Keypoints to Successful Newborn Hearing Screening. Thirty Years of Experience and Innovations
Abstract
:1. Introduction
2. Neonatal Hearing Screening
3. Types of Neonatal Hearing Screening
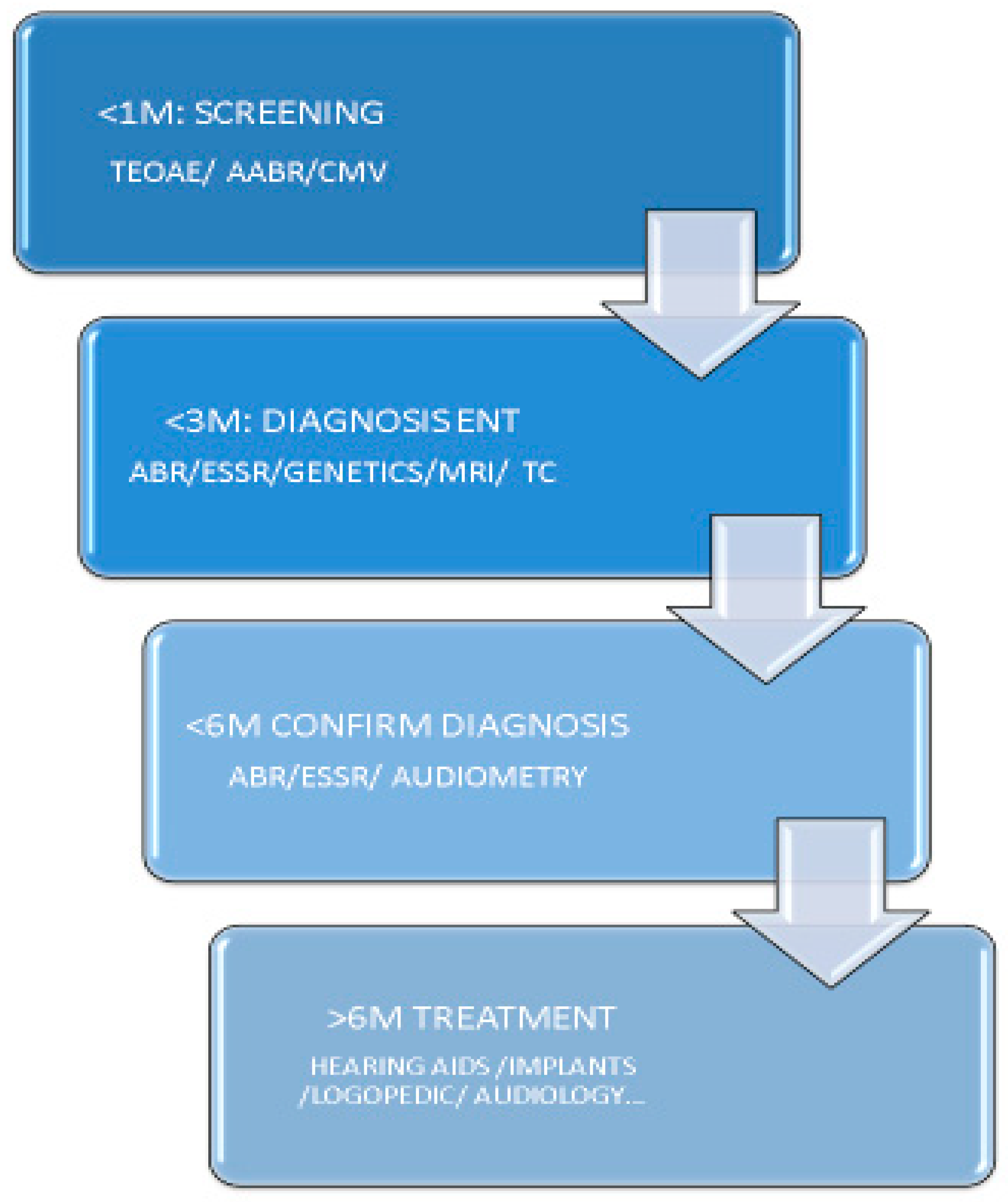
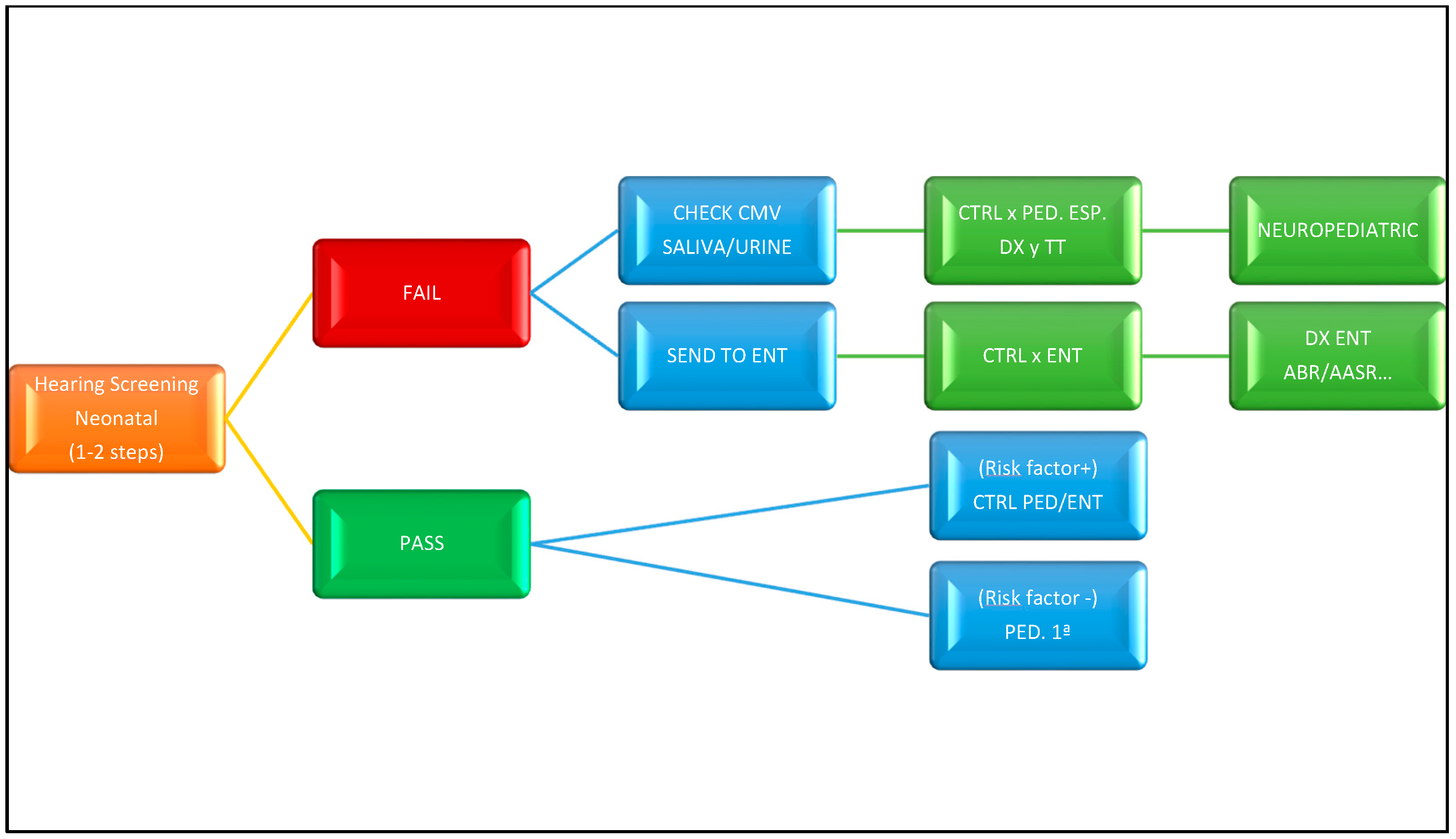
4. Guides and Action Algorithms
5. Some Details of Practical Interest in Hospital Hearing Screening
6. Additional Remarks
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wroblewska-Seniuk, K.E.; Dabrowski, P.; Szyfter, W.; Mazela, J. Universal newborn hearing screening: Methods and results, obstacles, and benefits. Pediatr. Res. 2016, 81, 415–442. [Google Scholar] [CrossRef]
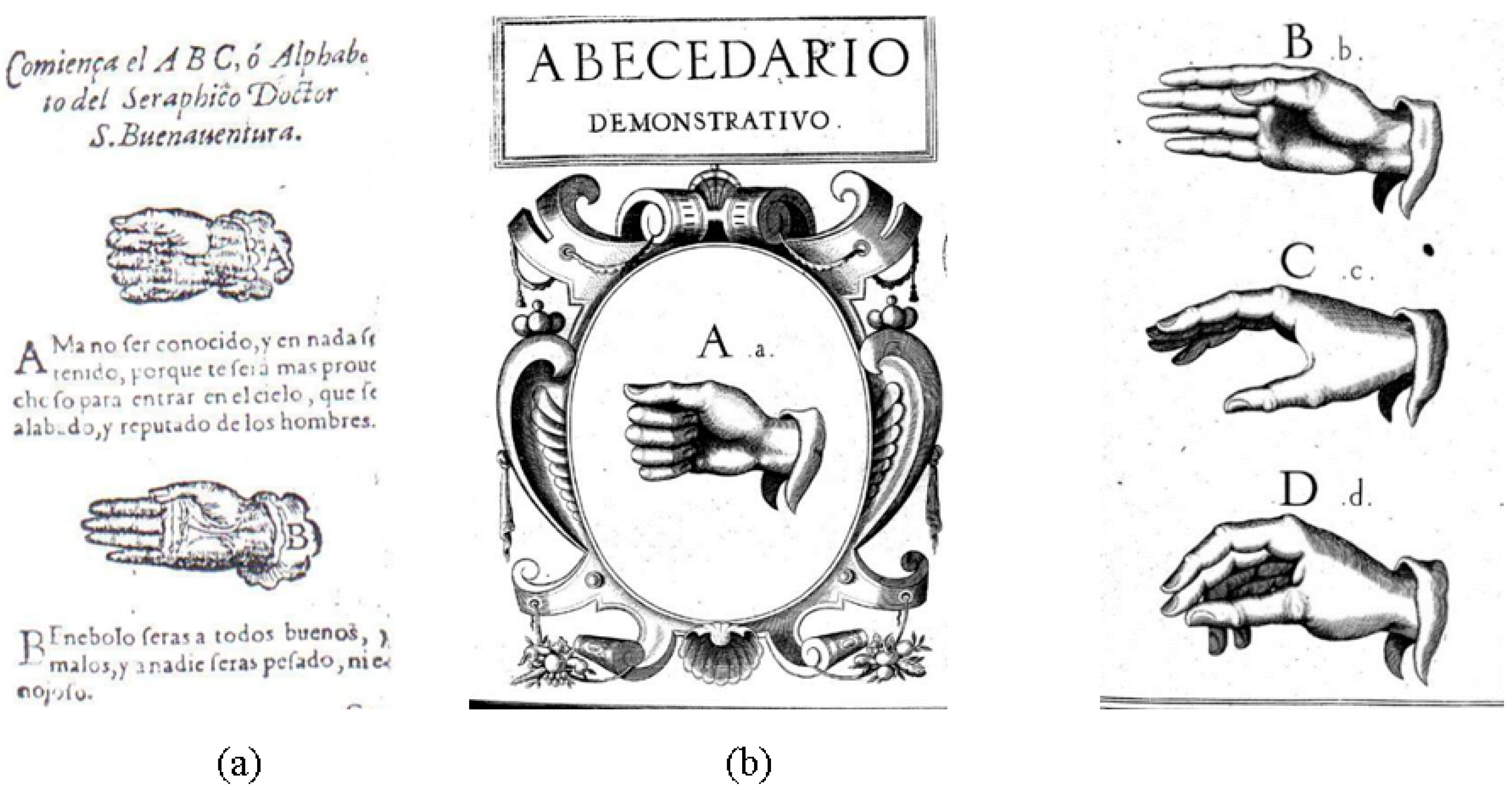
- de Yebra, M. Libro llamado Refugium Infirmorum, muy útil y Provechoso para Todo Genero de Gente, en el Qual se Contienen Muchos Avisos Espirituales para Socorro de los Afligidos Enfermos, y para Ayudar a bien Morir a los que Están en lo Ultimo de su vida; con un Alfabeto de S. Buenaventura para hablar por la mano; Luys Sanchez: Madrid, Spain, 1593. [Google Scholar]
- Oviedo, A. El libro “Refugium Infirmorum” (Madrid, 1593), del Monje Franciscano Fray Melchor de Yebra; Clásicos Cultura Sorda: Berlin, Germany, 2007. [Google Scholar]
- Bonet, J.P. Reduction de las Letras y arte para Enseñar a Ablar a los Mudos; [Simplification of the Letters of the Alphabet and Method of Teaching Deaf-Mutes to Speak]; Francisco Abarca de Angulo: Madrid, Spain, 1620; pp. 126–127. [Google Scholar]
- National Center for Hearing Assessment and Management. Home Page. 2021. Available online: http://www.infanthearing.org/ (accessed on 15 October 2021).
- White, K.R. The Evolution of EHDI: From Concept to Standard of Care. NCHAM ebook, 2021 eds. Available online: http://www.infanthearing.org/ehdi-ebook/2021_ebook/1b%20Chapter1EvolutionEHDI2021.pdf (accessed on 15 October 2021).
- Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. J. Early Hear. Detect. Interv. JEHDI 2019, 4, 1–44. [CrossRef]
- Bussé, A.M.L.; Mackey, A.R.; Hoeve, H.L.J.; Goedegebure, A.; Carr, G.; Uhlén, I.M.; Simonsz, H.J.; EUS€REEN Foundation. Assessment of hearing screening programmes across 47 countries or regions I: Provision of newborn hearing screening. Int. J. Audiol. 2021, 10, 1–10. [Google Scholar]
- Mackey, A.R.; Bussé, A.M.L.; Hoeve, H.L.J.; Goedegebure, A.; Carr, G.; Simonsz, H.J.; Uhlén, I.M.; Foundation, F.T.E. Assessment of hearing screening programmes across 47 countries or regions II: Coverage, referral, follow-up and detection rates from newborn hearing screening. Int. J. Audiol. 2021, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bussé, A.M.L.; Mackey, A.R.; Carr, G.; Hoeve, H.L.J.; Uhlén, I.M.; Goedegebure, A.; Simonsz, H.J.; EUS€REEN Foundation. Assessment of hearing screening programmes across 47 countries or regions III: Provision of childhood hearing screening after the newborn period. Int. J. Audiol. 2021, 9, 1–8. [Google Scholar]
- Sequí, J.M.; Mir, B.; Paredes, C.; Brines, J.; Marco, J. Resultados preliminares en la aplicación de las otoemisiones acústicas provocadas en el periodo neonatal. An. Esp. Pediatr. 1992, 33, 73–75. [Google Scholar]
- Sequí, J.M.; Mir, B.; Paredes, C.; Brines, J.; Marco, J. Resultados de un estudio sobre la presencia de otoemisiones espontáneas en el recién nacido. An. Esp. Pediatr. 1992, 37, 121–125. [Google Scholar]
- Sequí, J.M.; Brines, J.; Mir, B.; Paredes, C.; Marco, J. Estudio comparativo entre otoemisiones provocadas y potenciales auditivos tronculares en el periodo neonatal. An. Esp. Pediatr. 1992, 37, 457–460. [Google Scholar]
- White, K.R. Newborn hearing screening. In Handbook of Clinical Audiology, 7th ed.; Katz, J., Medwetsky, L., Burkard, R., Hood, L., Eds.; Lippincott, Williams, & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Schildroth, A.N.; Karchmer, M.A. Deaf Children in America; College Hill Press: San Diego, CA, USA, 1986. [Google Scholar]
- Yoshinaga-Itano, C.; Sedey, A.L.; Coulter, D.K.; Mehl, A.L. Language of early- and later-identified children with hearing loss. Pediatrics 1998, 102, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Núñez-Batalla, F.; Jáudenes-Casaubón, C.; Sequí-Canet, J.M.; Vivanco-Allende, A.; Zubicaray-Ugarteche, J. Early diagnosis and treatment of unilateral or asymmetrical hearing loss in children: CODEPEH recommendations. Acta Otorrinolaringol. Esp. 2020, 71, 45. [Google Scholar] [CrossRef]
- Trinidad-Ramos, G.; de Aguilar, V.A.; Jaudenes-Casaubón, C.; Núñez-Batalla, F.; Sequí-Canet, J.M. Comisión para la Detección Precoz de la Hipoacusia (CODEPEH). Early hearing detection and intervention: 2010 CODEPEH recommendation. Acta Otorrinolaringol. Esp. 2010, 61, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Maxon, A.B.; White, K.R.; Behrens, T.R.; Vohr, B.R. Referral rates and cost efficiency in a universal newborn hearing screening program using transient evoked otoacoustic emissions (TEOAE). J. Am. Acad. Audiol. 1995, 6, 217–277. [Google Scholar]
- Bubbico, L.; Tognola, G.; Grandori, F. Evolution of Italian Universal Newborn Hearing Screening Programs. Ann. Ig. 2017, 29, 116–122. [Google Scholar] [CrossRef]
- Sequi Canet, J.M.; Sala Langa, M.J.; Collar Del Castillo, J.I. “Resultados de una década de despistaje de hipoacusia en un hospital comarcal.” [Results from ten years newborn hearing screening in a secondary hospital]. An. Pediatr. 2016, 85, 189–196. [Google Scholar] [CrossRef]
- Shang, Y.; Hao, W.; Gao, Z.; Xu, C.; Ru, Y.; Ni, D. An effective compromise between cost and referral rate: A sequential hearing screening protocol using TEOAEs and AABRs for healthy newborns. Int. J. Pediatr. Otorhinolaryngol. 2016, 91, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Vos, B.; Lagasse, R.; Levêque, A. The organisation of universal newborn hearing screening in the Wallonia-BrusselsFederation. B-ENT 2013, 9 (Suppl. 21), 9–15. [Google Scholar]
- Neumann KEuler HA Chadha, S.; White, K.R. A Survey on the Global Status of Newborn and Infant Hearing Screening. J. Early Hear. Detect. Interv. 2020, 5, 63–84. [Google Scholar]
- Pacheco, M.D.C.M.; Canet, J.M.S.; Tobele, M.D. Programas de detección precoz de la hipoacusia infantil en España: Estado de la cuestión. Acta Otorrinolaringol. Española 2021, 72, 37–50. [Google Scholar] [CrossRef]
- Sequí Canet, J.M.; Collar del Castillo, J.; Lorente Mayor, L.; Oller Prieto, A.; Morant Barber, M.; Peñalver Giner, O.; Valdivieso Martínez, R. Hearing screening based on otoacoustic emissions in infants born in secondary-level hospitals: Feasible, efficient and effective. Acta Pediatr. Esp. 2005, 63, 465–470. [Google Scholar]
- Nuñez, F.; Jaudenes, C.; Sequí, J.M.; Vivanco, A.; Zubicaray, J.; (CODEPEH). “Actualización de los programas de detección precoz de la sordera infantil: Nivel 1 detección”. Rev. Española Discapac. 2019, 7, 201–220. [Google Scholar]
- Núñez-Batalla, F.; Jáudenes-Casaubón, C.; Sequí-Canet, J.M.; Vivanco-Allende, A.; Zubicaray-Ugarteche, J. Recomendaciones CODEPEH 2014 para la detección precoz de la hipoacusia diferida. An. Pediatría 2016, 85, 215.e1–215.e6. [Google Scholar] [CrossRef]
- Núñez-Batalla, F.; Jáudenes-Casaubón, C.; Sequí-Canet, J.M.; Vivanco-Allende, A.; Zubicaray-Ugarteche, J.; Lascarro, I.O. Programas de cribado de la hipoacusia congénita en 2020: Recomendaciones CODEPEH. Acta Otorrinolaringol. Española 2021, 72, 312–323. [Google Scholar] [CrossRef]
- Moresco, B.L.; Svoboda, M.D.; Ng, Y.-T. A Quiet Disease with Loud Manifestations. Semin. Pediatr. Neurol. 2017, 26, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Kummer, P.; Marcrum, S.C. Potential Benefit of Selective CMV Testing after Failed Newborn Hearing Screening. Int. J. Neonatal. Screen 2018, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing Loss and Congenital CMV Infection: A Systematic Review. Pediatrics 2014, 134, 972–982. [Google Scholar] [CrossRef] [Green Version]
- Sequi-Canet, J.M.; Sala-Langa, M.J.; Del Castillo, J.I.C. Factores perinatales que influyen en la detección de otoemisiones acústicas en recién nacidos sanos, por parto vaginal, en las primeras 48 horas de vida. Acta Otorrinolaringol. Española 2014, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sequi-Canet, J.M.; Sequi-Sabater, J.M.; Collar-Castillo, J.I.; Orta-Sibu, N. Breastfeeding results in better hearing in newborns compared to bottle-feeding. J. Clin. Transl. Res. 2020, 6, 81–86. [Google Scholar] [PubMed]
- Lu, C.-Y.; Tsao, P.-N.; Ke, Y.-Y.; Lin, Y.-H.; Lin, Y.-H.; Hung, C.-C.; Su, Y.-N.; Hsu, W.-C.; Hsieh, W.-S.; Huang, L.-M.; et al. Concurrent Hearing, Genetic, and Cytomegalovirus Screening in Newborns, Taiwan. J. Pediatr. 2018, 199, 144–150.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Hung, C.-C.; Lin, S.-Y.; Hsieh, W.-S.; Tsao, P.-N.; Lee, C.-N.; Su, Y.-N.; Hsu, C.J. Newborn Genetic Screening for Hearing Impairment: A Preliminary Study at a Tertiary Center. PLoS ONE 2011, 6, e22314. [Google Scholar] [CrossRef] [PubMed]
- Nist-Lund, C.; Pan, B.; Patterson, A.; Asai, Y.; Chen, T.; Zhou, W.; Zhu, H.; Romero, S.; Resnik, J.; Polley, D.B.; et al. Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders. Nat. Commun. 2019, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.P.; Ardinger, H.H.; Pagon, R.A. GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]
- Núñez-Batalla, F.; Jáudenes-Casaubón, C.; Sequí-Canet, J.M.; Vivanco-Allende, A.; Zubicaray-Ugarteche, J.; Cabanillas-Farpón, R. Diagnóstico etiológico de la sordera infantil: Recomendaciones de la CODEPEH. Acta Otorrinolaringol. Española 2017, 68, 43–55. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sequi-Canet, J.M.; Brines-Solanes, J. Keypoints to Successful Newborn Hearing Screening. Thirty Years of Experience and Innovations. Healthcare 2021, 9, 1436. https://doi.org/10.3390/healthcare9111436
Sequi-Canet JM, Brines-Solanes J. Keypoints to Successful Newborn Hearing Screening. Thirty Years of Experience and Innovations. Healthcare. 2021; 9(11):1436. https://doi.org/10.3390/healthcare9111436
Chicago/Turabian StyleSequi-Canet, Jose Miguel, and Juan Brines-Solanes. 2021. "Keypoints to Successful Newborn Hearing Screening. Thirty Years of Experience and Innovations" Healthcare 9, no. 11: 1436. https://doi.org/10.3390/healthcare9111436
APA StyleSequi-Canet, J. M., & Brines-Solanes, J. (2021). Keypoints to Successful Newborn Hearing Screening. Thirty Years of Experience and Innovations. Healthcare, 9(11), 1436. https://doi.org/10.3390/healthcare9111436





