Design, Assessment, and Validation of a Questionnaire to Estimate Food-Dependent Exercise-Induced Anaphylaxis Prevalence in Latin American Population
Abstract
1. Introduction
2. Materials and Methods
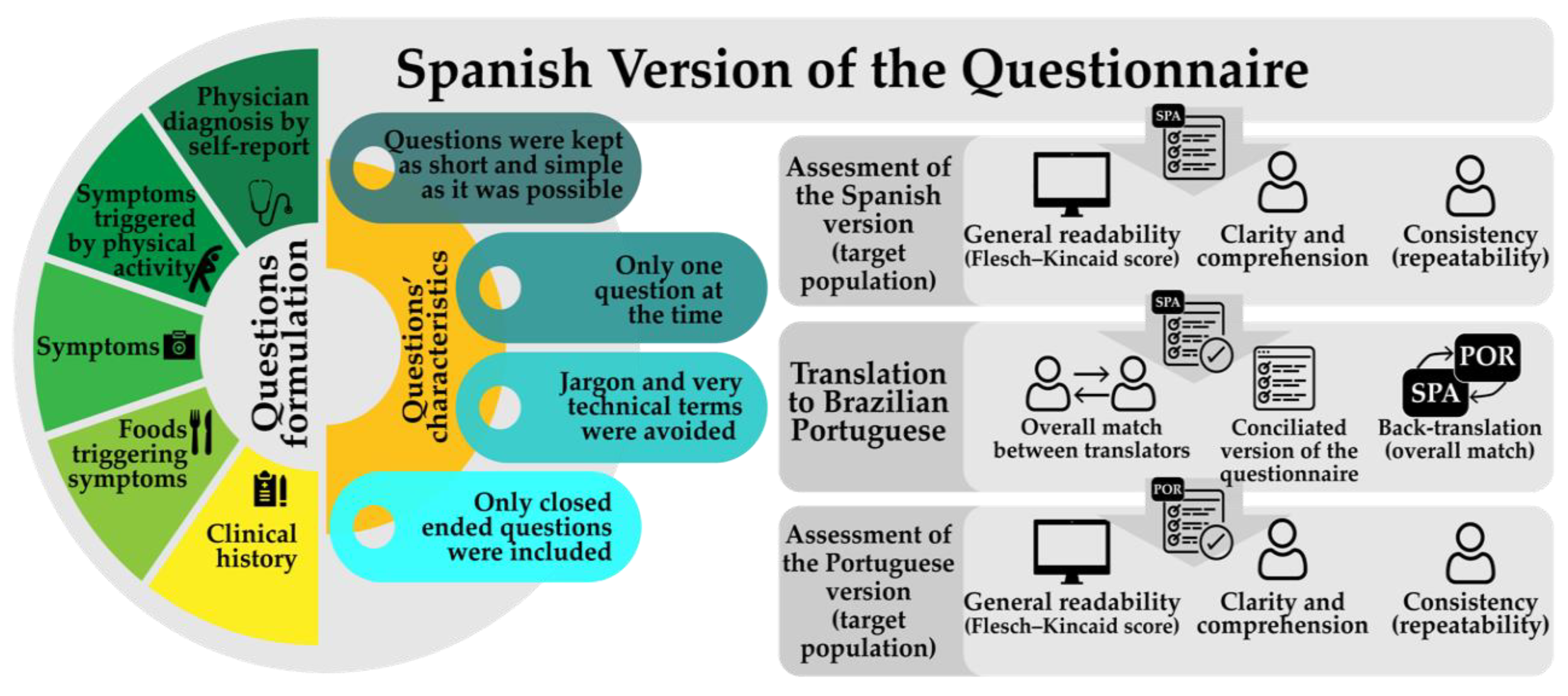
2.1. Questionnaire Design
2.2. Assessment of Clarity and Comprehension of the Spanish Version of the Questionnaire
2.3. Assessment of Consistency (Repeatability)
2.4. Questionnaire Translation to Portuguese Back-Translation Procedure
2.5. Statistical and Ethical Issues
3. Results
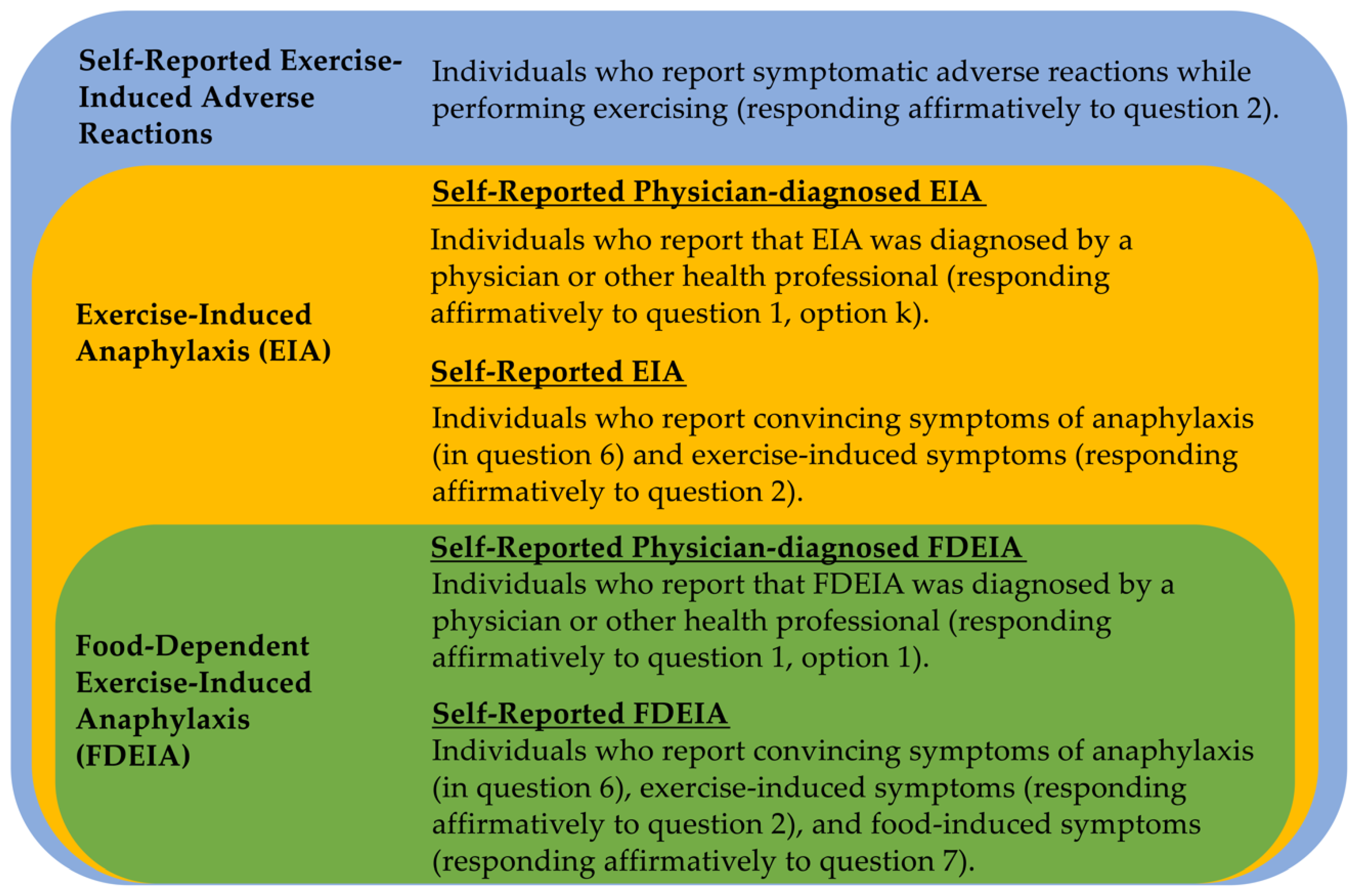
3.1. Questionnaire Design
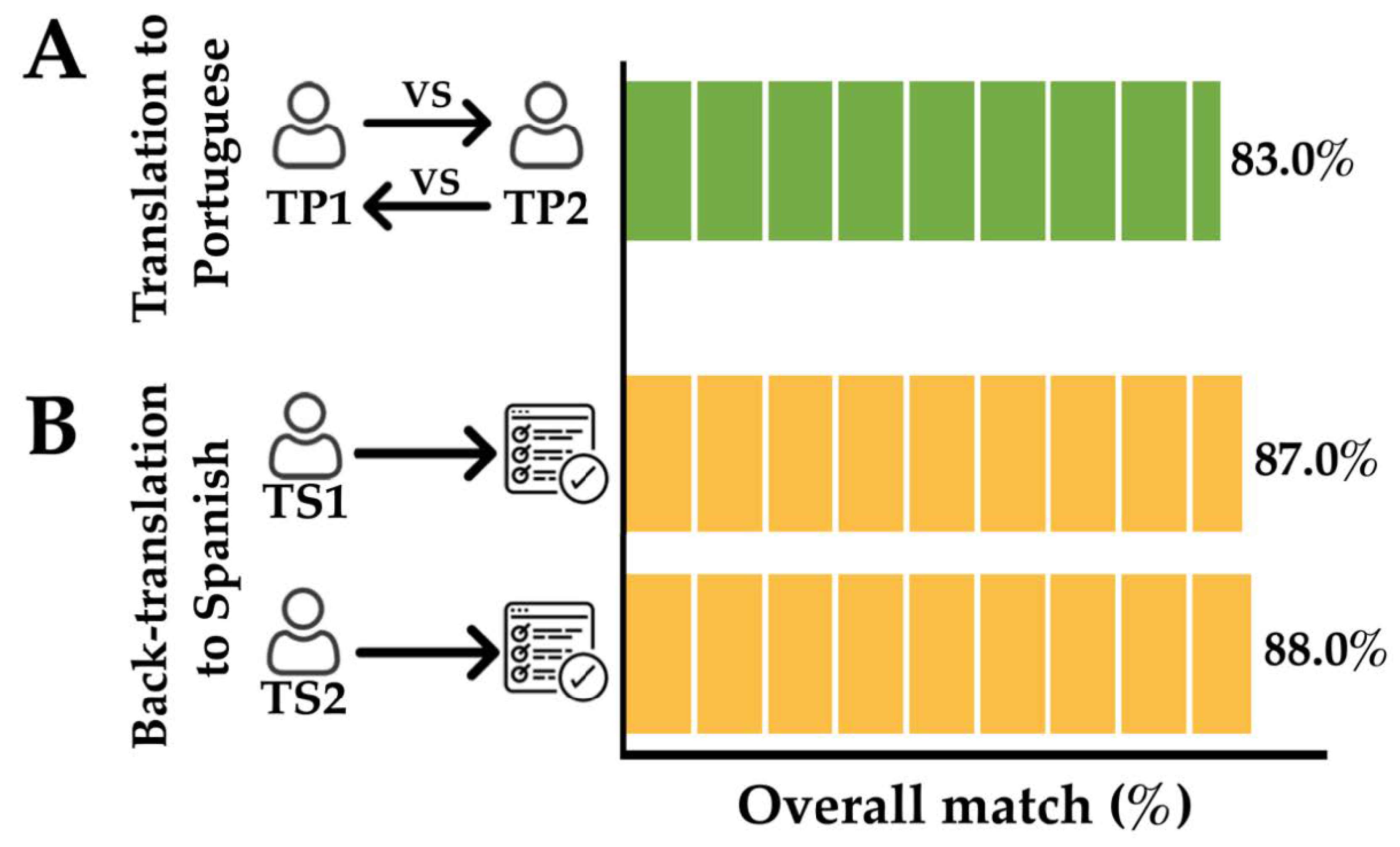
3.2. Translation of the Questionnaire to Portuguese/Back-Translation to Spanish
3.3. Characteristics of the Evaluators of the Spanish and Portuguese Versions of the Questionnaire
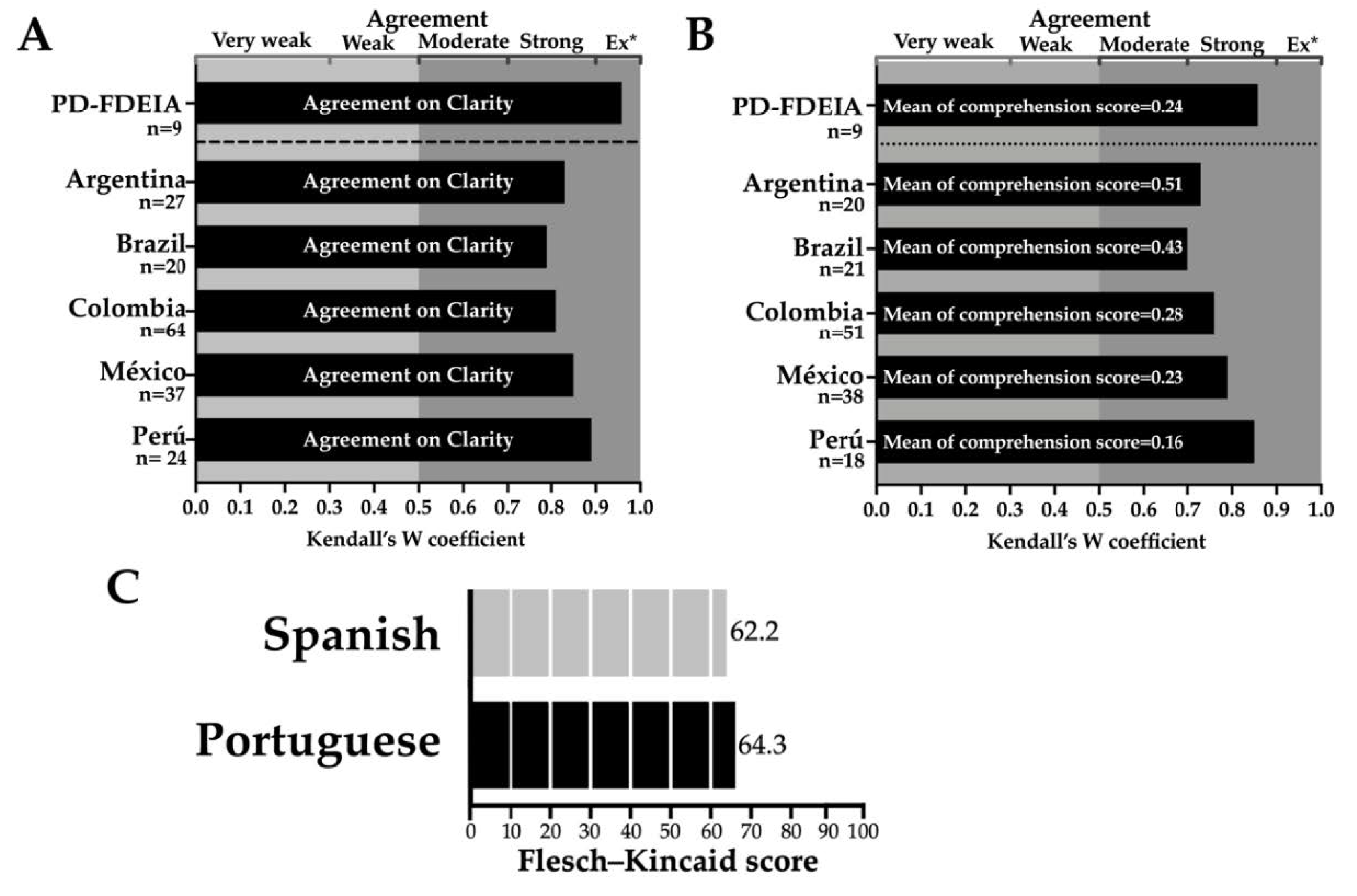
3.4. Clarity and Comprehension Assessment of the Spanish and Portuguese Versions of the Questionnaire
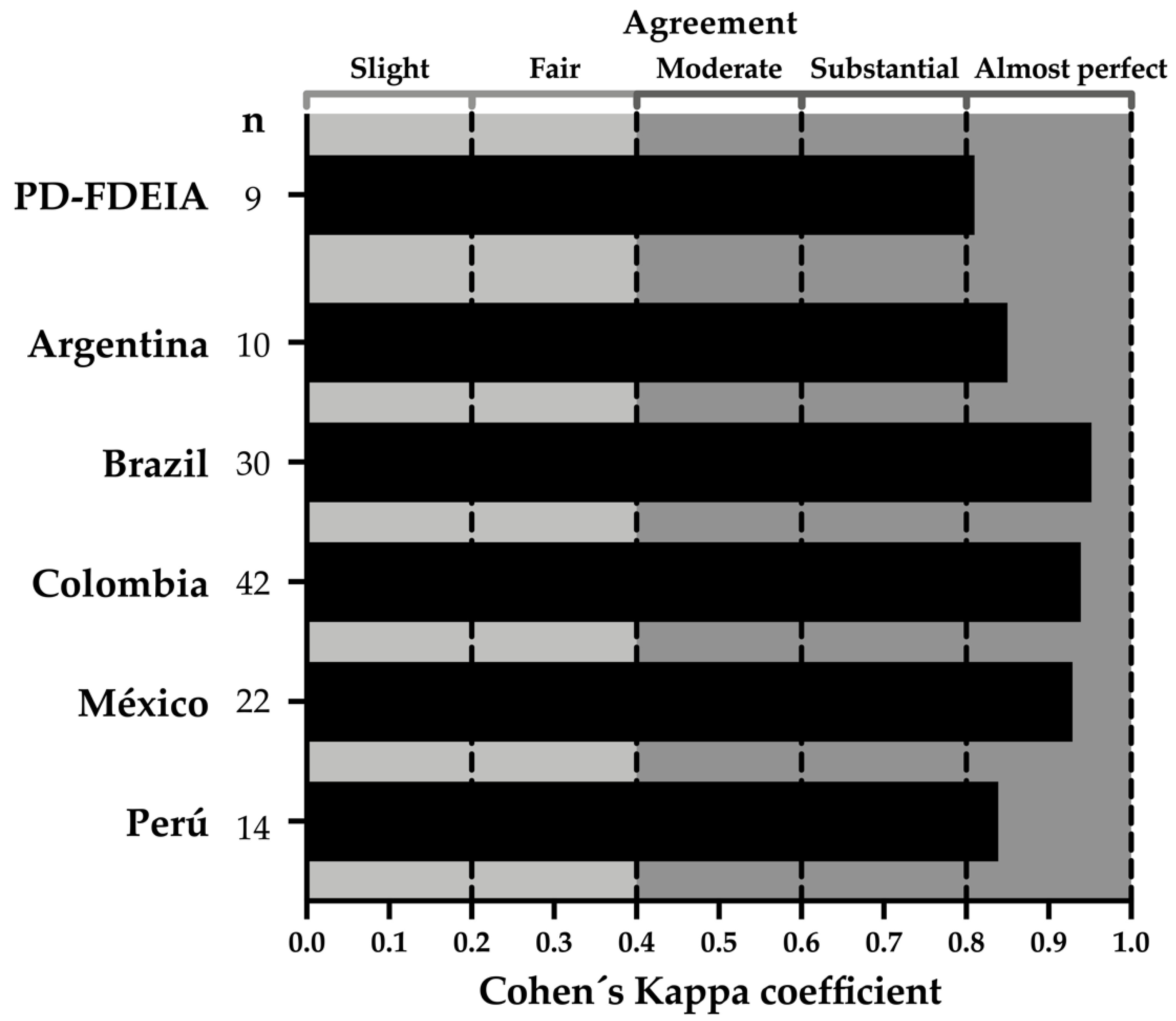
3.5. Consistency (Repeatability) Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minty, B. Food-dependent exercise-induced anaphylaxis. Can. Fam. Physician 2017, 63, 42–43. [Google Scholar] [PubMed]
- Aihara, Y.; Takahashi, Y.; Kotoyori, T.; Mitsuda, T.; Ito, R.; Aihara, M.; Ikezawa, Z.; Yokota, S. Frequency of food-dependent, exercise-induced anaphylaxis in Japanese junior-high-school students. J. Allergy Clin. Immunol. 2001, 108, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S. An Epidemiological Survey on Food-dependent Exercise-induced Anaphylaxis in Kindergartners, Schoolchildren and Junior High School Students. Asia Pac. J. Public Health 1994, 7, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Foong, R.-X.; Giovannini, M.; du Toit, G. Food-dependent exercise-induced anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Evoy, K.; Wong, A.; Simons, S. A cautionary tale for the physician: Case report and review of food-dependent exercise-induced anaphylaxis. Gen. Intern. Med. Clin. Innov. 2016, 1, 56–58. [Google Scholar] [CrossRef][Green Version]
- Ansley, L.; Bonini, M.; Delgado, L.; Giacco, S.D.; Toit, G.D.; Khaitov, M.; Kurowski, M.; Hull, J.H.; Moreira, A.; Robson-Ansley, P.J. Pathophysiological mechanisms of exercise-induced anaphylaxis: An EAACI position statement. Allergy 2015, 70, 1212–1221. [Google Scholar] [CrossRef]
- Greve, M. Food dependent exercise induced anaphylaxis triggered by inhaled antigen. Am. J. Emerg. Med. 2019, 37, 796.e1–796.e2. [Google Scholar] [CrossRef]
- Ukleja-Sokołowska, N.; Zacniewski, R.; Gawrońska-Ukleja, E.; Żbikowska-Gotz, M.; Lis, K.; Sokołowski, Ł.; Adamczak, R.; Bartuzi, Z. Food-dependent, exercise-induced anaphylaxis in a patient allergic to peach. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418803154. [Google Scholar] [CrossRef]
- Mobayed, H.M.S.; Ali Al-Nesf, M. Two cases of food-dependent exercise-induced anaphylaxis with different culprit foods. Ann. Thorac. Med. 2014, 9, 42–44. [Google Scholar] [CrossRef]
- Pravettoni, V.; Incorvaia, C. Diagnosis of exercise-induced anaphylaxis: Current insights. J. Asthma Allergy 2016, 9, 191–198. [Google Scholar] [CrossRef]
- Hoyos-Bachiloglu, R.; Ivanovic-Zuvic, D.; Álvarez, J.; Linn, K.; Thöne, N.; de los Ángeles Paul, M.; Borzutzky, A. Prevalence of parent-reported immediate hypersensitivity food allergy in Chilean school-aged children. Allergol. Immunopathol. 2014, 42, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, N.; Valdez-Meza, E.E.; Vergara-Jiménez, M.J.; Canizalez-Román, A.; Borzutzky, A.; Cabrera-Chávez, F. Parent-reported prevalence of food allergy in Mexican schoolchildren: A population-based study. Allergol. Immunopathol. 2016, 44, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Chávez, F.; Rodríguez-Bellegarrigue, C.I.; Figueroa-Salcido, O.G.; Lopez-Gallardo, J.A.; Arámburo-Gálvez, J.G.; de Vergara-Jiménez, M.J.; Castro-Acosta, M.L.; Sotelo-Cruz, N.; Gracia-Valenzuela, M.H.; Ontiveros, N. Food Allergy Prevalence in Salvadoran Schoolchildren Estimated by Parent-Report. Int. J. Environ. Res. Public Health 2018, 15, 2446. [Google Scholar] [CrossRef] [PubMed]
- Kazi, A.M.; Khalid, W. Questionnaire designing and validation. J. Pak Med. Assoc. 2012, 62, 514–516. [Google Scholar] [PubMed]
- Gusi, N.; Badía, X.; Herdman, M.; Olivares, P.R. Traducción y adaptación cultural de la versión española del cuestionario EQ-5D-Y en niños y adolescentes. Atención Primaria 2009, 41, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, N.; López-Gallardo, J.A.; Vergara-Jiménez, M.J.; Cabrera-Chávez, F. Self-Reported Prevalence of Symptomatic Adverse Reactions to Gluten and Adherence to Gluten-Free Diet in an Adult Mexican Population. Nutrients 2015, 7, 6000–6015. [Google Scholar] [CrossRef] [PubMed]
- Arámburo-Gálvez, J.G.; Carvalho Gomes, I.; André, T.G.; Beltrán-Cárdenas, C.E.; Macêdo-Callou, M.A.; Braga Rocha, É.M.; Mye-Takamatu-Watanabe, E.A.; Rahmeier-Fietz, V.; Figueroa-Salcido, O.G.; Cárdenas-Torres, F.I.; et al. Translation, Cultural Adaptation, and Evaluation of a Brazilian Portuguese Questionnaire to Estimate the Self-Reported Prevalence of Gluten-Related Disorders and Adherence to Gluten-Free Diet. Medicina 2019, 55, 593. [Google Scholar] [CrossRef]
- Farrell, A.; Judge, C.; Redenbaugh, V.; Awad, H.; Conlon, N. Food-dependent exercise-induced reactions: Lessons from a 15-year retrospective study. Ir. J. Med. Sci. 2019, 188, 815–819. [Google Scholar] [CrossRef]
- Shadick, N.A.; Liang, M.H.; Partridge, A.J.; Bingham, C.; Wright, E.; Fossel, A.H.; Sheffer, A.L. The natural history of exercise-induced anaphylaxis: Survey results from a 10-year follow-up study. J. Allergy Clin. Immunol. 1999, 104, 123–127. [Google Scholar] [CrossRef]
- Giannetti, M.P. Exercise-Induced Anaphylaxis: Literature Review and Recent Updates. Curr. Allergy Asthma Rep. 2018, 18, 72. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M.; Medicine, A.C. ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2018. [Google Scholar]
- Fernández Huerta, J. Medidas sencillas de lecturabilidad. Consigna 1959, 214, 29–32. [Google Scholar]
- Ferreira, D.; Carreira, H.; Silva, S.; Lunet, N. Assessment of the contents related to screening on Portuguese language websites providing information on breast and prostate cancer. Cad. Saude Publica 2013, 29, 2163–2176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barrio-Cantalejo, I.M.; Simón-Lorda, P.; Melguizo, M.; Escalona, I.; Marijuán, M.I.; Hernando, P. Validación de la Escala INFLESZ para evaluar la legibilidad de los textos dirigidos a pacientes. [Validation of the INFLESZ scale to evaluate readability of texts aimed at the patient]. An. Sist Sanit Navar. 2008, 31, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.R.; Ardusso, L.R.F.; Bilò, M.B.; El-Gamal, Y.M.; Ledford, D.K.; Ring, J.; Sanchez-Borges, M.; Senna, G.E.; Sheikh, A.; Thong, B.Y.; et al. World Allergy Organization anaphylaxis guidelines: Summary. J. Allergy Clin. Immunol. 2011, 127, 587–593. [Google Scholar] [CrossRef]
- Flesch, R. A new readability yardstick. J. Appl. Psychol. 1948, 32, 221–233. [Google Scholar] [CrossRef]
- Romano, A.; Fonso, M.D.; Giuffreda, F.; Papa, G.; Artesani, M.C.; Viola, M.; Venuti, A.; Palmieri, V.; Zeppilli, P. Food-Dependent Exercise-Induced Anaphylaxis: Clinical and Laboratory Findings in 54 Subjects. Int. Arch. Allergy Immunol. 2001, 125, 264–272. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme. In Codex Alimentarius Commission: Procedural Manual; Food & Agriculture Organization: Rome, Italy; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Asaumi, T.; Manabe, T.; Yanagida, N.; Sato, S.; Ebisawa, M. Wheat-Dependent Exercise-Induced Anaphylaxis. Curr. Treat Options Allergy 2017, 4, 291–302. [Google Scholar] [CrossRef]
- Harada, S.; Horikawa, T.; Icihashi, M. A study of food-dependent exercise-induced anaphylaxis by analyzing the Japanese cases reported in the literature. Arerugi 2000, 49, 1066–1073. [Google Scholar]
- Morita, E.; Kunie, K.; Matsuo, H. Food-dependent exercise-induced anaphylaxis. J. Dermatol. Sci. 2007, 47, 109–117. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Tadakamadla, S.K.; Mangal, G.; Quadri, M.F.A.; Nayeem, M.; Tadakamadla, J. Psychometric Analyses of the Indian (Hindi) Version of the Child Perception Questionnaire (CPQ11–14). Children 2020, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, D.; Poder, T.G.; Vasiliadis, H.-M.; Touati, N.; Fortin, B.; Lévesque, L.; Longo, C. Translation and Cultural Adaptation of the Patient Self-Administered Financial Effects (P-SAFE) Questionnaire to Assess the Financial Burden of Cancer in French-Speaking Patients. Healthcare 2020, 8, 366. [Google Scholar] [CrossRef] [PubMed]
- Risco, E.; Sauch, G.; Albero, A.; Acar-Denizli, N.; Zabalegui, A.; Kostov, B.; Amil, P.; Alonso, A.; Rios, A.; Martín, J.; et al. Spanish Validation of the “User Reported Measure of Care Coordination” Questionnaire for Older People with Complex, Chronic Conditions. Int. J. Environ. Res. Public Health 2020, 17, 6608. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.S.; Yoo, J.-E.; Kim, S.; Han, S.; Suh, H.S. Cultural Adaptation and Validation of the Korean Version of the iMTA Productivity Cost Questionnaire. Healthcare 2020, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Boore, J.R. Translation and back-translation in qualitative nursing research: Methodological review. J. Clin. Nurs. 2010, 19, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Henriques, E.R. Intercompreensão de texto escrito por falantes nativos de português e de espanhol. DELTA 2000, 16, 263–295. [Google Scholar] [CrossRef]
- Paegelow, R.S. Back Translation Revisited: Differences that Matter (and Those that Do Not). ATA Chron. 2008, 1, 22. [Google Scholar]
- Barboza, E.M.F.; de Nunes, E.M.A. A inteligibilidade dos websites governamentais brasileiros e o acesso para usuários com baixo nível de escolaridade. [The intelligibility of Brazilian government websites and access for users with low level of education]. Inclusao Soc. 2007, 2, 19–33. [Google Scholar]
- Rattray, J.; Jones, M.C. Essential elements of questionnaire design and development. J. Clin. Nurs. 2007, 16, 234–243. [Google Scholar] [CrossRef]
- Schmidt, R.C. Managing Delphi Surveys Using Nonparametric Statistical Techniques. Decis. Sci. 1997, 28, 763–774. [Google Scholar] [CrossRef]
| Clarity | Comprehension | Consistency | ||
|---|---|---|---|---|
| Participants (n) | 181 | 157 | 127 | |
| Male (n) | 87 | 75 | 58 | |
| Female (n) | 94 | 82 | 69 | |
| Age in years (range) | 18–66 | 18–66 | 18–66 | |
| Scholarly | Elementary (n) | 2 | 2 | 1 |
| Junior highschool (n) | 45 | 38 | 28 | |
| Highschool (n) | 13 | 12 | 11 | |
| University (n) | 99 | 87 | 76 | |
| Postgraduate (n) | 22 | 18 | 11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Santamaría, J.; Arámburo-Gálvez, J.G.; Beltrán-Cárdenas, C.E.; Mora-Melgem, J.A.; Figueroa-Salcido, O.G.; Ramírez-Torres, G.I.; Cárdenas-Torres, F.I.; Carvalho Gomes, I.; Geralda André, T.; Macêdo-Callou, M.A.; et al. Design, Assessment, and Validation of a Questionnaire to Estimate Food-Dependent Exercise-Induced Anaphylaxis Prevalence in Latin American Population. Healthcare 2020, 8, 519. https://doi.org/10.3390/healthcare8040519
González-Santamaría J, Arámburo-Gálvez JG, Beltrán-Cárdenas CE, Mora-Melgem JA, Figueroa-Salcido OG, Ramírez-Torres GI, Cárdenas-Torres FI, Carvalho Gomes I, Geralda André T, Macêdo-Callou MA, et al. Design, Assessment, and Validation of a Questionnaire to Estimate Food-Dependent Exercise-Induced Anaphylaxis Prevalence in Latin American Population. Healthcare. 2020; 8(4):519. https://doi.org/10.3390/healthcare8040519
Chicago/Turabian StyleGonzález-Santamaría, Jhonatan, Jesús Gilberto Arámburo-Gálvez, Carlos Eduardo Beltrán-Cárdenas, José Antonio Mora-Melgem, Oscar Gerardo Figueroa-Salcido, Giovanni Isaí Ramírez-Torres, Feliznando Isidro Cárdenas-Torres, Itallo Carvalho Gomes, Tatiane Geralda André, María Auxiliadora Macêdo-Callou, and et al. 2020. "Design, Assessment, and Validation of a Questionnaire to Estimate Food-Dependent Exercise-Induced Anaphylaxis Prevalence in Latin American Population" Healthcare 8, no. 4: 519. https://doi.org/10.3390/healthcare8040519
APA StyleGonzález-Santamaría, J., Arámburo-Gálvez, J. G., Beltrán-Cárdenas, C. E., Mora-Melgem, J. A., Figueroa-Salcido, O. G., Ramírez-Torres, G. I., Cárdenas-Torres, F. I., Carvalho Gomes, I., Geralda André, T., Macêdo-Callou, M. A., Braga Rocha, É. M., Ontiveros, N., & Cabrera-Chávez, F. (2020). Design, Assessment, and Validation of a Questionnaire to Estimate Food-Dependent Exercise-Induced Anaphylaxis Prevalence in Latin American Population. Healthcare, 8(4), 519. https://doi.org/10.3390/healthcare8040519







