Acute High Level Noise Exposure Can Cause Physiological Dysfunction in Macaque Monkeys: Insight on the Medical Protection for Special Working Environmental Personnel
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Groups
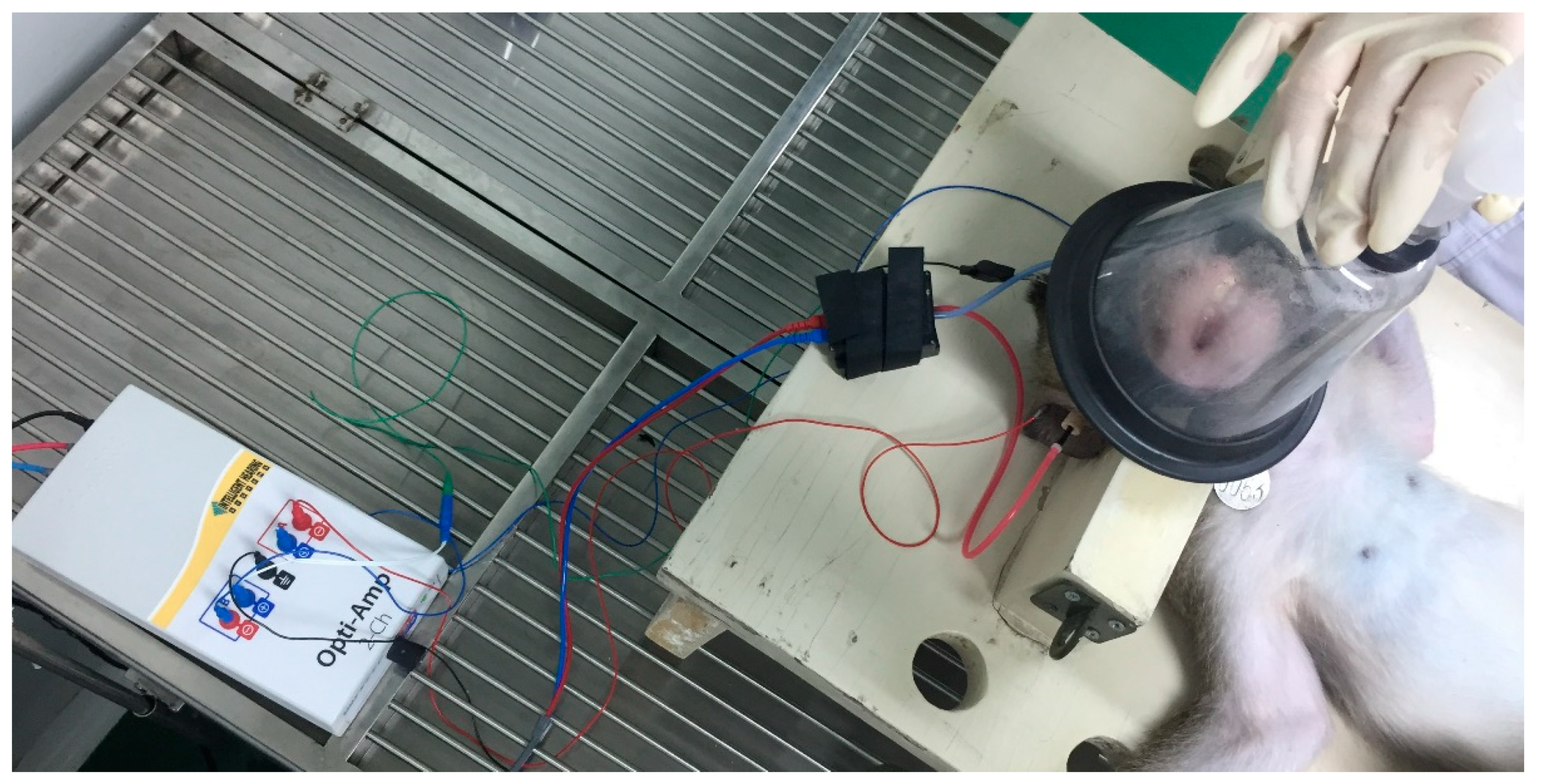
2.2. High Level Noise Exposure
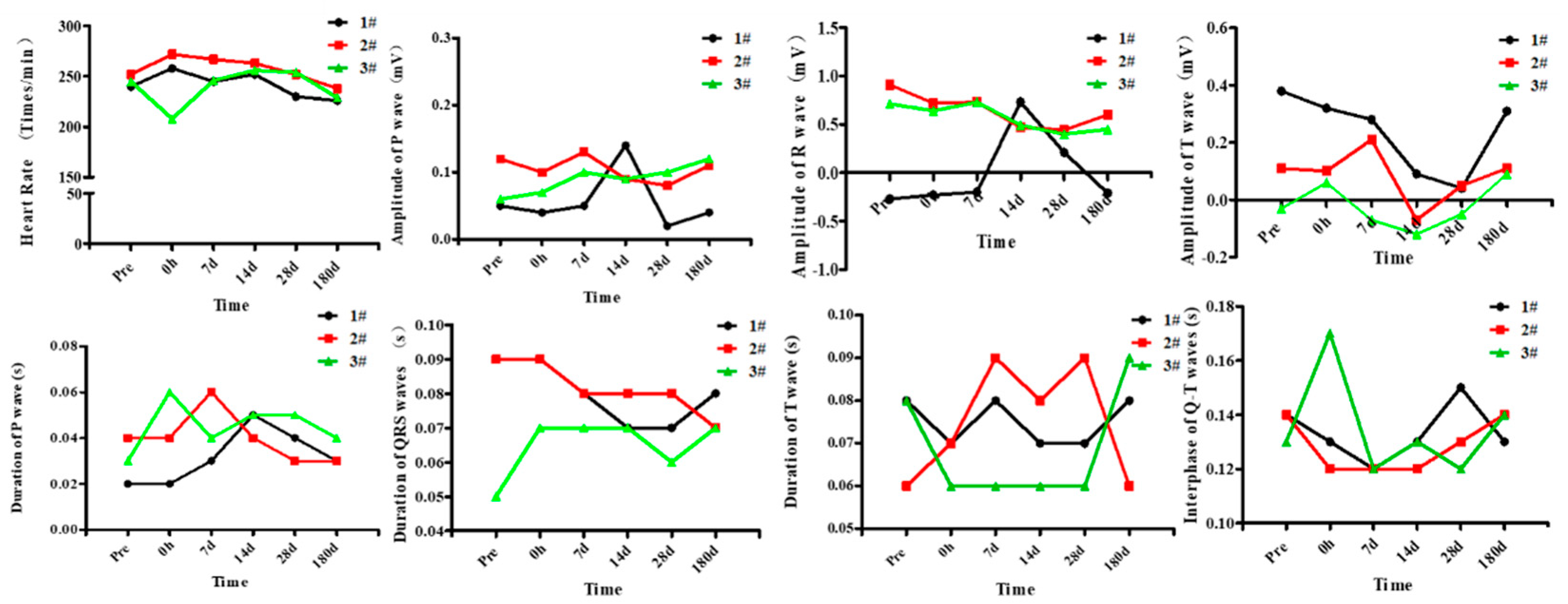
2.3. Measurement of ECG Indicators
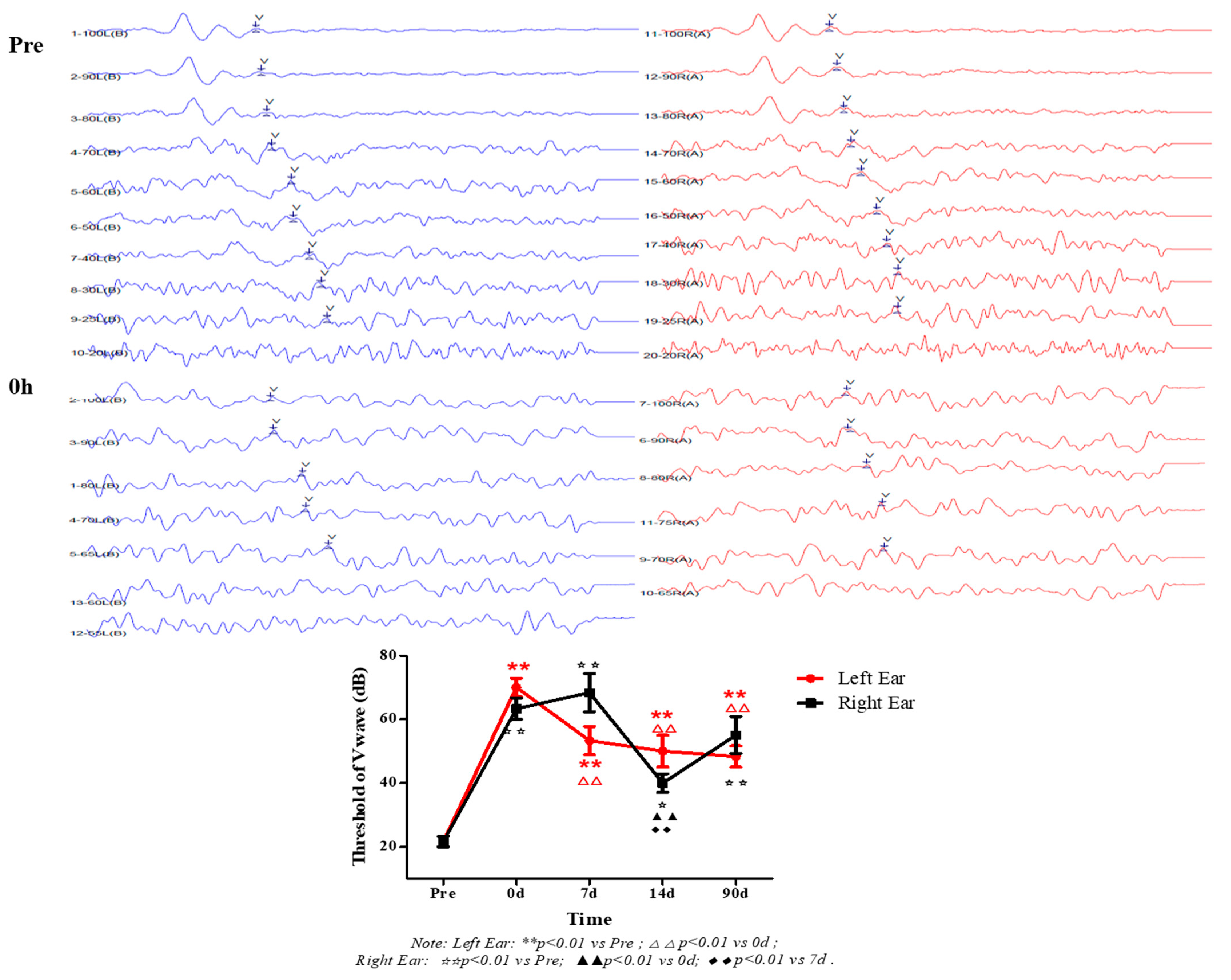
2.4. Measurement of Auditory Brainstem Response
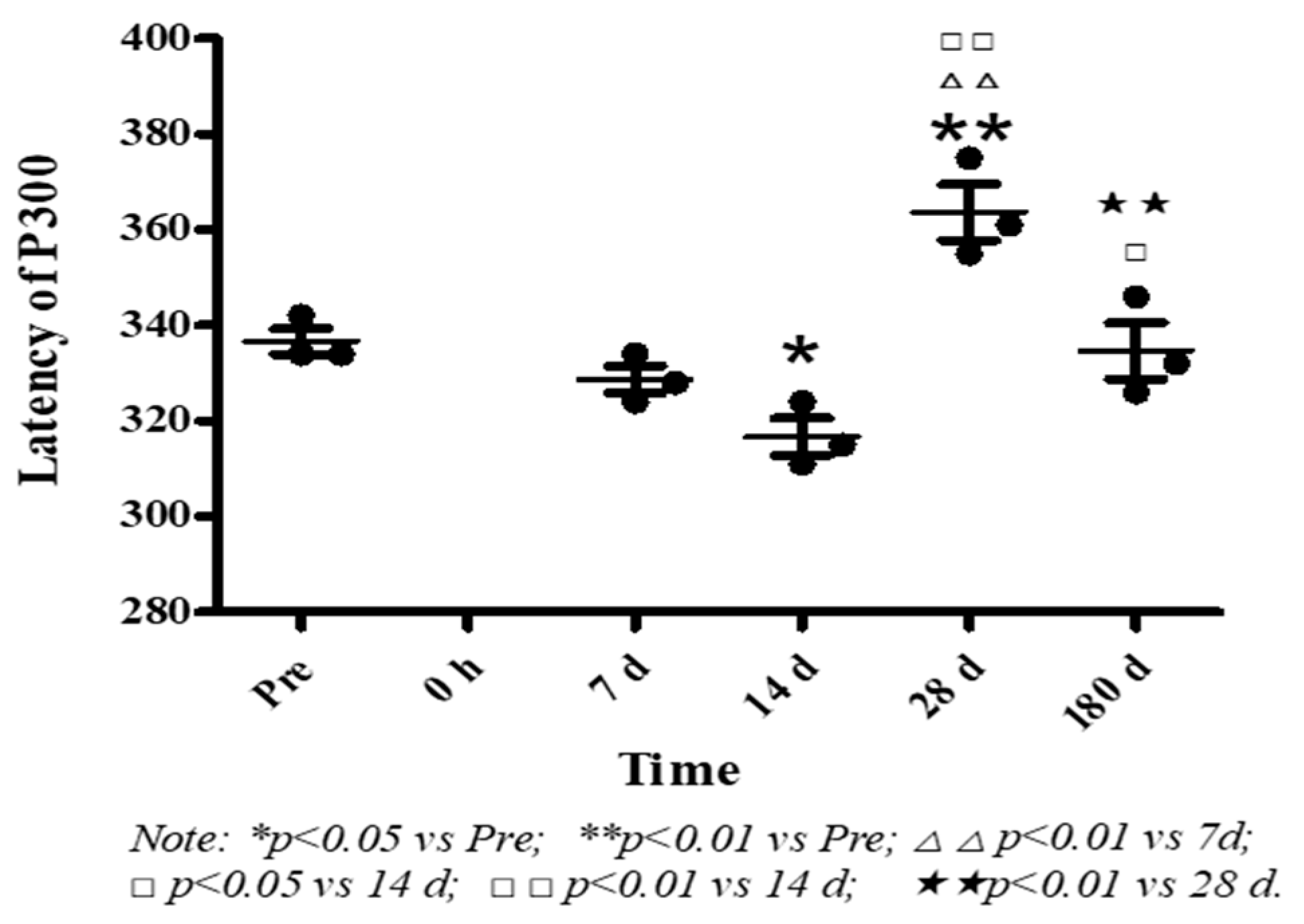
2.5. Collection and Analysis of Auditory P300 Event-Related Potential
2.6. Statistical Analysis
3. Results
3.1. Effects of High Level Noise on Auditory Brainstem Response in Macaque Monkeys
3.2. Effects of High Level Noise on ECG of Macaque Monkeys
3.3. Effects of High Level Noise on Auditory Event-Related Potential (P300) in Macaque Monkeys
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walstead, S.; Deane, G.B. Intensity statistics of very high frequency sound scattered from wind-driven waves. J. Acoust. Soc. Am. 2016, 139, 2784. [Google Scholar] [CrossRef]
- Yehudai, N.; Fink, N.; Shpriz, M.; Marom, T. Acute Acoustic Trauma among Soldiers during an Intense Combat. J. Am. Acad. Audiol. 2017, 28, 436–443. [Google Scholar] [CrossRef]
- Hemel, N.V.; Verzijlbergen, J.F. Acoustic shock waves, a new weapon against angina? Neth. Heart J. 2016, 24. [Google Scholar] [CrossRef]
- Lie, A.; Skogstad, M.; Johannessen, H.K.A.; Tynes, T.; Mehlum, I.S.; Nordby, K.C.; Engdahl, B.; Tambs, K. Occupational noise exposure and hearing: A systematic review. Int. Arch. Occup. Environ. Health 2016, 89, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, E.; Zhang, J.; Chen, S.; Yu, G.; Liu, X.; Peng, C.; Lavin, M.F.; Du, Z.; Shao, H. Relationship between occupational noise exposure and the risk factors of cardiovascular disease in China. Medicine 2018, 97, e11720. [Google Scholar] [CrossRef]
- Dijk, F. Epidemiological research on non-auditory effects of occupational noise exposure. Environ. Int. 1990, 16, 405–409. [Google Scholar] [CrossRef]
- Zajamšek, B.; Hansen, K.L.; Doolan, C.J.; Hansen, C.H. Characterisation of wind farm infrasound and low-frequency noise. J. Sound Vib. 2016, 176–190. [Google Scholar] [CrossRef]
- Oerlemans, S. Effect of wind shear on amplitude modulation of wind turbine noise. Int. J. Aeroacoustics 2015, 14, 715–728. [Google Scholar] [CrossRef]
- Baliatsas, C.; van Kamp, I.; van Poll, R.; Yzermans, J. Health effects from low-frequency noise and infrasound in the general population: Is it time to listen? A systematic review of observational studies. Sci. Total Environ. 2016, 557, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bowley, D.M.; Miles, J.; Muzaffar, J.; Orr, L. The Downrange Acoustic Toolbox: An Active Solution for Combat-Related Acute Acoustic Trauma. J. Spec. Oper. Med. Peer Rev. J. Sof. Med. Prof. 2020, 20, 104–111. [Google Scholar]
- Milford, C.; Theobald, M.R.; Nemitz, E.; Hargreaves, K.J.; Horvath, L.; Raso, J.; Dämmgen, U.; Neftel, A.; Jones, S.K.; Hensen, A. Ammonia fluxes in relation to cutting and fertilization of an intensively managed grassland derived from an inter-comparison of gradient measurements. Biogeosci. Discuss. 2009, 6, 819–834. [Google Scholar] [CrossRef]
- Medina-Garin, D.R.; Dia, A.; Bedubourg, G.; Deparis, X.; Berger, F.; Michel, R. Acute acoustic trauma in the French armed forces during 2007–2014. Noise Health 2016, 18, 297. [Google Scholar] [CrossRef]
- Eggermont, J.J. Auditory brainstem response. Handb Clin Neurol 2019, 160, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Holme, R.; Steel, K. Progressive Hearing Loss and Increased Susceptibility to Noise-Induced Hearing Loss in Mice Carrying a Cdh23 but not a Myo7a Mutation. J. Assoc. Res. Otolaryngol. Jaro 2004, 5, 66. [Google Scholar] [CrossRef][Green Version]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Clinical application of the P300 event-related brain potential. Phys. Med. Rehabil. Clin. N. Am. 2004, 15, 133–161. [Google Scholar] [CrossRef]
- Darley, D.S.; Kellman, R.M. Otologic considerations of blast injury. Disaster Med. Public Health Prep. 2010, 4, 145–152. [Google Scholar] [CrossRef]
- Hammill, T.; Le Prell, C.; Allen, F.R.; Kujawa, S.; Kil, J. Temporary and Permanent Noise-induced Threshold Shifts: A Review of Basic and Clinical Observations. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2016, 37, e271–e275. [Google Scholar] [CrossRef]
- Occupational Safety and Health Administration. Guidelines for Noise Enforcement: Appendix A (OSHA Directive CPL 2–2.35 A); U.S. Department of Labor: Washington, DC, USA, 1983.
- Shah, A.; Ayala, M.; Capra, G.; Fox, D.; Hoffer, M. Otologic assessment of blast and nonblast injury in returning Middle East-deployed service members. Laryngoscope 2014, 124, 272–277. [Google Scholar] [CrossRef]
- Remenschneider, A.K.; Lookabaugh, S.; Aliphas, A.; Brodsky, J.R.; Devaiah, A.K.; Dagher, W.; Grundfast, K.M.; Heman-Ackah, S.E.; Rubin, S.; Sillman, J. Otologic outcomes after blast injury: The Boston Marathon experience. Otol. Neurotol. 2014, 35, 1825–1834. [Google Scholar] [CrossRef]
- Smalt, C.J.; Lacirignola, J.; Davis, S.K.; Calamia, P.T.; Collins, P.P. Noise dosimetry for tactical environments. Hear. Res. 2016, 349, 42–54. [Google Scholar] [CrossRef]
- Zhang, J. Blast-induced tinnitus: Animal models. J. Acoust. Soc. Am. 2019, 146, 3811. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, A.; Ralli, M.; Gilardi, A.; Salvi, R. Occupational Noise: Auditory and Non-Auditory Consequences. Int. J. Environ. Res. Public Health 2020, 17, 8963. [Google Scholar] [CrossRef]
- Muenzel, T.; Schmidt, F.P.; Sebastian, S.; Herzog, J.; Daiber, A.; Sørensen, M. Environmental Noise and the Cardiovascular System. J. Am. Coll. Cardiol. 2018, 71, 688–697. [Google Scholar] [CrossRef]
- Choi, S.H.; Choi, C.H. Noise-Induced Neural Degeneration and Therapeutic Effect of Antioxidant Drugs. J. Audiol. Otol. 2015, 19, 111–119. [Google Scholar] [CrossRef]
- Lightfoot, G. Sloping ABR baselines and the ECG myogenic artefact. Int. J. Audiol. 2017, 56, 612–616. [Google Scholar] [CrossRef]
- Kellerhals, B. Acute acoustic trauma. Acta Oto Laryngol. 1987, 104, 225–233. [Google Scholar]
- Mu, Z.B.; Huang, Y.X.; Zhao, B.M.; Liu, Z.X.; Zhang, B.H.; Wang, Q.L. Effect of explosive noise on gastrointestinal transit and plasma levels of polypeptide hormones. World J. Gastroenterol. 2006, 12, 2284–2287. [Google Scholar] [PubMed]
- Alves-Pereira, M.; Ferreira, J.M.R.; de Melo, J.J.; Motylewski, J.; Branco, N.A.A.C. Noise and the respiratory system. Rev. Port. Pneumol. 2003, 9, 367–379. [Google Scholar] [CrossRef]
- Medina, M.O.; Barocio, A.O.; Montaño, A.F.; Ramos, N.O. Discussing the effects of environmental noise upon the immune system. J. Acoust. Soc. Am. 2010, 128, 2421. [Google Scholar] [CrossRef]
- Ahmadi, R.; Gohari, A.; Hooshmand, M. The effect of noise stress on serum levels of LH, FSH and testosterone in male rats. Feyz J. Kashan Univ. Med Sci. 2015, 19, 24–29. [Google Scholar]
- Aldo, A.F.; Luc, P.J.S.; Wolpert, D.M. Noise in the nervous system. Nat. Rev. Neurosci. 2008, 9, 292–303. [Google Scholar]
- Helfrich, R.F.; Knight, R.T. Cognitive neurophysiology: Event-related potentials. Handb. Clin. Neurol. 2019, 160, 543–558. [Google Scholar]
- Fabiani, M.; Karis, D.; Donchin, E. Effects of mnemonic strategy manipulation in a Von Restorff paradigm. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 22–35. [Google Scholar] [CrossRef]
- Kutas, M.; Mccarthy, G.; Donchin, E. Augmenting mental chronometry: The P300 as a measure of stimulus evaluation time. Science 1977, 197, 792–795. [Google Scholar] [CrossRef]
- Donchin, M.C. A metric for thought: A comparison of P300 latency and reaction time. Science 1981, 211, 77–80. [Google Scholar] [CrossRef]
- Verleger, R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology 1997, 34, 131–156. [Google Scholar] [CrossRef]
- Reinvang, I. Cognitive Event-Related Potentials in Neuropsychological Assessment. Neuropsychol. Rev. 1999, 9, 231–248. [Google Scholar] [CrossRef]
- Mark, K.M.; Murphy, D.; Stevelink, S.A.M.; Fear, N.T. Rates and Associated Factors of Secondary Mental Health Care Utilisation among Ex-Military Personnel in the United States: A Narrative Review. Healthcare 2019, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Babisch, W. Stress hormones in the research on cardiovascular effects of noise. Noise Health 2002, 5, 1–11. [Google Scholar]

| Detection Time | Hear Rate (bpm) | P (mV) | R (mV) | T (mV) | P (s) | QRS (s) | T (s) | Q-T (s) |
|---|---|---|---|---|---|---|---|---|
| Pre | 245.67 + 6.03 | 0.08 ± 0.04 | 0.45 ± 0.63 | 0.15 ± 0.21 | 0.03 ± 0.01 | 0.08 ± 0.02 | 0.07 ± 0.01 | 0.14 ± 0.01 |
| 0 d | 246.00 + 33.65 | 0.07 ± 0.03 | 0.38 ± 0.53 | 0.16 ± 0.14 | 0.04 ± 0.02 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.14 ± 0.03 |
| 7 d | 252.67 + 12.42 | 0.09 ± 0.04 | 0.42 ± 0.54 | 0.14 ± 0.19 | 0.04 ± 0.02 | 0.08 ± 0.01 | 0.08 ± 0.02 | 0.12 ± 0 |
| 14 d | 257.00 + 5.57 | 0.11 ± 0.03 | 0.56 ± 0.14 | (−)0.03 ± 0.11 | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.13 ± 0.01 |
| 28 d | 245.33 + 13.32 | 0.07 ± 0.04 | 0.35 ± 0.12 | 0.01 ± 0.06 | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.02 | 0.13 ± 0.02 |
| 180 d | 231.00 + 6.24 | 0.09 ± 0.04 | 0.28 ± 0.43 | 0.17 ± 0.12 | 0.03 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.02 | 0.14 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, W.; Wang, H.; Zou, Y.; Xu, X.; Yu, N.; Zhu, Y.; Ren, Y.; Ma, L.; Qiu, Y.; Hu, X.; et al. Acute High Level Noise Exposure Can Cause Physiological Dysfunction in Macaque Monkeys: Insight on the Medical Protection for Special Working Environmental Personnel. Healthcare 2021, 9, 840. https://doi.org/10.3390/healthcare9070840
Zhi W, Wang H, Zou Y, Xu X, Yu N, Zhu Y, Ren Y, Ma L, Qiu Y, Hu X, et al. Acute High Level Noise Exposure Can Cause Physiological Dysfunction in Macaque Monkeys: Insight on the Medical Protection for Special Working Environmental Personnel. Healthcare. 2021; 9(7):840. https://doi.org/10.3390/healthcare9070840
Chicago/Turabian StyleZhi, Weijia, Haoyu Wang, Yong Zou, Xinping Xu, Ning Yu, Yuyang Zhu, Yanling Ren, Lizhen Ma, Yefeng Qiu, Xiangjun Hu, and et al. 2021. "Acute High Level Noise Exposure Can Cause Physiological Dysfunction in Macaque Monkeys: Insight on the Medical Protection for Special Working Environmental Personnel" Healthcare 9, no. 7: 840. https://doi.org/10.3390/healthcare9070840
APA StyleZhi, W., Wang, H., Zou, Y., Xu, X., Yu, N., Zhu, Y., Ren, Y., Ma, L., Qiu, Y., Hu, X., & Wang, L. (2021). Acute High Level Noise Exposure Can Cause Physiological Dysfunction in Macaque Monkeys: Insight on the Medical Protection for Special Working Environmental Personnel. Healthcare, 9(7), 840. https://doi.org/10.3390/healthcare9070840






