli, P. Awareness and consideration of malnutrition among oncologists: Insights from an exploratory survey. Nutrition 2016, 32, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.; Weekes, C.E. Dietary advice with or without oral nutritional supplements for disease-related malnutrition in adults. Cochrane Database Syst. Rev. 2011, CD002008. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.; Spiro, A.; Ahern, R.; Emery, P.W. Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2012, 104, 371–385. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Alish, C.; Pereira, S.L.; Deutz, N.E.; Heyland, D.K.; Goodpaster, B.H.; Tappenden, K.; Heymsfield, S.B. Implications of low muscle mass across the continuum of care: A narrative review. Ann. Med. 2018, 50, 675–693. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.J.; Bolam, K.A.; Wright, O.R.L.; Skinner, T.L. The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients 2017, 9, 1003. [Google Scholar] [CrossRef]
- Miller, M.; Zrim, S.; Lawn, S.; Woodman, R.; Leggett, S.; Jones, L.; Karapetis, C.; Kichenadasse, G.; Sukumaran, S.; Roy, A.C.; et al. A Pilot Study of Self-Management-based Nutrition and Physical Activity Intervention in Cancer Survivors. Nutr. Cancer 2016, 68, 762–771. [Google Scholar] [CrossRef]
- Uster, A.; Ruehlin, M.; Mey, S.; Gisi, D.; Knols, R.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin. Nutr. 2018, 37, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Silvers, M.A.; Savva, J.; Huggins, C.E.; Truby, H.; Haines, T. Potential benefits of early nutritional intervention in adults with upper gastrointestinal cancer: A pilot randomised trial. Support. Care Cancer 2014, 22, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Y.; Fang, Y. Nutritional status and related factors of patients with advanced gastrointestinal cancer. Br. J. Nutr. 2014, 111, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Loan, B.T.H.; Nakahara, S.; Tho, B.A.; Dang, T.N.; Anh, L.N.; Huy, N.D.; Ichikawa, M. Nutritional status and postoperative outcomes in patients with gastrointestinal cancer in Vietnam: A retrospective cohort study. Nutrition 2018, 48, 117–121. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro-Grillo, I.; Camilo, M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: Long-term follow-up of a randomized controlled trial of nutritional therapy. Am. J. Clin. Nutr. 2012, 96, 1346–1353. [Google Scholar] [CrossRef]
- Kim, H.; Suh, E.E.; Lee, H.J.; Yang, H.K. The effects of patient participation-based dietary intervention on nutritional and functional status for patients with gastrectomy: A randomized controlled trial. Cancer Nurs. 2014, 37, 10–20. [Google Scholar] [CrossRef]
- Okada, S.; Yamazaki, S.; Kaiga, T.; Funada, T.; Kochi, M.; Takayama, T. Impact of nutritional status in the era of FOLFOX/FIRI-based chemotherapy. World J. Surg. Oncol. 2017, 15, 162. [Google Scholar] [CrossRef][Green Version]
- Du, Y.-P.; Li, L.-L.; He, Q.; Li, Y.; Song, H.; Lin, Y.-J.; Peng, J.-S. Nutritional risk screening and nutrition assessment for gastrointestinal cancer patients. Chin. J. Gastrointest. Surg. 2012, 15, 460–463. [Google Scholar]
- Hébuterne, X.; Lemarié, E.; Michallet, M. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef]
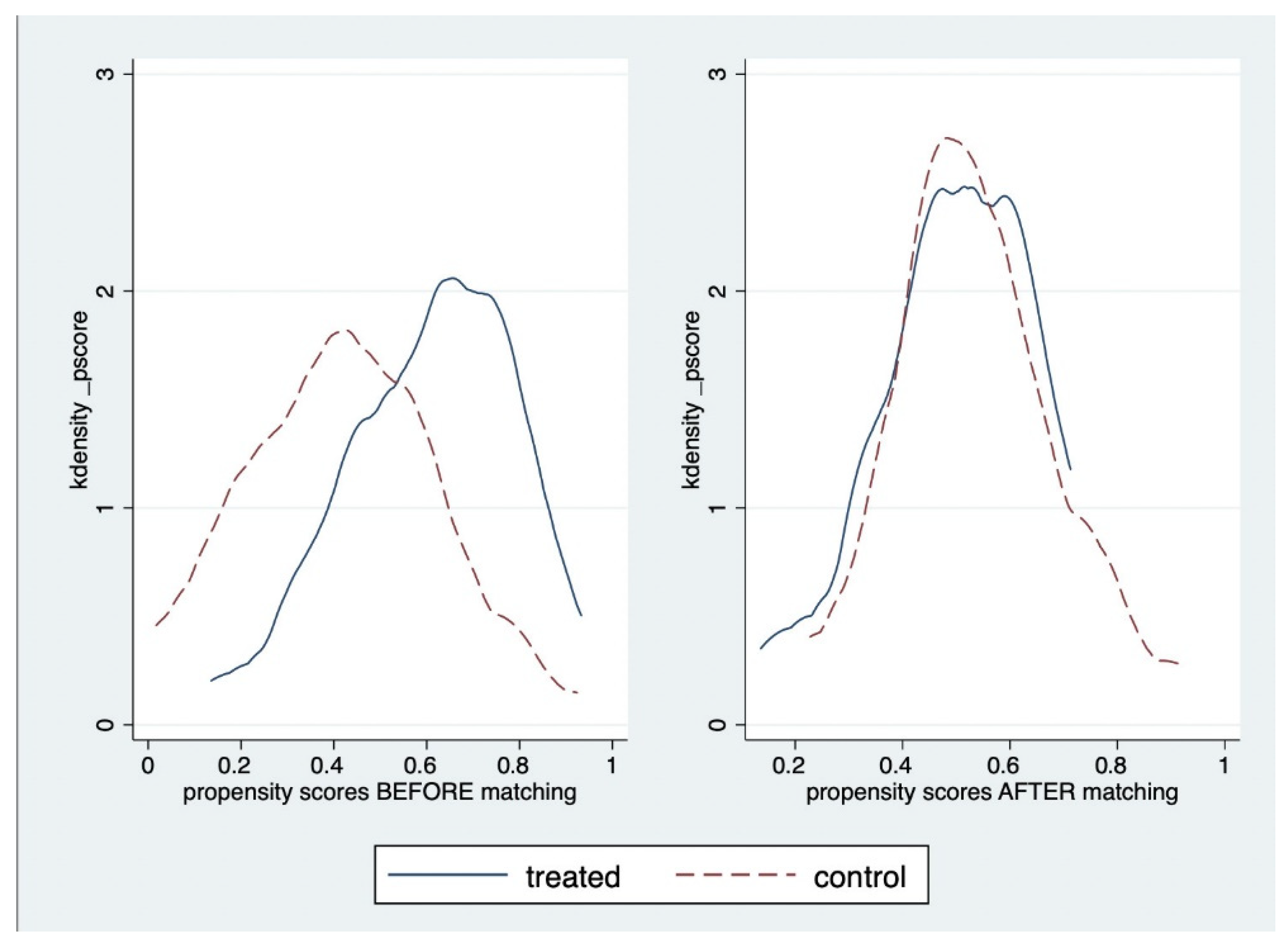
| Characteristics of Participants | Before Propensity Score Matching | After Propensity Score Matching (1:1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control Group (n = 50) | Intervention Group (n = 53) | p-Value | Control Group (n = 31) | Intervention Group (n = 31) | p-Value | |||||
| n | % | n | % | n | % | n | % | |||
| Gender | ||||||||||
| Males | 31 | 62.0 | 33 | 62.3 | 0.98 a | 22 | 71.0 | 22 | 71.0 | 1.00 a |
| Females | 19 | 38.0 | 20 | 37.7 | 9 | 29.0 | 9 | 29.0 | ||
| Educational level | ||||||||||
| Under high school | 25 | 50.0 | 25 | 47.2 | 0.60 a | 14 | 45.2 | 14 | 45.2 | 1.00 a |
| Highschool | 13 | 26.0 | 10 | 18.9 | 6 | 19.3 | 6 | 19.3 | ||
| Intermediate college | 4 | 8.0 | 8 | 15.0 | 4 | 12.9 | 4 | 12.9 | ||
| University/postgraduate | 8 | 16.0 | 10 | 18.9 | 7 | 22.6 | 7 | 22.6 | ||
| Occupation | ||||||||||
| Office workers | 4 | 8.0 | 7 | 13.2 | 0.60 a | 4 | 12.9 | 4 | 12.9 | 0.76 a |
| Farmers/workers | 12 | 24.0 | 14 | 26.4 | 5 | 16.1 | 8 | 25.8 | ||
| Retired | 22 | 44.0 | 18 | 34.0 | 13 | 41.9 | 10 | 32.3 | ||
| Doing household | 12 | 24.0 | 14 | 26.4 | 9 | 29.1 | 9 | 29.1 | ||
| Economic conditions | ||||||||||
| Poor/near-poor households | 2 | 4.0 | 2 | 3.8 | 0.14 b | 2 | 6.4 | 1 | 3.2 | 0.22 b |
| Normal/rich | 48 | 96.0 | 51 | 96.2 | 29 | 93.6 | 30 | 96.8 | ||
| Living area | ||||||||||
| Rural | 25 | 50.0 | 27 | 51.0 | 0.92 a | 15 | 48.4 | 14 | 45.2 | 0.80 a |
| Urban | 25 | 50.0 | 26 | 49.0 | 16 | 51.6 | 17 | 54.8 | ||
| Type of disease | ||||||||||
| Stomach cancer | 22 | 44.0 | 29 | 54.7 | 0.28 a | 15 | 48.4 | 15 | 48.4 | 1.00 a |
| Colon cancer | 28 | 56.0 | 24 | 45.3 | 16 | 51.6 | 16 | 51.6 | ||
| Stage of cancer | ||||||||||
| I–II | 9 | 18.0 | 8 | 15.1 | 0.69 a | 4 | 12.9 | 8 | 25.8 | 0.26 b |
| III–IV | 41 | 82.0 | 45 | 84.9 | 27 | 87.1 | 23 | 74.2 | ||
| Number of chemotherapy treatments | ||||||||||
| 0 | 19 | 38.0 | 21 | 39.6 | 0.87 a | 13 | 41.9 | 13 | 41.9 | 1.00 a |
| 1 | 31 | 62.0 | 32 | 60.4 | 18 | 58.1 | 18 | 58.1 | ||
| Having comorbidities | ||||||||||
| No | 36 | 72.0 | 49 | 92.5 | 0.02 a | 26 | 83.9 | 27 | 87.1 | 0.30 a |
| Yes | 14 | 28.0 | 4 | 7.5 | 5 | 16.1 | 4 | 12.9 | ||
| Mean | SD | Mean | SD | p-Value | Mean | SD | Mean | SD | p-Value | |
| Age | 58.2 | 10.0 | 54.9 | 10.6 | 0.11 c | 55.2 | 10.2 | 57.3 | 8.9 | 0.58 c |
| Control Group (n = 50) | Intervention Group (n = 53) | p-Value (1–2) | p-Value (3–4) | |||
|---|---|---|---|---|---|---|
| T0 (1) | T1 (2) | T0 (3) | T1 (4) | |||
| Weight | 50.5 ± 7.6 | 50.9 ±7.1 | 50.2 ± 7.4 | 51.6 ± 7.8 | 0.19 ¶ | <0.01 ¶ |
| Muscle mass | 37.0 ±5.7 | 37.6 ±5.6 | 36.5 ± 5.8 | 37.7 ±6.6 | 0.16¶ | 0.02 ¶ |
| MUAC | 25.2 ±3.1 | 24.6 ±3.1 | 25.3 ± 2.5 | 25.6 ±2.9 | 0.16 ¶ | 0.29 ¶ |
| Fat mass (%) | 23.2 ± 6.5 | 21.5 ± 6.6 | 23.0 ± 7.5 | 22.6 ± 7.5 | 0.02 ¶ | 0.30 ¶ |
| Albumin (g/L) | 35.8 ± 7.2 | 30.7 ± 11.5 | 39.2 ± 5.8 | 36.7 ± 5.5 | 0.01 * | 0.05 * |
| Protein (g/L) | 62.6 ± 13.6 | 52.1 ± 15.9 | 58.8 ± 10.6 | 57.8 ± 8.5 | 0.02 * | 0.57 * |
| PG-SGA score | 11.7 ± 5.1 | 10.9 ± 6.2 | 13.1 ± 5.6 | 8.9 ± 6.0 | 0.39 * | <0.01 * |
| PG-SGA classification | ||||||
| PG-SGA A (Well-nourished) | 9 (18.0) | 20 (40.0) | 12 (22.6) | 34 (64.2) | <0.01 # | <0.01 # |
| PG-SGA B and C (moderately/severely malnourished) | 41 (82.0) | 30 (60.0) | 41 (77.4) | 19 (35.8) | ||
| BMI score | 19.6 ± 2.4 | 19.8 ± 2.2 | 19.7 ± 2.2 | 20.3 ± 2.4 | 0.12 ¶ | <0.01 ¶ |
| BMI classification | ||||||
| ≤18.5 kg/m2 | 20 (40.0) | 13 (26.0) | 17 (32.1) | 10 (18.9) | 0.04 # | 0.02 # |
| >18.5 kg/m2 | 30 (60.0) | 37 (74.0) | 36 (67.9) | 43 (81.1) | ||
| Intervention (n = 53) (Mean ± SD) | Control (n = 50) (Mean ± SD) | P I-C | |||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | P1 | T0 | T1 | P2 | ||
| Weight (kg) | |||||||
| Stomach cancer | 49.5 ± 8.5 | 49.9 ± 8.6 | 0.46 | 51.0 ± 7.8 | 50.8 ± 6.4 | 0.66 | 0.4 |
| Colon cancer | 50.99 ± 6.1 | 53.5 ± 6.4 | <0.0001 | 50.1 ± 7.6 | 51.1 ± 7.6 | 0.02 | 0.007 |
| Stage I–II | 50.2 ± 5.5 | 52.0 ± 6.2 | 0.04 | 47.6 ± 5.9 | 48.2 ± 5.6 | 0.55 | 0.3 |
| Stage III–IV | 50.2 ± 7.8 | 51.5 ± 8.1 | 0.001 | 51.2 ± 7.9 | 51.6 ± 7.3 | 0.26 | 0.1 |
| Muscle mass (kg) | |||||||
| Stomach cancer | 36.3 ± 5.8 | 37.9 ± 7.1 | 0.04 | 38.2 ± 6.0 | 38.5 ± 5.7 | 0.52 | 0.29 |
| Colon cancer | 36.7 ± 5.9 | 37.4 ± 6.2 | 0.34 | 36.1 ± 5.4 | 36.8 ± 5.5 | 0.21 | 0.98 |
| Stage I–II | 35.2 ± 4.0 | 36.4 ± 4.4 | 0.15 | 33.8 ± 4.3 | 34.4 ± 4.4 | 0.36 | 0.56 |
| Stage III–IV | 36.8 ± 6.0 | 37.9 ± 7.0 | 0.04 | 37.7 ± 5.8 | 38.2 ± 5.8 | 0.2 | 0.14 |
| MUAC (cm) | |||||||
| Stomach cancer | 24.99 ± 2.8 | 24.8 ± 3.2 | 0.62 | 25.0 ± 2.5 | 24.3 ± 2.5 | 0.19 | 0.23 |
| Colon cancer | 25.7 ± 2.1 | 26.5 ± 2.1 | 0.0001 | 25.3 ± 3.6 | 24.8 ± 3.5 | 0.42 | 0.002 |
| Stage I–II | 25.6 ± 2.9 | 25.8 ± 3.2 | 0.77 | 25.3 ± 3.6 | 25.0 ± 4.3 | 0.8 | 0.56 |
| Stage III–IV | 25.3 ± 2.5 | 25.6 ± 2.8 | 0.3 | 25.2 ± 3.0 | 24.5 ± 2.9 | 0.14 | 0.006 |
| Fat mass (%) | |||||||
| Stomach cancer | 20.96 ± 7.3 | 20.4 ± 7.8 | 0.57 | 21.87 ± 5.5 | 20.3 ± 6.5 | 0.13 | 0.6 |
| Colon cancer | 25.5 ± 7.0 | 25.2 ± 6.4 | 0.47 | 24.2 ± 7.1 | 22.4 ± 6.7 | 0.02 | 0.25 |
| Stage I–II | 26.2 ± 10.2 | 25.2 ± 8.9 | 0.4 | 24.5 ± 9.6 | 24.7 ± 8.0 | 0.8 | 0.44 |
| Stage III–IV | 22.5 ± 6.9 | 22.2 ± 7.3 | 0.6 | 22.9 ± 5.7 | 20.7 ± 6.2 | 0.006 | 0.1 |
| TFT(mm) | |||||||
| Stomach cancer | 11.9 ± 5.9 | 15.0 ± 15.2 | 0.86 | 10.4 ± 4.9 | 11.9 ± 4.8 | 0.45 | 0.68 |
| Colon cancer | 15.4 ± 8.0 | 15.2 ± 7.2 | 0.87 | 11.5 ± 6.9 | 12.0 ± 5.7 | 0.52 | 0.63 |
| Stage I–II | 14.7 ± 10.1 | 16.4 ± 10.6 | 0.58 | 11.3 ± 8.4 | 14.4 ± 7.3 | 0.3 | 0.53 |
| Stage III–IV | 13.2 ± 6.6 | 14.9 ± 12.5 | 0.6 | 10.9 ± 5.6 | 11.4 ± 4.7 | 0.66 | 0.62 |
| PG–SGA score | |||||||
| Stomach cancer | 15.8 ± 5.3 | 11.0 ± 6.7 | 0.001 | 12.9 ± 5.3 | 12.4 ± 5.5 | 0.46 | 0.0013 |
| Colon cancer | 10.3 ± 3.9 | 6.3 ± 3.9 | 0.0007 | 11.1 ± 4.5 | 9.7 ± 6.5 | 0.33 | 0.003 |
| Stage I–II | 12.3 ± 3.4 | 9.3 ± 7.8 | 0.18 | 12.4 ± 6.8 | 9.9 ± 7.0 | 0.26 | 0.08 |
| Stage III–IV | 13.5 ± 5.7 | 8.8 ± 5.8 | 0.000 | 11.8 ± 4.5 | 11.1 ± 6.0 | 0.44 | 0.0001 |
| Albumin (g/L) | |||||||
| Stomach cancer | 38.4 ± 5.8 | 37.1 ± 4.8 | 0.21 | 35.0 ± 8.1 | 29.1 ± 14.1 | 0.11 | 0.04 |
| Colon cancer | 40.4 ± 6.0 | 36.1 ± 6.6 | 0.14 | 36.4 ± 6.6 | 32.0 ± 9.1 | 0.04 | 0.01 |
| Stage I–II | 34.7 ± 7.6 | 35.8 ± 8.9 | 0.29 | 35.9 ± 7.6 | 30.3 ± 17.1 | 0.48 | 0.72 |
| Stage III–IV | 39.7 ± 5.6 | 36.8 ± 5.2 | 0.04 | 35.8 ± 7.2 | 30.8 ± 9.8 | 0.009 | 0.001 |
| Total serum Protein (g/L) | |||||||
| Stomach cancer | 59.6 ± 11.2 | 57.3 ± 6.4 | 0.46 | 57.4 ± 13.2 | 48.6 ± 17.2 | 0.25 | 0.17 |
| Colon cancer | 59.3 ± 11.7 | 58.6 ± 10.6 | 0.94 | 66.6 ± 13.5 | 54.6 ± 14.3 | 0.0045 | 0.014 |
| Stage I–II | 49.9 ± 13.3 | 54.6 ± 8.0 | 0.46 | 63.7 ± 14.4 | 45.0 ± 17.4 | 0.25 | 0.16 |
| Stage III–IV | 60.8 ± 10.5 | 58.2 ± 8.3 | 0.94 | 62.3 ± 14.1 | 53.6 ± 15.0 | 0.005 | 0.018 |
| Weight | Muscle Mass | MUAC | Fat Mass | BMI | PG–SGA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef | 95%CI | Coef | 95%CI | Coef | 95%CI | Coef | 95%CI | Coef | 95%CI | Coef | 95%CI | |
| Average treatment effect on the treated (ATT) | 0.84 * | 0.47; 2.16 | 1.08 * | 0.09; 2.06 | 0.1 | −0.77; 0.97 | 1.06 | −1.03; 3.14 | 0.33 | –0.17; 1.83 | −1.28 * | –4.39; −0.84 |
| Before Propensity Score Matching | After Propensity Score Matching (1:1) | |||
|---|---|---|---|---|
| Moderately/Severely Malnourished by PG–SGA (Yes/No) | Moderately/Severely Malnourished by PG–SGA (Yes/No) | |||
| OR | 95%CI | OR | 95%CI | |
| Gender (Female vs. Male) | 1.70 * | 1.38; 7.66 | 2.58 * | 1.49; 6.51 |
| Age | 1.00 | 0.94; 1.06 | 1.03 | 0.98; 1.09 |
| Living area (Urban vs. Rural) | 0.82 * | 0.31; 0.91 | 0.91 * | 0.43; 0.98 |
| Type of cancer (Colon cancer vs. Stomach cancer) | 0.32 * | 0.11; 0.92 | 0.43 * | 0.16; 0.95 |
| Weight | 0.97 | 0.88; 1.06 | 0.99 | 0.90; 1.09 |
| Bone mass | 1.02 | 0.40; 2.65 | 1.45 | 0.58; 3.64 |
| Fat mass | 0.94 | 0.85; 1.03 | 0.92 | 0.84; 1.03 |
| MUAC | 0.98 | 0.82; 1.18 | 0.94 | 0.75; 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huong, L.T.; Phuong, D.T.; Anh, D.K.; Toi, P.L.; Anh, N.L.T.; Huy, T.L.; Linh, N.T. Nutritional Intervention Improves Nutrition Outcomes in Stomach and Colon Cancer Patients Receiving Chemotherapy: Finding from a Quasi-Experiment in Vietnam. Healthcare 2021, 9, 843. https://doi.org/10.3390/healthcare9070843
Huong LT, Phuong DT, Anh DK, Toi PL, Anh NLT, Huy TL, Linh NT. Nutritional Intervention Improves Nutrition Outcomes in Stomach and Colon Cancer Patients Receiving Chemotherapy: Finding from a Quasi-Experiment in Vietnam. Healthcare. 2021; 9(7):843. https://doi.org/10.3390/healthcare9070843
Chicago/Turabian StyleHuong, Le Thi, Duong Thi Phuong, Dang Kim Anh, Phung Lam Toi, Nguyen Le Tuan Anh, Trinh Le Huy, and Nguyen Thuy Linh. 2021. "Nutritional Intervention Improves Nutrition Outcomes in Stomach and Colon Cancer Patients Receiving Chemotherapy: Finding from a Quasi-Experiment in Vietnam" Healthcare 9, no. 7: 843. https://doi.org/10.3390/healthcare9070843
APA StyleHuong, L. T., Phuong, D. T., Anh, D. K., Toi, P. L., Anh, N. L. T., Huy, T. L., & Linh, N. T. (2021). Nutritional Intervention Improves Nutrition Outcomes in Stomach and Colon Cancer Patients Receiving Chemotherapy: Finding from a Quasi-Experiment in Vietnam. Healthcare, 9(7), 843. https://doi.org/10.3390/healthcare9070843






