Severity, Progress, and Related Factors of Mood Disorders in Patients with Coronary Artery Disease: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Measurement of Depression and Anxiety
2.3. CPET
2.4. Other Measurements
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Patients
3.2. Related Factors Associated with Depressive and Anxious Moods in Patients with CAD
3.3. Causative Factors of Depressive and Anxious Moods in Patients with CAD
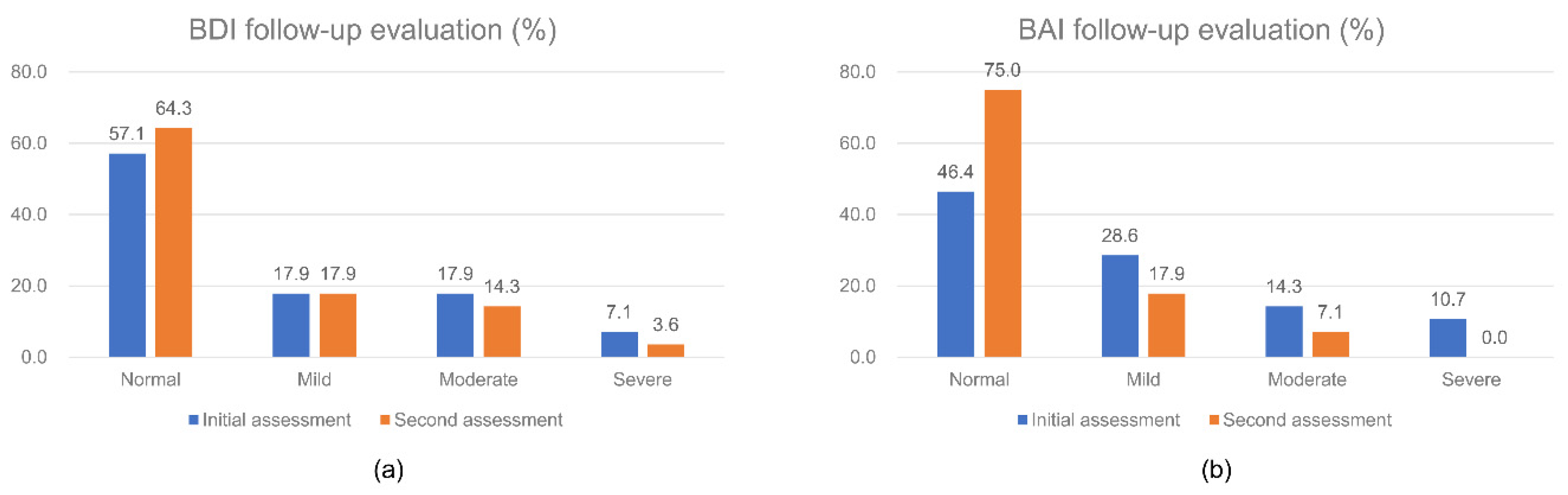
3.4. Follow-Up Data of Depressive and Anxious Moods
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Fact Sheets: The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 4 November 2020).
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A scientific statement from the American Heart Association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Batty, G.D.; Osborn, D.P.; Tynelius, P.; Rasmussen, F. Mental disorders across the adult life course and future coronary heart disease: Evidence for general susceptibility. Circulation 2014, 129, 186–193. [Google Scholar] [CrossRef]
- Carney, R.M.; Freedland, K.E. Depression and coronary heart disease. Nat. Rev. Cardiol. 2017, 14, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Rugulies, R. Depression as a predictor for coronary heart disease. a review and meta-analysis. Am. J. Prev. Med. 2002, 23, 51–61. [Google Scholar] [CrossRef]
- Herbst, S.; Pietrzak, R.H.; Wagner, J.; White, W.B.; Petry, N.M. Lifetime major depression is associated with coronary heart disease in older adults: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom. Med. 2007, 69, 729–734. [Google Scholar] [CrossRef]
- Elamragy, A.A.; Abdelhalim, A.A.; Arafa, M.E.; Baghdady, Y.M. Anxiety and depression relationship with coronary slow flow. PLoS ONE 2019, 14, e0221918. [Google Scholar] [CrossRef]
- Thombs, B.D.; Bass, E.B.; Ford, D.E.; Stewart, K.J.; Tsilidis, K.K.; Patel, U.; Fauerbach, J.A.; Bush, D.E.; Ziegelstein, R.C. Prevalence of depression in survivors of acute myocardial infarction. J. Gen. Intern. Med. 2006, 21, 30–38. [Google Scholar] [CrossRef]
- Egede, L.E. Major depression in individuals with chronic medical disorders: Prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen. Hosp. Psychiatry 2007, 29, 409–416. [Google Scholar] [CrossRef]
- Todaro, J.F.; Shen, B.J.; Raffa, S.D.; Tilkemeier, P.L.; Niaura, R. Prevalence of anxiety disorders in men and women with established coronary heart disease. J. Cardiopulm. Rehabil. Prev. 2007, 27, 86–91. [Google Scholar] [CrossRef]
- Lesperance, F.; Frasure-Smith, N.; Talajic, M.; Bourassa, M.G. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation 2002, 105, 1049–1053. [Google Scholar] [CrossRef]
- Rudisch, B.; Nemeroff, C.B. Epidemiology of comorbid coronary artery disease and depression. Biol. Psychiatry 2003, 54, 227–240. [Google Scholar] [CrossRef]
- Cohen, B.E.; Edmondson, D.; Kronish, I.M. State of the Art Review: Depression, Stress, Anxiety, and Cardiovascular Disease. Am. J. Hypertens 2015, 28, 1295–1302. [Google Scholar] [CrossRef]
- Roest, A.M.; Martens, E.J.; Denollet, J.; de Jonge, P. Prognostic association of anxiety post myocardial infarction with mortality and new cardiac events: A meta-analysis. Psychosom. Med. 2010, 72, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, A.; Blumenthal, J.A.; Davidson, K.W.; Saab, P.G.; Kubzansky, L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: The emerging field of behavioral cardiology. J. Am. Coll. Cardiol. 2005, 45, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Dempe, C.; Junger, J.; Hoppe, S.; Katzenberger, M.L.; Moltner, A.; Ladwig, K.H.; Herzog, W.; Schultz, J.H. Association of anxious and depressive symptoms with medication nonadherence in patients with stable coronary artery disease. J. Psychosom. Res. 2013, 74, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Frasure-Smith, N.; Lespérance, F. Depression and Anxiety as Predictors of 2-Year Cardiac Events in Patients With Stable Coronary Artery Disease. Arch. Gen. Psychiatry 2008, 65, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, J.H.; Bigger, J.T., Jr.; Blumenthal, J.A.; Frasure-Smith, N.; Kaufmann, P.G.; Lesperance, F.; Mark, D.B.; Sheps, D.S.; Taylor, C.B.; Froelicher, E.S. Depression and coronary heart disease: Recommendations for screening, referral, and treatment: A science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: Endorsed by the American Psychiatric Association. Circulation 2008, 118, 1768–1775. [Google Scholar] [CrossRef]
- Walters, P.; Barley, E.A.; Mann, A.; Phillips, R.; Tylee, A. Depression in primary care patients with coronary heart disease: Baseline findings from the UPBEAT UK study. PLoS ONE 2014, 9, e98342. [Google Scholar] [CrossRef]
- De Heer, E.W.; Palacios, J.E.; Adèr, H.J.; van Marwijk, H.W.J.; Tylee, A.; van der Feltz-Cornelis, C.M. Chest pain, depression and anxiety in coronary heart disease: Consequence or cause? A prospective clinical study in primary care. J. Psychosom. Res. 2020, 129, 109891. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck depression inventory–II. Psychol. Assess. 1996. [Google Scholar] [CrossRef]
- Steer, R.A.; Beck, A.T. Beck Anxiety Inventory. In Evaluating Stress: A Book of Resources; American Psychological Association: Washington, DC, USA, 1997. [Google Scholar]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Julian, L.J. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. 2011, 63 (Suppl. 11), S467–S472. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kohli, P.; Gulati, M. An update on exercise stress testing. Curr. Probl. Cardiol. 2012, 37, 177–202. [Google Scholar] [CrossRef]
- Seo, M.H.; Lee, W.-Y.; Kim, S.S.; Kang, J.-H.; Kang, J.-H.; Kim, K.K.; Kim, B.-Y.; Kim, Y.-H.; Kim, W.-J.; Kim, E.M. 2018 Korean Society for the Study of Obesity guideline for the management of obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J. An Overview of Current Physical Activity Recommendations in Primary Care. Korean J. Fam. Med. 2019, 40, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; On, Y.K.; Kim, H.S.; Chae, I.H.; Sohn, D.W.; Oh, B.H.; Lee, M.M.; Park, Y.B.; Choi, Y.S.; Lee, Y.W. Development of Korean activity scale/index (KASI). Korean Circ. J. 2000, 30, 1004–1009. [Google Scholar] [CrossRef]
- AbuRuz, M.E.; Al-Dweik, G.; Al-Akash, H.Y. Checking the moderating effect of perceived control on the relationship between anxiety and postoperative hospital length of stay among coronary artery bypass graft patients. Int. J. Gen. Med. 2019, 12, 79–85. [Google Scholar] [CrossRef]
- Stafford, L.; Berk, M.; Jackson, H.J. Tobacco smoking predicts depression and poorer quality of life in heart disease. BMC Cardiovasc. Disord. 2013, 13, 35. [Google Scholar] [CrossRef]
- Doyle, F.; Rohde, D.; Rutkowska, A.; Morgan, K.; Cousins, G.; McGee, H. Systematic review and meta-analysis of the impact of depression on subsequent smoking cessation in patients with coronary heart disease: 1990 to 2013. Psychosom. Med. 2014, 76, 44–57. [Google Scholar] [CrossRef]
- Frasure-Smith, N.; Lespérance, F.; Juneau, M.; Talajic, M.; Bourassa, M.G. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom. Med. 1999, 61, 26–37. [Google Scholar] [CrossRef]
- Bagherian-Sararoudi, R.; Gilani, B.; Bahrami Ehsan, H.; Sanei, H. Relationship between left ventricular ejection fraction and depression following myocardial infarction: An original article. ARYA Atheroscler. 2013, 9, 16–21. [Google Scholar] [PubMed]
- Ferketich, A.K.; Ferguson, J.P.; Binkley, P.F. Depressive symptoms and inflammation among heart failure patients. Am. Heart J. 2005, 150, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.T.; Hebert, P.R.; Coffey, C.S.; Sedrakyan, A.; Curtis, J.P.; Krumholz, H.M. β-Blocker Therapy and Symptoms of Depression, Fatigue, and Sexual Dysfunction. JAMA 2002, 288, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ranchord, A.M.; Spertus, J.A.; Buchanan, D.M.; Gosch, K.L.; Chan, P.S. Initiation of β-blocker therapy and depression after acute myocardial infarction. Am. Heart J. 2016, 174, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Shankman, S.A.; Nadelson, J.; McGowan, S.K.; Sovari, A.A.; Vidovich, M.I. The predictive power of depression screening procedures for veterans with coronary artery disease. Vasc. Health Risk Manag. 2012, 8, 233–238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mallik, S.; Spertus, J.A.; Reid, K.J.; Krumholz, H.M.; Rumsfeld, J.S.; Weintraub, W.S.; Agarwal, P.; Santra, M.; Bidyasar, S.; Lichtman, J.H.; et al. Depressive Symptoms After Acute Myocardial Infarction: Evidence for Highest Rates in Younger Women. Arch. Intern. Med. 2006, 166, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Spijkerman, T.A.; van den Brink, R.H.; Jansen, J.H.; Crijns, H.J.; Ormel, J. Who is at risk of post-MI depressive symptoms? J. Psychosom. Res. 2005, 58, 425–432. [Google Scholar] [CrossRef]
- AHA. Cardiovascular Disease: Women’s No. 1 Health Threat. Available online: https://www.heart.org/idc/groups/heart-public/@wcm/@adv/documents/downloadable/ucm_472728.pdf (accessed on 4 November 2020).
- Gupta, A.; Wang, Y.; Spertus, J.A.; Geda, M.; Lorenze, N.; Nkonde-Price, C.; D’Onofrio, G.; Lichtman, J.H.; Krumholz, H.M. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J. Am. Coll. Cardiol. 2014, 64, 337–345. [Google Scholar] [CrossRef]
- Ali, S.S.; Khan, S.A.; Khosa, F.; Aneni, E.C.; Jones, A.; St Leger, A.S.; Feiz, H.R.; Cury, R.C.; Agatston, A.S.; Nasir, K. Noninvasive assessment of subclinical atherosclerosis in persons with symptoms of depression. Atherosclerosis 2017, 264, 92–99. [Google Scholar] [CrossRef]
- Stillman, A.N.; Moser, D.J.; Fiedorowicz, J.; Robinson, H.M.; Haynes, W.G. Association of anxiety with resistance vessel dysfunction in human atherosclerosis. Psychosom. Med. 2013, 75, 537–544. [Google Scholar] [CrossRef]
- Richardson, S.; Shaffer, J.A.; Falzon, L.; Krupka, D.; Davidson, K.W.; Edmondson, D. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am. J. Cardiol. 2012, 110, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Kollia, N.; Panagiotakos, D.; Georgousopoulou, E.; Chrysohoou, C.; Yannakoulia, M.; Stefanadis, C.; Chatterji, S.; Haro, J.M.; Papageorgiou, C.; Pitsavos, C. Exploring the path between depression, anxiety and 10-year cardiovascular disease incidence, among apparently healthy Greek middle-aged adults: The ATTICA study. Maturitas 2017, 106, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Van Melle, J.P.; de Jonge, P.; Spijkerman, T.A.; Tijssen, J.G.; Ormel, J.; van Veldhuisen, D.J.; van den Brink, R.H.; van den Berg, M.P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: A meta-analysis. Psychosom. Med. 2004, 66, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.H.; Anderson, L.; Jenkinson, C.E.; Whalley, B.; Rees, K.; Davies, P.; Bennett, P.; Liu, Z.; West, R.; Thompson, D.R.; et al. Psychological interventions for coronary heart disease. Cochrane Database Syst. Rev. 2017, 4, Cd002902. [Google Scholar] [CrossRef]
- Mooney, M.E.; Sofuoglu, M. Bupropion for the treatment of nicotine withdrawal and craving. Expert Rev. Neurother. 2006, 6, 965–981. [Google Scholar] [CrossRef]
- Busch, A.M.; Borrelli, B.; Leventhal, A.M. The Relationship between Smoking and Depression Post-Acute Coronary Syndrome. Curr. Cardiovasc. Risk Rep. 2012, 5, 510–518. [Google Scholar] [CrossRef][Green Version]
- Ski, C.F.; Jelinek, M.; Jackson, A.C.; Murphy, B.M.; Thompson, D.R. Psychosocial interventions for patients with coronary heart disease and depression: A systematic review and meta-analysis. Eur. J. Cardiovasc. Nurs. 2016, 15, 305–316. [Google Scholar] [CrossRef]
- Khawaja, I.S.; Westermeyer, J.J.; Gajwani, P.; Feinstein, R.E. Depression and coronary artery disease: The association, mechanisms, and therapeutic implications. Psychiatry 2009, 6, 38–51. [Google Scholar]
- Del Pino, A.; Gaos, M.T.; Dorta, R.; García, M. Modification of coronary-prone behaviors in coronary patients of low socio-economic status. Span. J. Psychol. 2005, 8, 68–78. [Google Scholar] [CrossRef][Green Version]
| Characteristics | Value |
|---|---|
| Sex (male; female) | 95 (80.5); 23 (19.5) |
| Age | 58.5 ± 11.0 |
| Diagnosis (STEMI; NSTEMI; unstable angina) | 33 (28.0); 32 (27.1); 53 (44.9) |
| Number of diseased vessels | 1.8 ± 0.9 |
| Number of treated vessels | 1.1 ± 0.6 |
| LVEF (%) | 54.8 ± 11.6 |
| Hypertension | 61 (51.7) |
| Diabetes mellitus | 43 (36.4) |
| Dyslipidemia | 38 (32.2) |
| Previous history of CAD | 30 (25.4) |
| Previous history of depression or anxiety | 0 (0.0) |
| Family history of CAD | 13 (11.0) |
| Smoking status (never; smoker; ex-smoker) | 49 (41.5); 44 (37.3); 25 (21.2) |
| Alcohol | 62 (52.5) |
| Physical activity (high; medium; low) | 21 (17.8); 41 (34.7); 56 (47.5) |
| KASI raw score | 43.7 ± 13.7 |
| BMI (normal; overweight; obese; extremely obese) | 32 (27.1); 25 (21.2); 54 (45.8); 7 (5.9) |
| Residence type (married; single) | 100 (84.7); 18 (15.3) |
| Education level of raw years | 12.0 ± 3.6 |
| Working status (working; not working) | 77 (65.3); 41 (34.7) |
| Use of beta-blockers | 60 (50.8) |
| Length of hospital stay | 3.6 ± 2.6 |
| Depressive mood (normal; mild; moderate; severe) | 78 (66.1); 18 (15.3); 16 (13.6); 6 (5.1) |
| Anxious mood (normal; mild; moderate; severe) | 67 (56.8); 31 (26.3); 12 (10.2); 8 (6.8) |
| Depression (+) (n = 40) | Depression (−) (n = 78) | p-Value | Anxiety (+) (n = 51) | Anxiety (−) (n = 67) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Sex | Male | 32 (80.0) | 63 (80.8) | 0.92 | 37 (72.5) | 58 (86.6) | 0.06 |
| Female | 8 (20.0) | 15 (19.2) | 14 (27.5) | 9 (13.4) | |||
| Age | 58.0 ± 13.0 | 58.8 ± 9.8 | 0.72 | 58.2 ± 11.7 | 58.8 ± 10.4 | 0.75 | |
| Diagnosis | STEMI | 15 (37.5) | 18 (23.1) | <0.001 * | 20 (39.2) | 13 (19.4) | 0.02 * |
| NSTEMI | 14 (35.0) | 18 (23.1) | 15 (29.4) | 17 (25.4) | |||
| Unstable angina | 11 (27.5) | 42 (53.8) | 16 (31.4) | 37 (55.2) | |||
| Number of vessels involved | 1.8 ± 0.8 | 1.7 ± 0.8 | 0.48 | 1.9 ± 0.8 | 1.7 ± 0.8 | 0.19 | |
| Number of stent-insertion vessels | 1.0 ± 0.4 | 1.1 ± 0.6 | 0.52 | 1.1 ± 0.6 | 1.1 ± 0.6 | 0.85 | |
| LVEF (%) | 50.6 ± 13.0 | 57.0 ± 10.1 | 0.004 * | 50.3 ± 13.2 | 58.3 ± 8.7 | 0.001 * | |
| Hypertension | 21 (52.5) | 40 (51.3) | 0.90 | 23 (45.1) | 38 (56.7) | 0.21 | |
| Diabetes mellitus | 17 (42.5) | 26 (33.3) | 0.33 | 19 (37.3) | 24 (35.8) | 0.78 | |
| Dyslipidemia | 17 (42.5) | 21 (26.9) | 0.09 | 20 (39.2) | 18 (26.9) | 0.16 | |
| Previous history of CAD | 13 (32.5) | 17 (21.8) | 0.20 | 15 (29.4) | 15 (22.4) | 0.39 | |
| Previous history of depression or anxiety | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - | |
| Family history of CAD | 8 (20.0) | 5 (6.4) | 0.03 * | 6 (11.8) | 7 (10.4) | 0.82 | |
| Smoking status | Never | 12 (30.0) | 37 (47.4) | <0.001 * | 22 (43.1) | 27 (40.3) | 0.43 |
| Smoker | 20 (50.0) | 24 (30.8) | 21 (41.2) | 23 (34.3) | |||
| Ex-smoker | 8 (20.0) | 17 (21.8) | 8 (15.7) | 17 (25.4) | |||
| Alcohol | 22 (55.0) | 40 (51.3) | 0.70 | 25 (49.0) | 37 (55.2) | 0.50 | |
| Physical activity | High | 5 (12.5) | 16 (20.5) | 0.003 * | 10 (19.6) | 11 (16.4) | 0.57 |
| Medium | 12 (30.0) | 29 (37.2) | 15 (29.4) | 26 (38.8) | |||
| Low | 23 (57.5) | 33 (42.3) | 26 (51.0) | 30 (44.8) | |||
| KASI raw score | 39.6 ± 13.5 | 45.8 ± 13.4 | 0.02 * | 39.9 ± 13.7 | 46.6 ± 13.1 | 0.01 * | |
| BMI | Normal | 7 (17.5) | 25 (32.1) | 0.24 | 12 (23.5) | 20 (29.9) | 0.59 |
| Overweight | 10 (25.0) | 15 (19.2) | 13 (25.5) | 12 (17.9) | |||
| Obesity | 19 (47.5) | 35 (44.9) | 22 (43.1) | 32 (47.8) | |||
| Extreme obesity | 4 (10.0) | 3 (3.8) | 4 (7.8) | 3 (4.5) | |||
| Residence type | Married or living with partner | 31 (77.5) | 69 (88.5) | 0.12 | 42 (82.4) | 58 (86.6) | 0.53 |
| Single, separated, or widowed | 9 (22.5) | 9 (11.5) | 9 (17.6) | 9 (13.4) | |||
| Education level raw year | 11.3 ± 3.6 | 12.3 ± 3.6 | 0.07 | 11.8 ± 4.0 | 12.0 ± 3.2 | 0.90 | |
| Working status | Working | 22 (55.0) | 55 (70.5) | 0.09 | 30 (58.8) | 47 (70.1) | 0.20 |
| Not working | 18 (45.0) | 23 (29.5) | 21 (41.2) | 20 (29.9) | |||
| Use of beta-blockers | 28 (70.0) | 32 (41.0) | 0.003 * | 32 (62.7) | 28 (41.8) | 0.02 * | |
| Length of hospital stay | 4.3 ± 3.5 | 3.2 ± 2.0 | 0.02 * | 4.1 ± 2.5 | 3.2 ± 2.7 | 0.002 * | |
| Depression (+) (n = 16) | Depression (−) (n = 25) | p-Value | Anxiety (+) (n = 20) | Anxiety (−) (n = 21) | p-Value | |
|---|---|---|---|---|---|---|
| VO2peak/kg (mL/kg/min) | 25.7 ± 5.0 | 27.2 ± 5.2 | 0.630 | 26.3 ± 5.4 | 27.0 ± 5.0 | 0.664 |
| METs | 7.3 ± 1.4 | 7.8 ± 1.5 | 0.536 | 7.5 ± 1.5 | 7.8 ± 1.4 | 0.551 |
| RPE on Borg’s scale | 15.5 ± 1.1 | 15.2 ± 1.1 | 0.682 | 15.3 ± 1.1 | 15.3 ± 1.2 | 0.816 |
| Peak HR (bpm) | 145.3 ± 19.4 | 153.5 ± 26.2 | 0.162 | 143.6 ± 23.3 | 158.0 ± 23.7 | 0.057 |
| Peak SBP (mmHg) | 172.5 ± 20.9 | 190.2 ± 28.0 | 0.022 * | 176.5 ± 26.0 | 192.4 ± 26.2 | 0.058 |
| Peak DBP (mmHg) | 83.4 ± 10.0 | 84.1 ± 8.8 | 0.807 | 83.2 ± 9.3 | 84.6 ± 9.0 | 0.622 |
| RPP (mmHg·bpm) | 22,837.3 ± 5558.6 | 25,789.1 ± 6127.3 | 0.074 | 23,241.0 ± 6735.6 | 26,388.6 ± 4991.7 | 0.054 |
| AT (mL/min) | 1402.5 ± 233.8 | 1497.3 ± 362.8 | 0.567 | 1408.7 ± 301.3 | 1523.0 ± 348.7 | 0.269 |
| VE/VCO2 | 30.1 ± 7.4 | 26.3 ± 4.1 | 0.101 | 28.3 ± 5.7 | 26.7 ± 5.4 | 0.354 |
| RQ | 1.18 ± 0.09 | 1.21 ± 0.10 | 0.248 | 1.19 ± 0.07 | 1.21 ± 0.11 | 0.375 |
| Univariate Analyses | Multivariate Analyses | |||||
|---|---|---|---|---|---|---|
| Factor | Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value |
| Diagnosis | ||||||
| STEMI | - | - | 0.028 * | |||
| NSTEMI | 0.933 | 0.351–2.483 | 0.890 | |||
| UA | 0.314 | 0.121–0.816 | 0.017 * | |||
| LVEF (%) | 0.953 | 0.921–0.986 | 0.006 * | |||
| Family history of CAD | 3.650 | 1.108–12.023 | 0.033 * | 4.050 | 1.158–14.166 | 0.029 * |
| Smoking status | ||||||
| Smoker | - | - | 0.106 | |||
| Ex-smoker | 0.389 | 0.161–0.939 | 0.036 * | |||
| Non-smoker | 0.565 | 0.202–1.580 | 0.276 | |||
| Physical activity | ||||||
| Low | - | - | 0.273 | |||
| Medium | 0.594 | 0.252–1.400 | 0.234 | |||
| High | 0.448 | 0.144–1.397 | 0.167 | |||
| KASI raw score | 0.967 | 0.939–0.996 | 0.024 * | 0.967 | 0.937–0.997 | 0.034 * |
| Use of beta-blockers | 3.354 | 1.488–7.562 | 0.004 * | 3.022 | 1.295–7.052 | 0.011 * |
| Length of hospital stay | 1.172 | 0.995–1.380 | 0.058 | |||
| Univariate Analyses | Multivariate Analyses | |||||
|---|---|---|---|---|---|---|
| Factor | Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value |
| Diagnosis | ||||||
| STEMI | - | - | 0.022 * | |||
| NSTEMI | 0.574 | 0.214–1.535 | 0.268 | |||
| UA | 0.281 | 0.113–0.700 | 0.006 * | |||
| LVEF (%) | 0.937 | 0.903–0.972 | 0.000 * | 0.940 | 0.906–0.976 | 0.001 * |
| KASI raw score | 0.963 | 0.936–0.991 | 0.010 * | 0.969 | 0.940–0.998 | 0.036 * |
| Use of beta-blockers | 2.346 | 1.112–1.951 | 0.025 * | |||
| Length of hospital stay | 1.149 | 0.974–1.354 | 0.099 | |||
| r (p-Value) | ||
|---|---|---|
| Change values of BDI | LVEF (%) | −0.402 (0.034) |
| Length of hospital stay | 0.576 (0.001) | |
| Change values of BAI | Length of hospital stay | 0.481 (0.010) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Lee, S.C.; Shin, Y.S.; Park, S.; Won, K.B.; Ann, S.H.; Ko, E.J. Severity, Progress, and Related Factors of Mood Disorders in Patients with Coronary Artery Disease: A Retrospective Study. Healthcare 2020, 8, 568. https://doi.org/10.3390/healthcare8040568
Lee C, Lee SC, Shin YS, Park S, Won KB, Ann SH, Ko EJ. Severity, Progress, and Related Factors of Mood Disorders in Patients with Coronary Artery Disease: A Retrospective Study. Healthcare. 2020; 8(4):568. https://doi.org/10.3390/healthcare8040568
Chicago/Turabian StyleLee, Changbae, Sang Cheol Lee, Yeon Seob Shin, Sangwoo Park, Ki Bum Won, Soe Hee Ann, and Eun Jae Ko. 2020. "Severity, Progress, and Related Factors of Mood Disorders in Patients with Coronary Artery Disease: A Retrospective Study" Healthcare 8, no. 4: 568. https://doi.org/10.3390/healthcare8040568
APA StyleLee, C., Lee, S. C., Shin, Y. S., Park, S., Won, K. B., Ann, S. H., & Ko, E. J. (2020). Severity, Progress, and Related Factors of Mood Disorders in Patients with Coronary Artery Disease: A Retrospective Study. Healthcare, 8(4), 568. https://doi.org/10.3390/healthcare8040568






