The Relationship between Poor Pulmonary Function and Irregular Pulse in the Elderly: Findings from a Nationwide Cross-Sectional Survey in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Sample
2.3. Measurements
2.3.1. General Characteristics
2.3.2. Physiological Data
2.3.3. Pulse Palpation
2.3.4. Spirometry Measurement
2.4. Data Analysis
3. Results
3.1. General Characteristics
3.2. Clinical Data Comparisons
3.3. Pulmonary Function Test and Irregular Pulse
3.4. The Effects of Reduced Pulmonary Function on IP
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.Z.; Safford, M.M.; Muntner, P.; Khodneva, Y.; Dawood, F.Z.; Zakai, N.A.; Thacker, E.L.; Judd, S.; Howard, V.J.; Howard, G.; et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern. Med. 2014, 174, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.B.; Sears, S.F. Cardiology patient pages. A patient’s guide to living with atrial fibrillation. Circulation 2008, 117, e340. [Google Scholar] [CrossRef]
- Cooke, G.; Doust, J.; Sanders, S. Is pulse palpation helpful in detecting atrial fibrillation? A systematic review. J. Fam. Pract. 2006, 55, 130–134. [Google Scholar]
- Virtanen, R.; Kryssi, V.; Vasankari, T.; Salminen, M.; Kivelä, S.L.; Airaksinen, K.E. Self-detection of atrial fibrillation in an aged population: The LietoAF Study. Eur. J. Prev. Cardiol. 2014, 21, 1437–1442. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Pérula-de-Torres, L.; Martínez-Adell, M.; González-Blanco, V.; Baena-Díez, J.M.; Martín-Rioboó, E.; Parras-Rejano, J.M.; González-Lama, J.; Martín-Alvarez, R.; Ruiz-Moral, R.; Fernández-García, J.; et al. Opportunistic detection of atrial fibrillation in subjects aged 65 years or older in primare care: A randomised clinical trial of efficacy. DOFA-AP study protocol. BMC Fam. Pract. 2012, 13, 106. [Google Scholar] [CrossRef]
- Hobbs, F.D.; Fitzmaurice, D.A.; Mant, J.; Murray, E.; Jowett, S.; Bryan, S.; Raftery, J.; Davies, M.; Lip, G. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol. Assess 2005, 9, 1–74. [Google Scholar] [CrossRef]
- Fitzmaurice, D.A.; Hobbs, F.D.; Jowett, S.; Mant, J.; Murray, E.T.; Holder, R.; Raftery, J.P.; Bryan, S.; Davies, M.; Lip, G.Y.; et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: Cluster randomised controlled trial. BMJ 2007, 335, 383. [Google Scholar] [CrossRef]
- Ryu, J.H.; Krowka, M.J.; Pellikka, P.A.; Swanson, K.L.; McGoon, M.D. Pulmonary hypertension in patients with interstitial lung diseases. Mayo Clin. Proc. 2007, 82, 342–350. [Google Scholar] [CrossRef]
- Sideris, D.A.; Katsadoros, D.P.; Valianos, G.; Assioura, A. Type of cardiac dysrhythmias in respiratory failure. Am. Heart J. 1975, 89, 32–35. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, J.-K.; Lee, Y.; Shin, J.H.; Lim, Y.-H.; Park, H.-C.; Kim, C.K.; Shin, J. The relationship between decreased pulmonary function and atrial fibrillation in general population: Findings from Ansung–Ansan cohort of the Korean Genome and Epidemiology Study. J. Cardiol. 2019, 74, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Buch, P.; Friberg, J.; Scharling, H.; Lange, P.; Prescott, E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur. Respir. J. 2003, 21, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van der Grinten, C.P.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef]
- Lee, J.; Reyes, B.A.; McManus, D.D.; Maitas, O.; Chon, K.H. Atrial fibrillation detection using an iPhone 4S. IEEE Trans. Biomed. Eng. 2013, 60, 203–206. [Google Scholar] [CrossRef]
- McManus, D.D.; Lee, J.; Maitas, O.; Esa, N.; Pidikiti, R.; Carlucci, A.; Harrington, J.; Mick, E.; Chon, K.H. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm. 2013, 10, 315–319. [Google Scholar] [CrossRef]
- Dagher, L.; Shi, H.; Zhao, Y.; Marrouche, N.F. Wearables in cardiology: Here to stay. Heart Rhythm. 2020, 17, 889–895. [Google Scholar] [CrossRef]
- Li, J.; Agarwal, S.K.; Alonso, A.; Blecker, S.; Chamberlain, A.M.; London, S.J.; Loehr, L.R.; McNeill, A.M.; Poole, C.; Soliman, E.Z.; et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2014, 129, 971–980. [Google Scholar] [CrossRef]
- Johnson, L.S.; Juhlin, T.; Engström, G.; Nilsson, P.M. Reduced forced expiratory volume is associated with increased incidence of atrial fibrillation: The Malmo Preventive Project. Europace 2014, 16, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Han, K.-D.; Choi, J.-I.; Boo, K.Y.; Kim, D.Y.; Lee, K.-N.; Shim, J.; Kim, J.S.; Kim, Y.-H. Frequent drinking is a more important risk factor for new-onset atrial fibrillation than binge drinking: A nationwide population-based study. Europace 2020, 22, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Kallmünzer, B.; Bobinger, T.; Kahl, N.; Kopp, M.; Kurka, N.; Hilz, M.J.; Marquardt, L.; Schwab, S.; Köhrmann, M. Peripheral pulse measurement after ischemic stroke: A feasibility study. Neurology 2014, 83, 598–603. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.C.; Hobbs, F.D.; Kenkre, J.E.; Roalfe, A.K.; Iles, R.; Lip, G.Y.; Davies, M.K. Prevalence of atrial fibrillation in the general population and in high-risk groups: The ECHOES study. Europace 2012, 14, 1553–1559. [Google Scholar] [CrossRef]
- Lowres, N.; Neubeck, L.; Redfern, J.; Freedman, S.B. Screening to identify unknown atrial fibrillation. A systematic review. Thromb. Haemost. 2013, 110, 213–222. [Google Scholar] [CrossRef]
- Park, H.C.; Park, J.K.; Choi, S.I.; Kim, S.G.; Kim, M.K.; Choi, B.Y.; Shin, J. Prevalence of atrial fibrillation and relation to echocardiographic parameters in a healthy asymptomatic rural Korean Population. J. Korean Med. Sci. 2015, 30, 1078–1084. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Buist, A.S.; McBurnie, M.A.; Vollmer, W.M.; Gillespie, S.; Burney, P.; Mannino, D.M.; Menezes, A.M.; Sullivan, S.D.; Lee, T.A.; Weiss, K.B.; et al. International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence study. Lancet 2007, 370, 741–750. [Google Scholar] [CrossRef]
- Yoo, K.H.; Kim, Y.S.; Sheen, S.S.; Park, J.H.; Hwang, Y.I.; Kim, S.H.; Yoon, H.I.; Lim, S.C.; Park, J.Y.; Park, S.J.; et al. Prevalence of chronic obstructive pulmonary disease in Korea: The fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology 2011, 16, 659–665. [Google Scholar] [CrossRef]
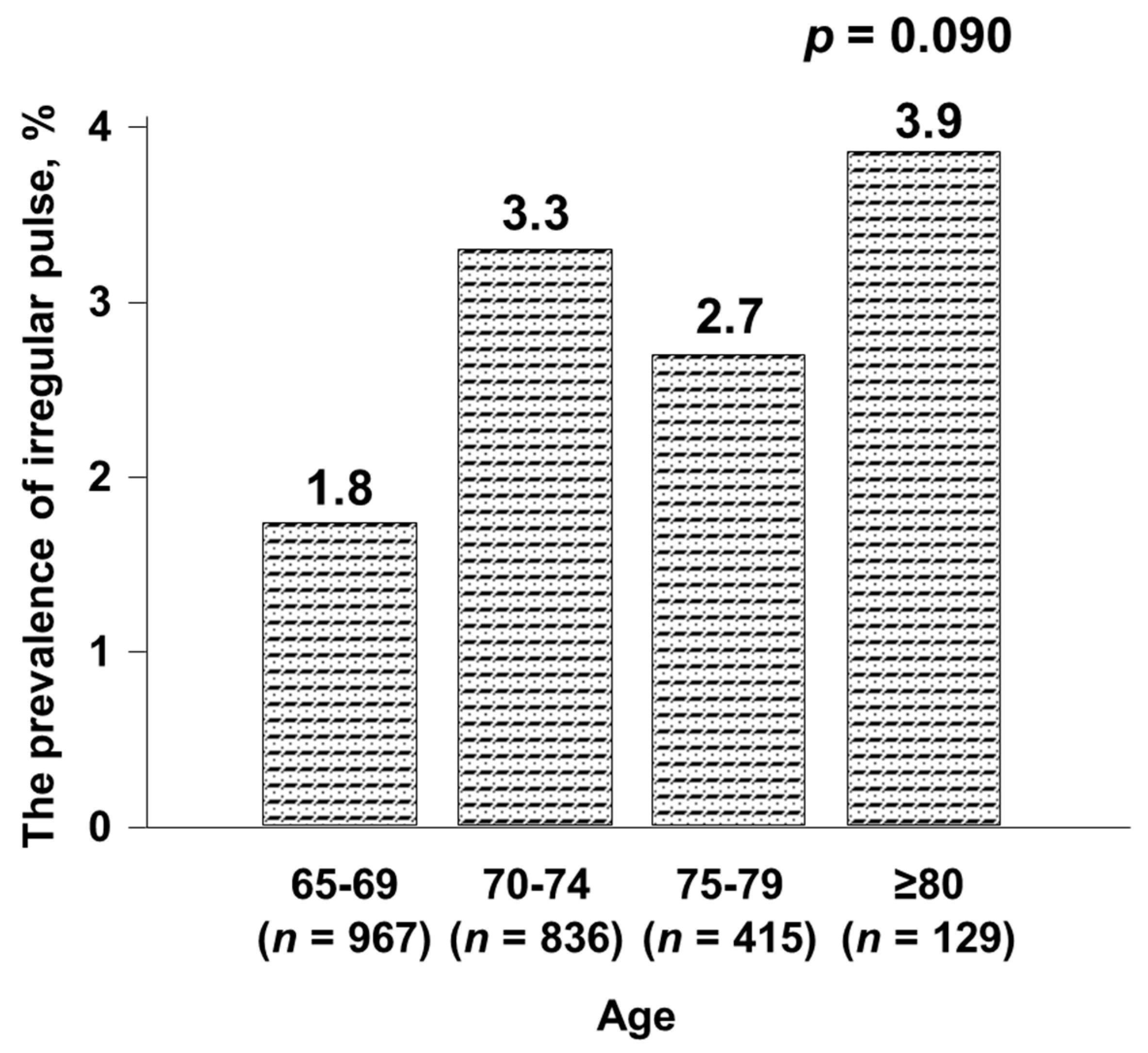
| Characteristics | Irregular Pulse (n = 61) | Regular Pulse (n = 2286) | p |
|---|---|---|---|
| n (%) | n (%) | ||
| Age (yr) | 73.0 (71.7–74.3) | 72.2 (71.9–72.5) | 0.214 |
| Age ≥75 years | 16 (32.2) | 528 (31.0) | 0.881 |
| Sex (male) | 29 (45.8) | 979 (40.5) | 0.493 |
| Chronic disease | |||
| Hypertension | 45 (75.6) | 1384 (62.6) | 0.088 |
| Diabetes mellitus | 15 (28.1) | 407 (18.9) | 0.154 |
| History of COPD | 2 (2.0) | 19 (1.0) | 0.373 |
| Congestive heart failure | 2 (3.5) | 47 (2.5) | 0.660 |
| Angina | 2 (1.6) | 112 (4.5) | 0.126 |
| Previous stroke | 4 (6.2) | 68 (3.1) | 0.200 |
| Previous myocardial infarction | 2 (2.3) | 50 (2.3) | 0.987 |
| Hyperlipidemia | |||
| Hypercholesterolemia | 8 (13.5) | 502 (22.8) | 0.150 |
| Hypertriglyceridemia | 5 (9.4) | 331 (18.2) | 0.151 |
| Hypo-HDL cholesterolemia | 15 (31.9) | 614 (30.3) | 0.855 |
| Thyroid disease | 0 (0.0) | 112 (5.1) | 0.155 |
| Medication use, n (%) | |||
| Antihypertensive drugs | 37 (58.5) | 1152 (51.2) | 0.477 |
| Antidiabetic drugs (including insulin) | 11 (21.4) | 366 (16.0) | 0.379 |
| Lipid-lowering drugs | 4 (5.7) | 327 (12.8) | 0.136 |
| Cigarette smoking status | 0.703 | ||
| Current smoker | 8 (10.1) | 254 (11.7) | |
| Ex-smoker | 17 (32.3) | 627 (26.8) | |
| Non-smoker | 34 (57.6) | 1367 (61.5) | |
| Alcohol consumption frequency | 0.022 | ||
| ≤1 time/month | 44 (74.6) | 1590 (70.8) | |
| 2–4 times/month | 2 (3.4) | 284 (12.7) | |
| 2–3 times/week | 3 (5.1) | 184 (8.2) | |
| ≥4 times/week | 10 (16.9) | 187 (8.3) | |
| Amount of alcohol | 0.611 | ||
| None | 32 (57.9) | 1051 (49.0) | |
| 1–4 servings | 20 (34.6) | 977 (42.0) | |
| 5–9 servings | 6 (5.8) | 185 (7.6) | |
| ≥10 servings | 1 (1.7) | 34 (1.4) | |
| Physical activity | |||
| Walking | 21 (27.6) | 885 (38.1) | 0.202 |
| Moderate activity | 2 (6.6) | 207 (9.1) | 0.640 |
| Strenuous activity | 3 (4.0) | 204 (8.2) | 0.205 |
| Characteristics | Irregular Pulse (n = 61) | Regular Pulse (n = 2286) | p |
|---|---|---|---|
| Body mass index, kg/m2 | 24.8 (23.9–25.8) | 24.2 (24.0–24.4) | 0.168 |
| Waist circumference, cm | 87.6 (84.7–90.5) | 84.9 (84.2–85.5) | 0.065 |
| Blood pressure, mmHg | |||
| Systolic | 130.8 (125.8–135.9) | 130.2 (129.3–131.1) | 0.811 |
| Diastolic | 72.1 (68.5–75.8) | 74.3 (73.8–74.9) | 0.235 |
| Heart rate, bpm | 66.0 (62.4–69.6) | 69.2 (68.6–69.8) | 0.085 |
| Pulse pressure, mmHg | 58.7 (54.4–62.9) | 55.9 (55.2–56.6) | 0.193 |
| White blood cell count, ×103/mm3 | 6.3 (5.6–7.1) | 6.1 (6.0–6.3) | 0.616 |
| Fasting glucose, mg/dL | 104.7 (98.4–110.9) | 102.7 (101.6–103.9) | 0.546 |
| eGFR, mL/min/1.73 m2 | 76.3 (72.7–79.9) | 78.9 (77.7–80.1) | 0.165 |
| Total cholesterol, mg/dL | 185.7 (174.4–197.0) | 193.0 (190.9–195.1) | 0.208 |
| HDL-C, mg/dL | 46.8 (43.5–50.1) | 46.6 (46.0–47.2) | 0.875 |
| Triglycerides, mg/dL | 125.6 (106.7–144.6) | 141.0 (136.7–145.3) | 0.117 |
| Characteristics | Irregular Pulse (n = 61) | Regular Pulse (n = 2286) | p |
|---|---|---|---|
| FVC, L | 2.84 (2.67–3.01) | 2.95 (2.91–2.99) | 0.208 |
| Quartile 1 (1.25–2.439) | 20 (36.5) | 562 (27.6) | 0.210 |
| Quartile 2 (2.44–2.899) | 12 (21.6) | 586 (26.0) | |
| Quartile 3 (2.90–3.529) | 21 (30.5) | 559 (23.8) | |
| Quartile 4 (3.53–5.46) | 8 (11.4) | 579 (22.6) | |
| FVC/predicted FVC, % | 85.9 (82.6–89.2) | 90.6 (89.9–91.2) | 0.005 |
| FVC/predicted FVC, n (%) | <0.001 | ||
| ≥80% | 36 (57.3) | 1837 (80.7) | |
| <80% | 25 (42.7) | 449 (19.3) | |
| FEV1/FVC | 0.72 (0.70–0.75) | 0.73 (0.73–0.74) | 0.656 |
| FEV1/FVC, n (%) | 0.293 | ||
| ≥0.7 | 42 (62.5) | 1636 (70.1) | |
| <0.7 | 19 (37.5) | 650 (29.9) | |
| FEV1, L | 2.03 (1.93–2.13) | 2.14 (2.11–2.17) | 0.040 |
| Quartile 1 (0.74–1.82) | 17 (33.3) | 563 (28.2) | 0.122 |
| Quartile 2 (1.83–2.14) | 19 (35.2) | 565 (26.1) | |
| Quartile 3 (2.15–2.53) | 17 (23.0) | 587 (23.9) | |
| Quartile 4 (2.54–3.99) | 8 (8.5) | 571 (21.8) | |
| FEV1/predicted FEV1, % | 88.7 (84.8–92.7) | 93.3 (92.4–94.2) | 0.027 |
| Interpretation, n (%) | 0.037 | ||
| Normal | 27 (40.1) | 1334 (57.7) | |
| Restrictive | 15 (22.4) | 302 (12.4) | |
| Obstructive | 19 (37.5) | 650 (29.9) |
| Characteristics | Univariate Logistic Models | Multivariate Logistic Models | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR | 95% CI | p | Adjusted OR † | 95% CI | p | |
| FVC, L | ||||||
| Quartile 1 (1.25–2.43) | 2.62 | 1.04–6.57 | 0.041 | 2.57 | 0.96–6.88 | 0.061 |
| Quartile 2 (2.44–2.89) | 1.64 | 0.55–4.91 | 0.378 | 1.65 | 0.51–5.36 | 0.404 |
| Quartile 3 (2.90–3.52) | 2.53 | 0.94–6.82 | 0.066 | 2.41 | 0.89–6.51 | 0.082 |
| Quartile 4 (3.53–5.46) | 1 | 1 | ||||
| FVC/predicted FVC, % | 0.971 | 0.952–0.991 | 0.005 | 0.976 | 0.956–0.997 | 0.023 |
| FVC/predicted FVC < 80% | 3.11 | 1.73–5.61 | <0.001 | 2.68 | 1.50–4.80 | 0.001 |
| FEV1, L | ||||||
| Quartile 1 (0.74–1.82) | 3.04 | 1.15–8.06 | 0.025 | 3.09 | 1.02–9.37 | 0.047 |
| Quartile 2 (1.83–2.14) | 3.47 | 1.30–9.23 | 0.013 | 3.55 | 1.24–10.13 | 0.018 |
| Quartile 3 (2.15–2.53) | 2.47 | 0.97–6.30 | 0.059 | 2.44 | 0.90–6.59 | 0.079 |
| Quartile 4 (2.54–3.99) | 1 | 1 | ||||
| FEV1/predicted FEV1, % | 0.985 | 0.973–0.998 | 0.022 | 0.986 | 0.973–0.999 | 0.040 |
| Interpretation | ||||||
| Normal | 1 | 1 | ||||
| Restrictive | 2.61 | 1.22–5.58 | 0.014 | 2.31 | 1.01–5.27 | 0.047 |
| Obstructive | 1.81 | 0.88–3.70 | 0.105 | 1.86 | 0.89–3.85 | 0.097 |
| Restrictive or obstructive | 2.04 | 1.09–3.83 | 0.026 | 1.95 | 1.02–3.72 | 0.043 |
| History of COPD | 2.14 | 0.47–9.64 | 0.468 | 2.56 | 0.57–11.60 | 0.221 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Lee, Y.; Hwang, S.Y.; Shin, J.; Kim, C.K.; Park, J.-K. The Relationship between Poor Pulmonary Function and Irregular Pulse in the Elderly: Findings from a Nationwide Cross-Sectional Survey in Korea. Healthcare 2020, 8, 312. https://doi.org/10.3390/healthcare8030312
Kim SH, Lee Y, Hwang SY, Shin J, Kim CK, Park J-K. The Relationship between Poor Pulmonary Function and Irregular Pulse in the Elderly: Findings from a Nationwide Cross-Sectional Survey in Korea. Healthcare. 2020; 8(3):312. https://doi.org/10.3390/healthcare8030312
Chicago/Turabian StyleKim, Sun Hwa, Yonggu Lee, Seon Young Hwang, Jinho Shin, Chun Ki Kim, and Jin-Kyu Park. 2020. "The Relationship between Poor Pulmonary Function and Irregular Pulse in the Elderly: Findings from a Nationwide Cross-Sectional Survey in Korea" Healthcare 8, no. 3: 312. https://doi.org/10.3390/healthcare8030312
APA StyleKim, S. H., Lee, Y., Hwang, S. Y., Shin, J., Kim, C. K., & Park, J.-K. (2020). The Relationship between Poor Pulmonary Function and Irregular Pulse in the Elderly: Findings from a Nationwide Cross-Sectional Survey in Korea. Healthcare, 8(3), 312. https://doi.org/10.3390/healthcare8030312





