Toxoplasmic Lymphadenitis Presenting as a Tiny Neck Tumor
Abstract
1. Introduction
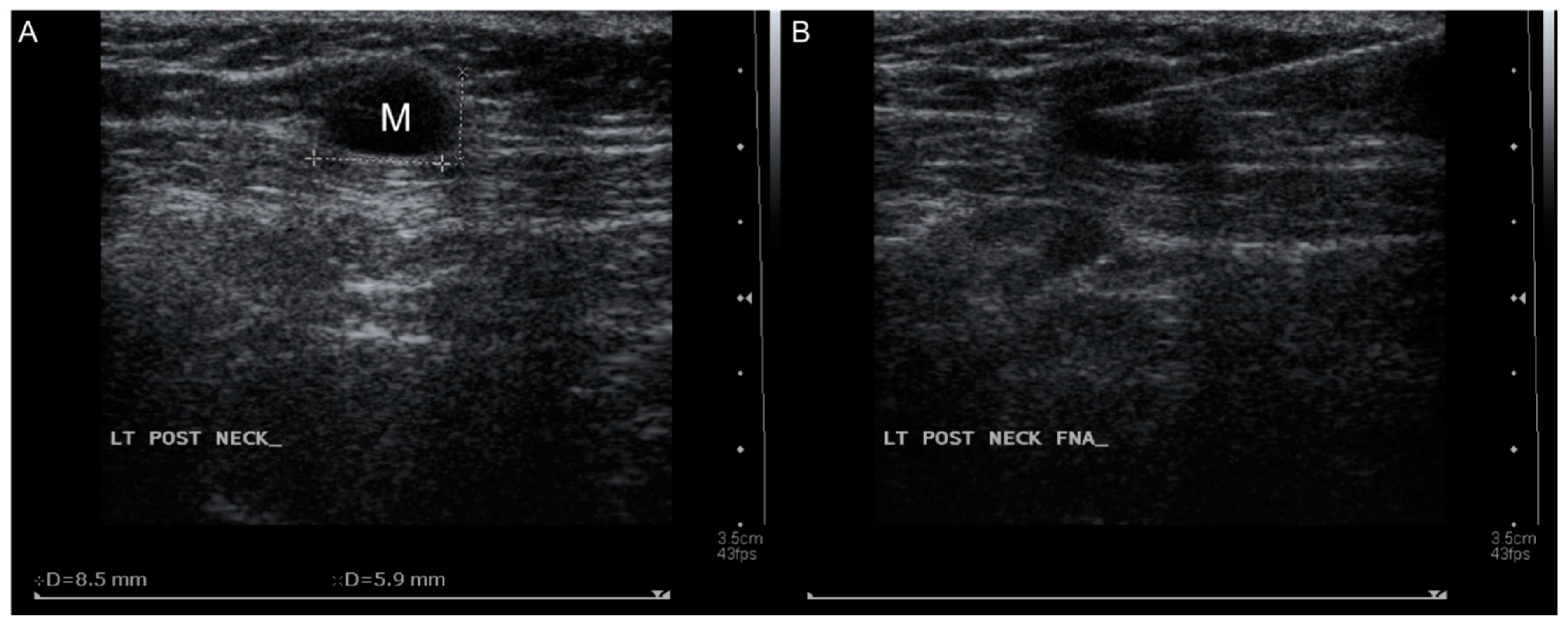
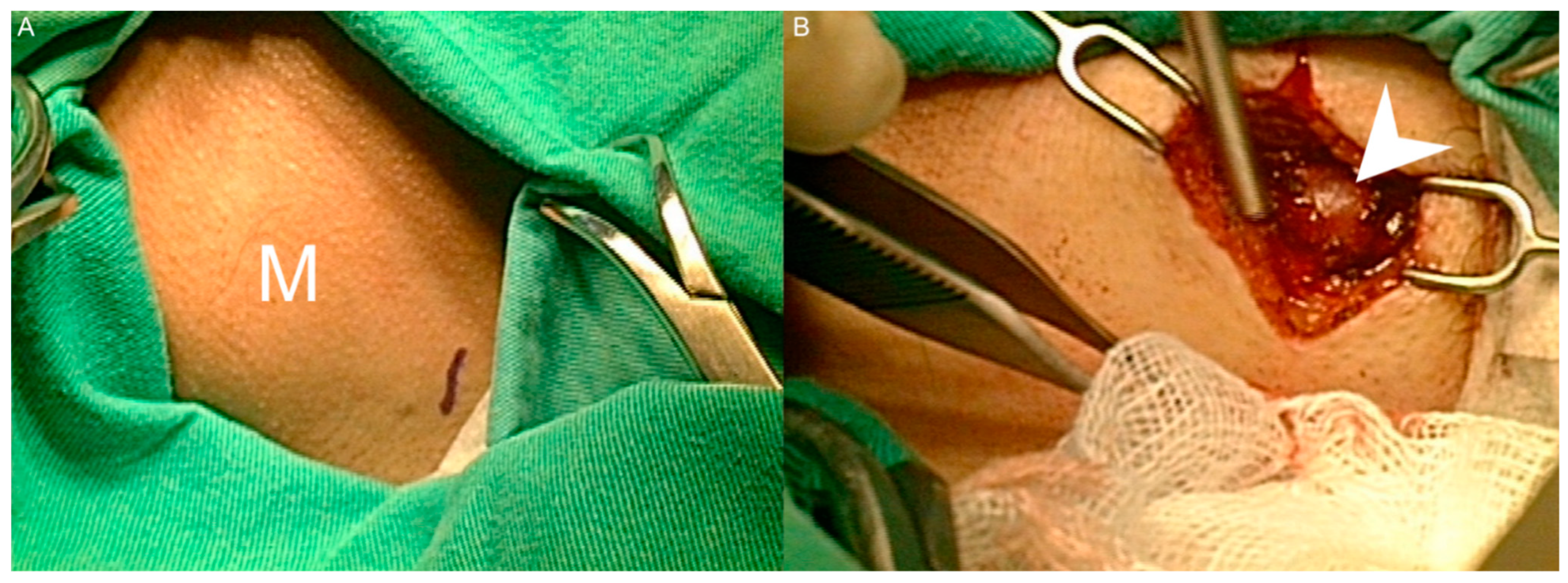
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chiang, T.Y.; Kuo, M.C.; Chen, C.H.; Yang, J.Y.; Kao, C.F.; Ji, D.D.; Fang, C.T. Risk factors for acute Toxoplasma gondii diseases in Taiwan: A population-based case-control study. PLoS ONE 2014, 9, e90880. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, S.; Chrishan Shivanthan, M.; Samaranayake, N.; Rodrigo, C.; Deepika Fernando, S. Antibiotics for human toxoplasmosis: A systematic review of randomized trials. Pathog. Glob. Health 2013, 107, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Aisner, S.C.; Aisner, J.; Moravec, C.; Arnett, E.N. Acquired toxoplasmic lymphadenitis with demonstration of the cyst form. Am. J. Clin. Pathol. 1983, 79, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Giuliodori, M.; Cultrera, R.; Seraceni, S. Detection of clinical-stage specific molecular Toxoplasma gondii gene patterns in patients with toxoplasmic lymphadenitis. J. Med. Microbiol. 2006, 55, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.M.; Chen, Y.Y.; Berry, G.J.; Strickler, J.G.; Dorfman, R.F.; Warnke, R.A. Infrequent detection of Toxoplasma gondii genome in toxoplasmic lymphadenitis: A polymerase chain reaction study. Hum. Pathol. 1992, 23, 154–158. [Google Scholar] [CrossRef]
- Kikuchi, M.; Yoshizumi, T.; Nakamura, H. Necrotizing lymphadenitis: Possible acute toxoplasmic infection. Virchows Arch. A Pathol. Anat. Histol. 1977, 376, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Stansfeld, A.G. The histological diagnosis of toxoplasmic lymphadenitis. J. Clin. Pathol. 1961, 14, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Iqbal, J.; Mansoor, A.; Khan, A.H. Toxoplasmic lymphadenitis—A clinicopathological study. J. Pak. Med. Assoc. 1991, 41, 303–305. [Google Scholar] [PubMed]
- McCabe, R.E.; Brooks, R.G.; Dorfman, R.F.; Remington, J.S. Clinical spectrum in 107 cases of toxoplasmic lymphadenopathy. Rev. Infect. Dis. 1987, 9, 754–774. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Trapuckd, S. Toxoplasma cyst with toxoplasmic lymphadenitis. Hum. Pathol. 1984, 15, 396–397. [Google Scholar] [CrossRef]
- Kojima, M.; Shimizu, K.; Kaba, S.; Itoh, H. Imprint cytology specimen of toxoplasmic lymphadenitis containing numerous large clusters of monocytoid B-cells: Two case reports. Diagn. Cytopathol. 2008, 36, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Kim, M.K.; Sim, J.S. Ultrasound-guided core needle biopsy of cervical lymph nodes in the diagnosis of toxoplasmosis. J. Clin. Ultrasound 2017, 45, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Hara, K.; Saga, S.; Asai, J.; Iijima, S. Two cases of acquired toxoplasmic lymphadenitis. Light and electron microscopic and immunohistochemical studies. Acta Pathol. Jpn. 1988, 38, 1565–1573. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-L.; Chen, J.-R.; Yang, S.-W. Toxoplasmic Lymphadenitis Presenting as a Tiny Neck Tumor. Healthcare 2021, 9, 487. https://doi.org/10.3390/healthcare9050487
Chen S-L, Chen J-R, Yang S-W. Toxoplasmic Lymphadenitis Presenting as a Tiny Neck Tumor. Healthcare. 2021; 9(5):487. https://doi.org/10.3390/healthcare9050487
Chicago/Turabian StyleChen, Shih-Lung, Jim-Ray Chen, and Shih-Wei Yang. 2021. "Toxoplasmic Lymphadenitis Presenting as a Tiny Neck Tumor" Healthcare 9, no. 5: 487. https://doi.org/10.3390/healthcare9050487
APA StyleChen, S.-L., Chen, J.-R., & Yang, S.-W. (2021). Toxoplasmic Lymphadenitis Presenting as a Tiny Neck Tumor. Healthcare, 9(5), 487. https://doi.org/10.3390/healthcare9050487





