Smart Protocols for Physical Therapy of Foot Drop Based on Functional Electrical Stimulation: A Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject
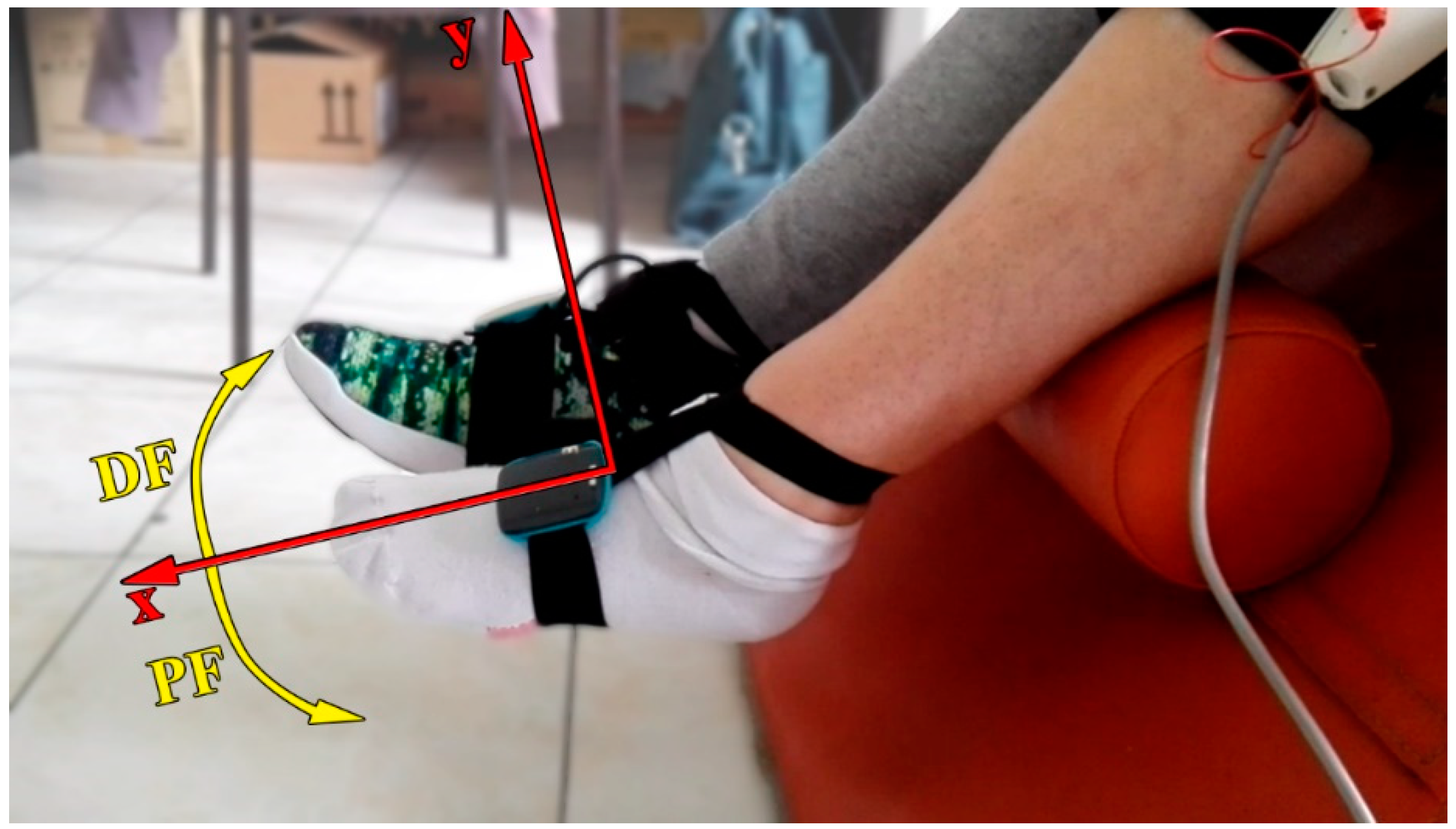
2.2. Hardware
2.3. Smart Protocols
2.4. Treatment
2.5. Assessment
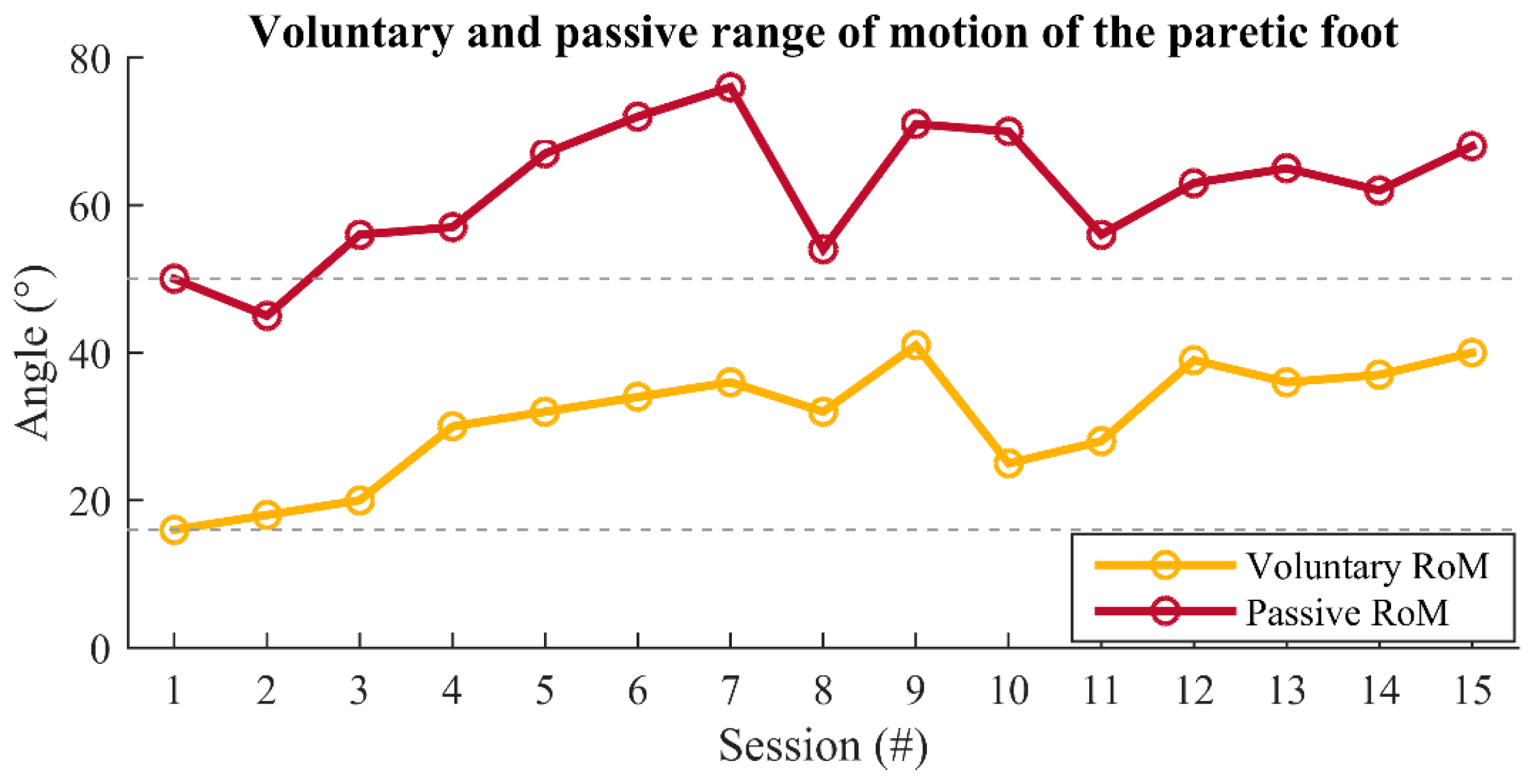
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burridge, J.; McLellan, D. Relation between abnormal patterns of muscle activation and response to common peroneal nerve stimulation in hemiplegia. J. Neurol. Neurosurg. Psychiatry 2000, 69, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Laufer, Y.; Ring, H.; Sprecher, E.; Hausdorff, J.M. Gait in Individuals with Chronic Hemiparesis: One-Year Follow-up of the Effects of a Neuroprosthesis That Ameliorates Foot Drop. J. Neurol. Phys. Ther. 2009, 33, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Burridge, J.H.; Dunkerley, A.L.; Wood, D.E.; Norton, J.A.; Singleton, C.; Swain, I.D. Clinical use of the odstock dropped foot stimulator: Its effect on the speed and effort of walking. Arch. Phys. Med. Rehabil. 1999, 80, 1577–1583. [Google Scholar] [CrossRef]
- Weber, D.J.; Stein, R.B.; Chan, K.M.; Loeb, G.E.; Richmond, F.J.; Rolf, R.; James, K.; Chong, S.L.; Thompson, A.K.; Misiaszek, J. Functional electrical stimulation using microstimulators to correct foot drop: A case study. Can. J. Physiol. Pharmacol. 2004, 82, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Burridge, J.; Taylor, P.; Hagan, S.; Swain, I. Experience of Clinical Use of the Odstock DroppedFoot Stimulator. Artif. Organs 1997, 21, 254–260. [Google Scholar] [CrossRef]
- Stein, R.B.; Everaert, D.G.; Thompson, A.K.; Chong, S.L.; Whittaker, M.; Robertson, J.; Kuether, G. Long-Term Therapeutic and Orthotic Effects of a Foot Drop Stimulator on Walking Performance in Progressive and Nonprogressive Neurological Disorders. Neurorehabilit. Neural Repair 2009, 24, 152–167. [Google Scholar] [CrossRef]
- Stefanovska, A.; Vodovnik, L.; Gros, N.; Rebersek, S.; Acimovic-Janezic, R. FES and spasticity. IEEE Trans. Biomed. Eng. 1989, 36, 738–745. [Google Scholar] [CrossRef]
- Sheffler, L.R.; Bailey, S.N.; Wilson, R.D.; Chae, J. Spatiotemporal, kinematic, and kinetic effects of a peroneal nerve stimulator versus an ankle foot orthosis in hemiparetic gait. Neurorehabilit. Neural Repair 2013, 27, 403–410. [Google Scholar] [CrossRef]
- Ring, H.; Treger, I.; Gruendlinger, L.; Hausdorff, J.M. Neuroprosthesis for Footdrop Compared with an Ankle-Foot Orthosis: Effects on Postural Control during Walking. J. Stroke Cerebrovasc. Dis. 2009, 18, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cozean, C.; Pease, W.S.; Hubbell, S. Biofeedback and functional electric stimulation in stroke rehabilitation. Arch. Phys. Med. Rehabil. 1988, 69, 401–405. [Google Scholar] [PubMed]
- Teixeira-Salmela, L.F.; Nadeau, S.; Mcbride, I.; Olney, S.J. Effects of muscle strengthening and physical conditioning training on temporal, kinematic and kinetic variables during gait in chronic stroke survivors. J. Rehabil. Med. 2001, 33, 53–60. [Google Scholar] [PubMed]
- Kerrigan, D.C.; Karvosky, M.E.; Riley, P.O. Spastic paretic stiff-legged gait: Joint kinetics. Am. J. Phys. Med. Rehabil. 2001, 80, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.L.; Hall, A.L.; Kautz, S.A.; Neptune, R.R. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J. Biomech. 2010, 43, 2348–2355. [Google Scholar] [CrossRef]
- Arch, E.S.; Reisman, D.S. Passive-Dynamic Ankle-Foot Orthoses with Personalized Bending Stiffness Can Enhance Net Plantarflexor Function for Individuals Poststroke. JPO J. Prosthet. Orthot. 2016, 28, 60–67. [Google Scholar] [CrossRef]
- Perez, M.A.; Lungholt, B.K.S.; Nyborg, K.; Nielsen, J.B. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp. Brain Res. 2004, 159, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J. Postinfarct Cortical Plasticity and Behavioral Recovery. Stroke 2007, 38, 840–845. [Google Scholar] [CrossRef]
- Keith, R.A. Treatment strength in rehabilitation. Arch. Phys. Med. Rehabil. 1997, 78, 1298–1304. [Google Scholar] [CrossRef]
- Duncan, P.W. Synthesis of Intervention Trials To Improve Motor Recovery following Stroke. Top. Stroke Rehabil. 1997, 3, 1–20. [Google Scholar] [CrossRef]
- Cauraugh, J.H.; Summers, J.J. Neural plasticity and bilateral movements: A rehabilitation approach for chronic stroke. Prog. Neurobiol. 2005, 75, 309–320. [Google Scholar] [CrossRef]
- Burgar, C.G.; Lum, P.S.; Shor, P.C.; Van Der Loos, H.F.M. Development of robots for rehabilitation therapy: The Palo Alto VA/Stanford experience. J. Rehabil. Res. Dev. 2001, 37, 663–674. [Google Scholar]
- Whitall, J.; Waller, S.M.; Silver, K.H.C.; Macko, R.F. Repetitive Bilateral Arm Training with Rhythmic Auditory Cueing Improves Motor Function in Chronic Hemiparetic Stroke. Stroke 2000, 31, 2390–2395. [Google Scholar] [CrossRef]
- Knutson, J.S.; Harley, M.Y.; Hisel, T.Z.; Chae, J. Improving hand function in stroke survivors: A pilot study of contralaterally controlled functional electric stimulation in chronic hemiplegia. Arch. Phys. Med. Rehabil. 2007, 88, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Knutson, J.S.; Harley, M.Y.; Hisel, T.Z.; Hogan, S.D.; Maloney, M.M.; Chae, J. Contralaterally controlled functional electrical stimulation for upper extremity hemiplegia: An early-phase randomized clinical trial in subacute stroke patients. Neurorehabil. Neural Repair 2012, 26, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Knutson, J.S.; Gunzler, D.D.; Wilson, R.D.; Chae, J. Contralaterally Controlled Functional Electrical Stimulation Improves Hand Dexterity in Chronic Hemiparesis. Stroke 2016, 47, 2596–2602. [Google Scholar] [CrossRef]
- Chan, M.K.-L.; Tong, R.K.-Y.; Chung, K.Y.-K. Bilateral Upper Limb Training with Functional Electric Stimulation in Patients with Chronic Stroke. Neurorehabilit. Neural Repair 2008, 23, 357–365. [Google Scholar] [CrossRef]
- Knutson, J.S.; Hansen, K.; Nagy, J.; Bailey, S.N.; Gunzler, D.D.; Sheffler, L.R.; Chae, J. Contralaterally controlled neuromuscular electrical stimulation for recovery of ankle dorsiflexion: A pilot randomized controlled trial in chronic stroke patients. Am. J. Phys. Med. Rehabil. Assoc. Acad. Phys. 2013, 92, 656. [Google Scholar] [CrossRef] [PubMed]
- Kesar, T.M.; Perumal, R.; Reisman, D.S.; Jancosko, A.; Rudolph, K.S.; Higginson, J.S.; Binder-Macleod, S.A. Functional Electrical Stimulation of Ankle Plantarflexor and Dorsiflexor Muscles. Stroke 2009, 40, 3821–3827. [Google Scholar] [CrossRef]
- Bae, S.; Lee, J.; Lee, B.-H. Effect of an EMG–FES Interface on Ankle Joint Training Combined with Real-Time Feedback on Balance and Gait in Patients with Stroke Hemiparesis. Healthcare 2020, 8, 292. [Google Scholar] [CrossRef]
- Lotze, M.; Braun, C.; Birbaumer, N.; Anders, S.; Cohen, L.G. Motor learning elicited by voluntary drive. Brain 2003, 126, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index: A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Malešević, J.; Malešević, N.; Bijelić, G.; Keller, T.; Konstantinović, L. Multi-pad stimulation device for treating foot drop: Case study. In Proceedings of the 2014 IEEE 19th International Functional Electrical Stimulation Society Annual Conference (IFESS), Kuala Lumpur, Malaysia, 17–19 September 2014; pp. 1–4. [Google Scholar]
- Malešević, N.M.; Maneski, L.Z.P.; Ilić, V.; Jorgovanović, N.; Bijelić, G.; Keller, T.; Popović, D.B. A multi-pad electrode based functional electrical stimulation system for restoration of grasp. J. Neuroeng. Rehabil. 2012, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Liu, H.; Yan, T.; Jin, D.; He, X.; Zheng, X.; Xu, S.; Tan, C. The Effectiveness of Functional Electrical Stimulation Based on a Normal Gait Pattern on Subjects with Early Stroke: A Randomized Controlled Trial. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Rozman, J.; Sovinec, B.; Trlep, M.; Zorko, B. Multielectrode spiral cuff for ordered and reversed activation of nerve fibres. J. Biomed. Eng. 1993, 15, 113–120. [Google Scholar] [CrossRef]
- Brurok, B.; Tãrhaug, T.; Karlsen, T.; Leivseth, G.; Helgerud, J.; Hoff, J. Effect of lower extremity functional electrical stimulation pulsed isometric contractions on arm cycling peak oxygen uptake in spinal cord injured individuals. J. Rehabil. Med. 2013, 45, 254–259. [Google Scholar] [CrossRef]
- Malešević, J.; Dujović, S.D.; Savić, A.M.; Konstantinović, L.; Vidaković, A.; Bijelić, G.; Malešević, N.; Keller, T. A decision support system for electrode shaping in multi-pad FES foot drop correction. J. Neuroeng. Rehabil. 2017, 14, 1–14. [Google Scholar] [CrossRef]
- Peurala, S.H.; Tarkka, I.M.; Pitkänen, K.; Sivenius, J. The Effectiveness of Body Weight-Supported Gait Training and Floor Walking in Patients With Chronic Stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1557–1564. [Google Scholar] [CrossRef]
- Thompson, A.K.; Stein, R.B. Short-term effects of functional electrical stimulation on motor-evoked potentials in ankle flexor and extensor muscles. Exp. Brain Res. 2004, 159, 491–500. [Google Scholar] [CrossRef]
- Tuck, K. Tilt sensing using linear accelerometers. In Freescale Semiconductor Application Note AN3107; Freescale Semiconductor Inc.: Austin, TX, USA, 2007. [Google Scholar]
- Biernaskie, J.; Chernenko, G.; Corbett, D. Efficacy of Rehabilitative Experience Declines with Time after Focal Ischemic Brain Injury. J. Neurosci. 2004, 24, 1245–1254. [Google Scholar] [CrossRef]
- Lee, R.G.; van Donkelaar, P. Mechanisms underlying functional recovery following stroke. Can. J. Neurol. Sci. 1995, 22, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Shea, C.H.; Kohl, R.M. Composition of Practice: Influence on the Retention of Motor Skills. Res. Q. Exerc. Sport 1991, 62, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, R.E. Motor learning following unilateral stroke. Arch. Phys. Med. Rehabil. 1996, 77, 811–815. [Google Scholar] [CrossRef]
- Sabut, S.K.; Bhattacharya, S.D.; Manjunatha, M. Functional Electrical Stimulation on Improving Foot Drop Gait in Poststroke Rehabilitation: A Review of its Technology and Clinical Efficacy. Crit. Rev. Biomed. Eng. 2013, 41, 149–160. [Google Scholar] [CrossRef]
- Merletti, R.; Andina, A.; Galante, M.; Furlan, I. Clinical experience of electronic peroneal stimulators in 50 hemiparetic patients. Scand. J. Rehabil. Med. 1979, 11, 111–121. [Google Scholar]
- Kafri, M.; Laufer, Y. Therapeutic Effects of Functional Electrical Stimulation on Gait in Individuals Post-Stroke. Ann. Biomed. Eng. 2014, 43, 451–466. [Google Scholar] [CrossRef]
- Dobkin, B.H.; Firestine, A.; West, M.; Saremi, K.; Woods, R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. NeuroImage 2004, 23, 370–381. [Google Scholar] [CrossRef]
- Sabut, S.K.; Sikdar, C.; Kumar, R.; Mahadevappa, M. Functional electrical stimulation of dorsiflexor muscle: Effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation 2011, 29, 393–400. [Google Scholar] [CrossRef]
- Yan, T.; Hui-Chan, C.W.; Li, L.S. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: A randomized placebo-controlled trial. Stroke 2005, 36, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Wagenaar, R.C.; Koelman, T.W.; Lankhorst, G.J.; Koetsier, J.C. Effects of intensity of rehabilitation after stroke: A research synthesis. Stroke 1997, 28, 1550–1556. [Google Scholar] [CrossRef]
- Knutson, J.S.; Chae, J. A novel neuromuscular electrical stimulation treatment for recovery of ankle dorsiflexion in chronic hemiplegia: A case series pilot study. Am. J. Phys. Med. Rehabil. Assoc. Acad. Phys. 2010, 89, 672. [Google Scholar] [CrossRef] [PubMed]
- Salhab, G.; Sarraj, A.R.; Saleh, S. Mirror therapy combined with functional electrical stimulation for rehabilitation of stroke survivors’ ankle dorsiflexion. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4699–4702. [Google Scholar]
- Kelso, J.A. Phase transitions and critical behavior in human bimanual coordination. Am. J. Physiol. Integr. Comp. Physiol. 1984, 246, R1000–R1004. [Google Scholar] [CrossRef]
- Connolly, K.; Stratton, P. Developmental Changes in Associated Movements. Dev. Med. Child Neurol. 2008, 10, 49–56. [Google Scholar] [CrossRef]
- Cincotta, M.; Ziemann, U. Neurophysiology of unimanual motor control and mirror movements. Clin. Neurophysiol. 2008, 119, 744–762. [Google Scholar] [CrossRef]
- Lawrence, D.G.; Kuypers, H.G. The functional organization of the motor system in the monkey: II. The effects of lesions of the descending brain-stem pathways. Brain 1968, 91, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, N.; Xu, J.; Branscheidt, M.; Hertler, B.; Schambra, H.; Widmer, M.; Faria, A.V.; Harran, M.D.; Cortés, J.C.; Kim, N.; et al. Evidence for a subcortical origin of mirror movements after stroke: A longitudinal study. Brain 2018, 141, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Mesci, N.; Ozdemir, F.; Kabayel, D.D.; Tokuc, B. The effects of neuromuscular electrical stimulation on clinical improvement in hemiplegic lower extremity rehabilitation in chronic stroke: A single-blind, randomised, controlled trial. Disabil. Rehabil. 2009, 31, 2047–2054. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Meng, F.; Zhang, Y.; Xu, M.-Y.; Yue, S.-W. Full-movement neuromuscular electrical stimulation improves plantar flexor spasticity and ankle active dorsiflexion in stroke patients: A randomized controlled study. Clin. Rehabil. 2016, 30, 577–586. [Google Scholar] [CrossRef]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef]
- Hesse, S.; Bertelt, C.; Jahnke, M.T.; Schaffrin, A.; Baake, P.; Malezic, M.; Mauritz, K.H. Treadmill Training With Partial Body Weight Support Compared With Physiotherapy in Nonambulatory Hemiparetic Patients. Stroke 1995, 26, 976–981. [Google Scholar] [CrossRef]
- Richards, C.L.; Malouin, F.; Wood-Dauphinee, S.; Williams, J.; Bouchard, J.-P.; Brunet, D. Task-specific physical therapy for optimization of gait recovery in acute stroke patients. Arch. Phys. Med. Rehabil. 1993, 74, 612–620. [Google Scholar] [CrossRef]
- Yekutiel, M.; Guttman, E. A controlled trial of the retraining of the sensory function of the hand in stroke patients. J. Neurol. Neurosurg. Psychiatry 1993, 56, 241–244. [Google Scholar] [CrossRef]
- Dean, C.M.; Shepherd, R.B. Task-Related Training Improves Performance of Seated Reaching Tasks after Stroke. Stroke 1997, 28, 722–728. [Google Scholar] [CrossRef]
- Carr, J.H. Movement Science: Foundations for Physical Therapy in Rehabilitation; Aspen Publishers: New York, NY, USA, 1987. [Google Scholar]
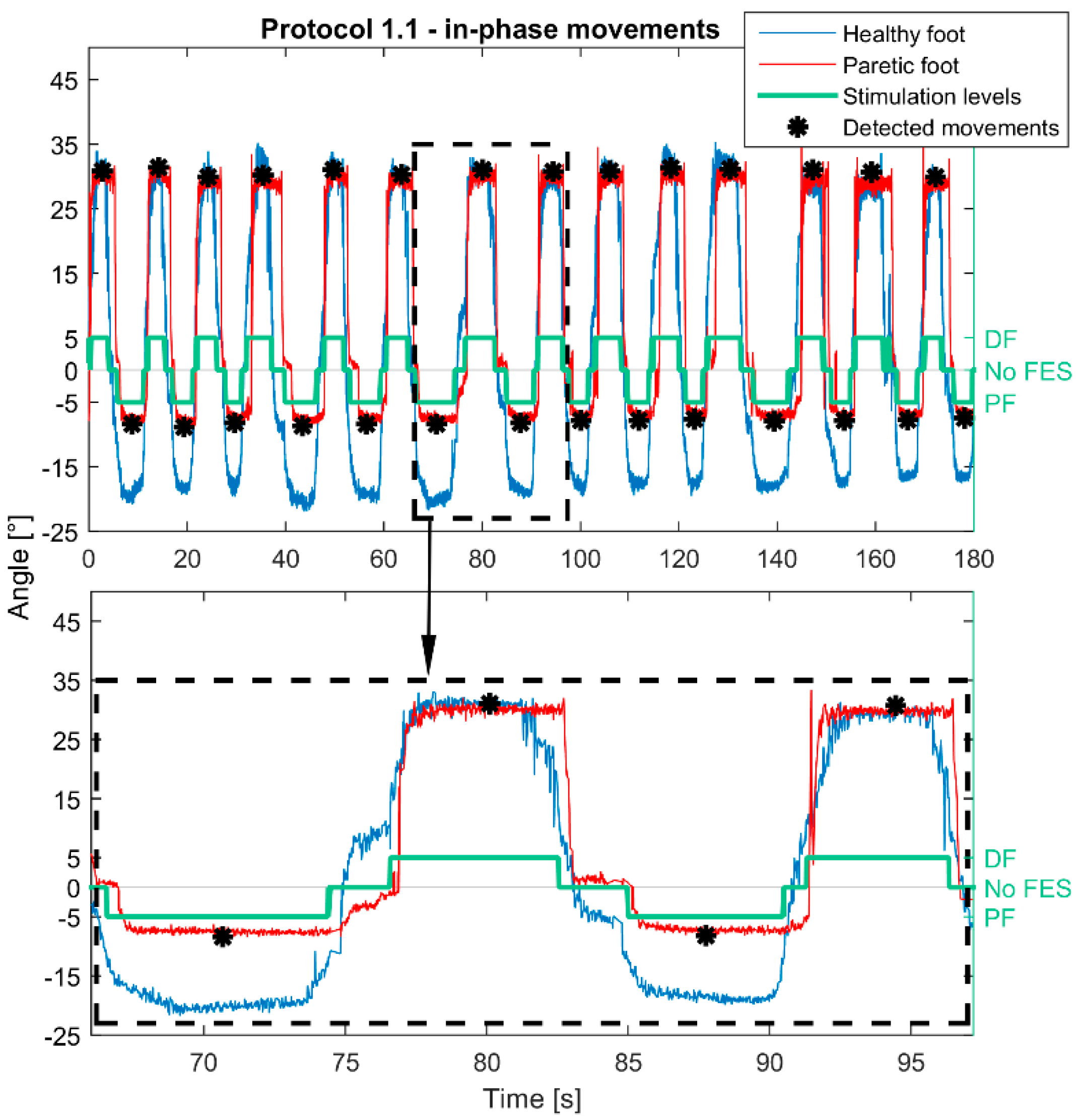



| Protocol 1.1 | ||
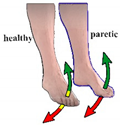 | Control | Contralaterally |
| Phase | In-phase | |
| Stimulation activation | Moving above/below positive (10°)/negative (−10°) threshold | |
| Stimulation termination | Return to the neutral zone | |
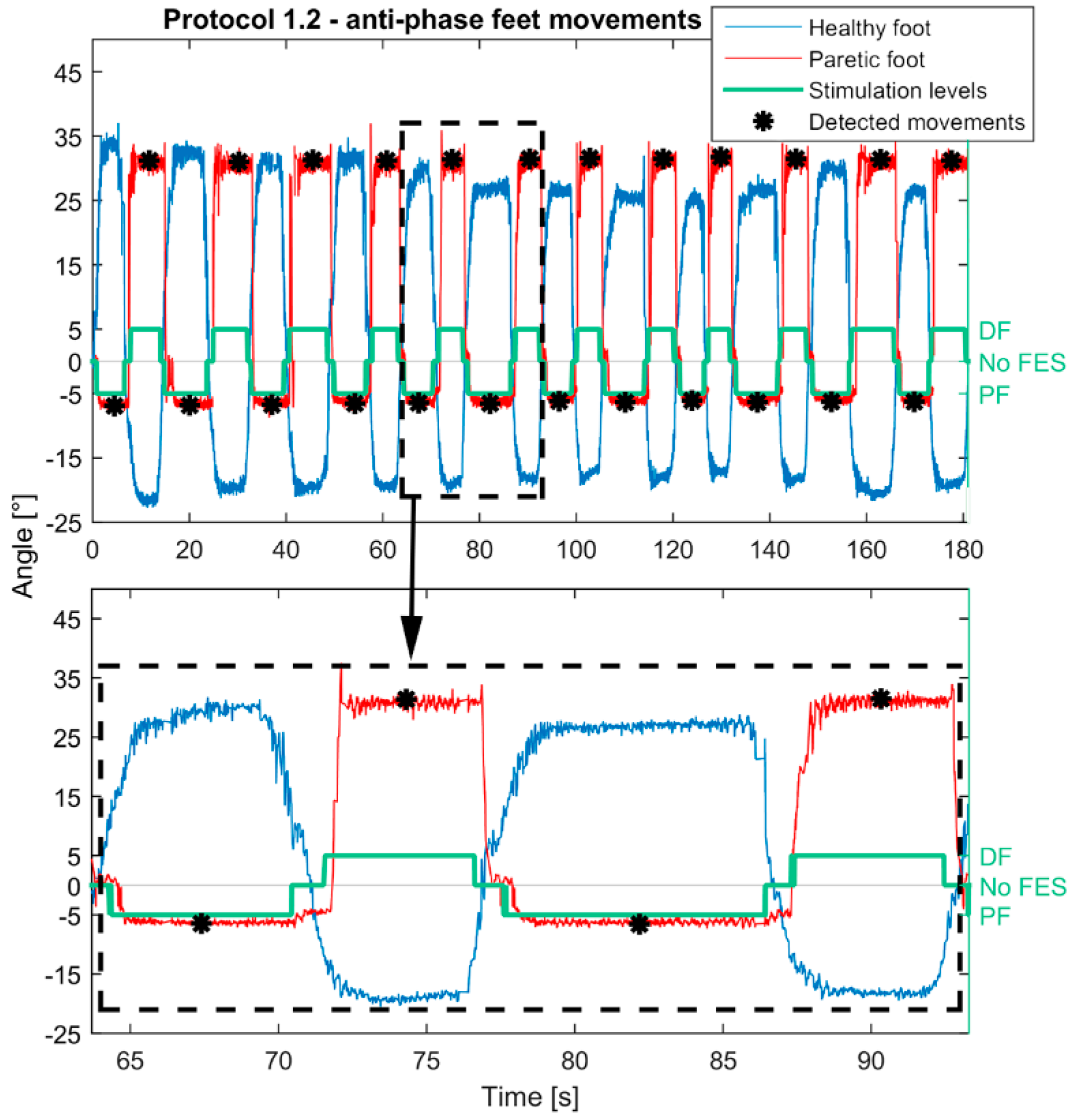
| Protocol 1.2 | ||
 | Control | Contralaterally |
| Phase | Anti-phase | |
| Stimulation activation | Moving above/below positive (10°)/negative (−10°) threshold | |
| Stimulation termination | Return to the neutral zone | |
| Protocol 2.1 | ||
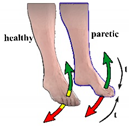 | Control | Contralaterally |
| Phase | In-phase | |
| Stimulation activation | Moving foot above/below positive (10°)/negative (−10°) threshold | |
| Stimulation termination | After a predefined time (3 s). Before subsequent stimulation, the healthy foot must be returned to the neutral zone. | |
| Protocol 2.2 | ||
 | Control | Contralaterally |
| Phase | Anti-phase | |
| Stimulation activation | Moving above/below positive (10°)/negative (−10°) threshold | |
| Stimulation termination | After a predefined time (3 s). Before subsequent stimulation, the healthy foot must be returned to the neutral zone. | |
| Protocol 3.1 | ||
 | Control | Contralaterally |
| Phase | In-phase | |
| Stimulation activation | The range between an upper threshold (10°) and maximal DF of the healthy foot was divided into five equal levels. The maximal DF and PF angles of the healthy foot were recorded at the beginning of the protocol. In the first level (nearest to the upper threshold), current amplitudes of all pads within DF pad configuration were deducted by 4 mA, in the second by 3 mA, etc. Analogous was for PF movements. | |
| Stimulation termination | Return to the neutral zone | |
| Protocol 3.2 | ||
 | Control | Contralaterally |
| Phase | Anti-phase | |
| Stimulation activation | The range between an upper threshold (10°) and maximal DF of the healthy foot was divided into five equal levels. The maximal DF and PF angles of the healthy foot were recorded at the beginning of the protocol. In the first level (nearest to the upper threshold), current amplitudes of all pads within PF pad configuration were deducted by 4 mA, in the second by 3 mA, etc. Analogous was for PF movements. | |
| Stimulation termination | Return to the neutral zone | |
| Protocol 4 | ||
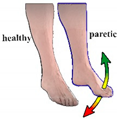 | Control | Voluntary movements of the paretic foot |
| Stimulation activation | The pad configuration for DF was activated when the foot was moved above the positive threshold (3°) and there was no increase in movement amplitude more than 0.5 s. Analogous was for PF movement, where the threshold was set to 2°. | |
| Stimulation termination | After a predefined time (3 s) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malešević, J.; Konstantinović, L.; Bijelić, G.; Malešević, N. Smart Protocols for Physical Therapy of Foot Drop Based on Functional Electrical Stimulation: A Case Study. Healthcare 2021, 9, 502. https://doi.org/10.3390/healthcare9050502
Malešević J, Konstantinović L, Bijelić G, Malešević N. Smart Protocols for Physical Therapy of Foot Drop Based on Functional Electrical Stimulation: A Case Study. Healthcare. 2021; 9(5):502. https://doi.org/10.3390/healthcare9050502
Chicago/Turabian StyleMalešević, Jovana, Ljubica Konstantinović, Goran Bijelić, and Nebojša Malešević. 2021. "Smart Protocols for Physical Therapy of Foot Drop Based on Functional Electrical Stimulation: A Case Study" Healthcare 9, no. 5: 502. https://doi.org/10.3390/healthcare9050502
APA StyleMalešević, J., Konstantinović, L., Bijelić, G., & Malešević, N. (2021). Smart Protocols for Physical Therapy of Foot Drop Based on Functional Electrical Stimulation: A Case Study. Healthcare, 9(5), 502. https://doi.org/10.3390/healthcare9050502







