Determination of the Minimum Detectable Change in the Total and Segmental Volumes of the Upper Limb, Evaluated by Perimeter Measurements
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Participants
2.1.1. Design
2.1.2. Ethical Approval
2.1.3. Participants
2.2. Measurements and Methods
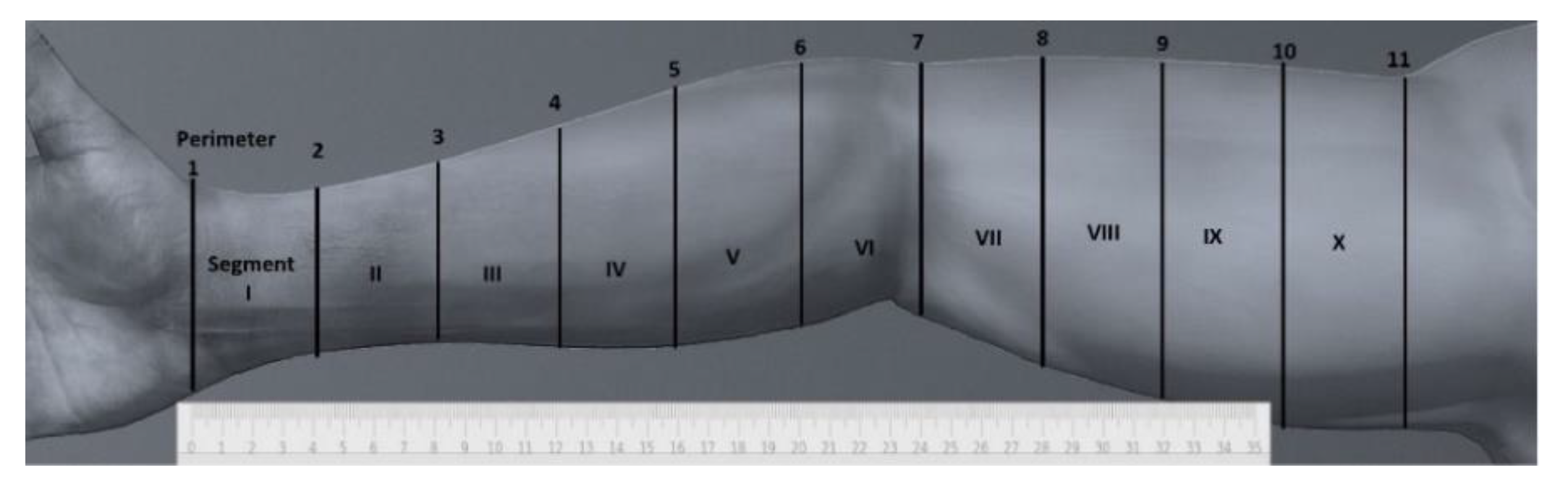
2.2.1. Arm Perimeter Measurements
2.2.2. Arm Volume Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Cancer Observatory. Available online: https://gco.iarc.fr/today/home/ (accessed on 1 June 2020).
- Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/populations/458-malaysia-fact-sheets.pdf/ (accessed on 1 June 2019).
- Sancho-Garnier, H.; Colonna, M. Breast cancer epidemiology. Presse Medicale 2019, 48, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global trend of breast cancer mortality rate: A 25-year study. Asian Pacific J. Cancer Prev. 2019, 20, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Sims, J.N.; Miele, L.; Noubissi, F.; Lowe, L.; Fonseca, D.D.; Alo, R.A.; Payton, M.; Tchounwou, P.B. Health and Racial Disparity in Breast Cancer. Adv. Exp. Med. Biol. 2019, 1152, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 43–73. [Google Scholar] [CrossRef] [PubMed]
- McDuff, S.; Mina, A.; Brunelle, C.; Salama, L.; Warren, L.; Abouegylah, M.; Swaroop, M.; Skolny, M.; Asdourian, M.; Gillespie, T.; et al. Timing of Lymphedema After Treatment for Breast Cancer: When Are Patients Most At Risk? Int. J. Radiat Oncol. Biol. Phys. 2019, 103, 62–70. [Google Scholar] [CrossRef]
- Allam, O.; Park, K.; Chandler, L.; Mozaffari, M.; Ahmad, M.; Lu, X.; Alperovich, M. The impact of radiation on lymphedema: A review of the literature. Gland Surg. 2020, 9, 596–602. [Google Scholar] [CrossRef]
- Li, C.Y.; Kataru, R.P.; Mehrara, B.J. Histopathologic features of lymphedema: A molecular review. Int. J. Mol. Sci. 2020, 21, 2546. [Google Scholar] [CrossRef]
- Norman, S.; Localio, A.; Potashnik, S.; Torpey, H.; Kallan, M.; Weber, A.; Miller, L.; Demichele, A.; Solin, L. Lymphedema in Breast Cancer Survivors: Incidence, Degree, Time Course, Treatment, and Symptoms. J. Clin. Oncol. 2009, 27, 390–397. [Google Scholar] [CrossRef]
- Zou, L.; Liu, F.; Shen, P.; Hu, Y.; Liu, X.; Xu, Y.; Pen, Q.; Wang, B.; Zhu, Y.; Tian, Y. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: A 2-year follow-up prospective cohort study. Breast Cancer 2018, 25, 309–314. [Google Scholar] [CrossRef]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Larocque, G.; McDiarmid, S. The legacy of lymphedema: Impact on nursing practice and vascular access. Can. Oncol. Nurs. J. 2019, 29, 194. [Google Scholar] [PubMed]
- Sierla, R.; Dylke, E.S.; Kilbreath, S. A Systematic Review of the Outcomes Used to Assess Upper Body Lymphedema. Cancer Investig. 2018, 36, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Hidding, J.T.; Viehoff, P.B.; Beurskens, C.H.G.; Van Laarhoven, H.W.M.; Nijhuis-van der Sanden, M.W.G.; Van der Wees, P.J. Measurement Properties of Instruments for Measuring of Lymphedema: Systematic Review. Phys. Ther. 2016, 96, 1965–1981. [Google Scholar] [CrossRef]
- Armer, J.; Ballman, K.; McCall, L.; Armer, N.; Sun, Y.; Udmuangpia, T.; Hunt, K.; Mittendorf, E.; Byrd, D.; Julian, T.; et al. Lymphedema symptoms and limb measurement changes in breast cancer survivors treated with neoadjuvant chemotherapy and axillary dissection: Results of American College of Surgeons Oncology Group (ACOSOG) Z1071 (Alliance) substudy. Support Care Cancer. 2019, 27, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Executive Committee of the International Society of Lymphology. The Diagnosis and Treatment of Peripheral Lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Sharkey, A.R.; King, S.W.; Kuo, R.Y.; Bickerton, S.B.; Ramsden, A.J.; Furniss, D. Measuring Limb Volume: Accuracy and Reliability of Tape Measurement Versus Perometer Measurement. Lymphat. Res. Biol. 2018, 16, 182–186. [Google Scholar] [CrossRef]
- Walter, S.D.; Eliasziw, M.; Donner, A. Sample size and optimal designs for reliability studies. Stat. Med. 1998, 17, 101–110. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Tidhar, D.; Armer, J.M.; Deutscher, D.; Shyu, C.R.; Azuri, J.; Madsen, R. Measurement issues in anthropometric measures of limb volume change in persons at risk for and living with lymphedema: A reliability study. J. Pers. Med. 2015, 5, 341–353. [Google Scholar] [CrossRef]
- Chen, Y.W.; Tsai, H.J.; Hung, H.C.; Tsauo, J.Y. Reliability study of measurements for lymphedema in breast cancer patients. Am. J. Phys. Med. Rehabil. 2008, 87, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Deltombe, T.; Jamart, J.; Recloux, S.; Legrand, X.; Vanderbroeck, N.; Theys, S.; Hanson, P. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology 2007, 40, 26–34. [Google Scholar] [PubMed]
- Karges, J.R.; Mark, B.E.; Stikeleather, S.J.; Worrell, T.W. Concurrent Validity of Upper-Extremity Volume Estimates. Phys. Ther. 2003, 83, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Sander, A.P.; Hajer, N.M.; Hemenway, K.; Miller, A.C. Upper-extremity volume measurements in women with lymphedema: A comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther. 2002, 82, 1201–1212. [Google Scholar] [CrossRef]
- Megens, A.M.; Harris, S.R.; Kim-Sing, C.; McKenzie, D.C. Measurement of upper extremity volume in women after axillary dissection for breast cancer. Arch. Phys. Med. Rehabil. 2001, 82, 1639–1644. [Google Scholar] [CrossRef]
- Borthwick, Y.; Paul, L.; Sneddon, M.; Mcalpine, L.; Miller, C. Reliability and validity of the figure-of-eight method of measuring hand size in patients with breast cancer-related lymphoedema. Eur. J. Cancer Care (Engl.) 2013, 22, 196–201. [Google Scholar] [CrossRef]
- Devoogdt, N.; Lemkens, H.; Geraerts, I.; Van Nuland, I.; Flour, M.; Coremans, T.; Christiaens, M.; Van Kampen, M. A new device to measure upper limb circumferences: Validity and reliability. Int. Angiol. 2010, 29, 401–407. [Google Scholar]
- Smoot, B.J.; Wong, J.F.; Dodd, M.J. Comparison of Diagnostic Accuracy of Clinical Measures of Breast Cancer-Related Lymphedema: Area Under the Curve. Arch. Phys. Med. Rehabil. 2011, 92, 603–610. [Google Scholar] [CrossRef]
- Czerniec, S.A.; Ward, L.C.; Lee, M.J.; Refshauge, K.M.; Beith, J.; Kilbreath, S.L. Segmental measurement of breast cancer-related arm lymphoedema using perometry and bioimpedance spectroscopy. Support Care Cancer 2011, 19, 703–710. [Google Scholar] [CrossRef]
- Meijer, R.; Rietman, J.; Geertzen, J.; Bosmans, J.; Dijkstra, P. Validity and intra- and interobserver reliability of an indirect volume measurements in patients with upper extremity lymphedema. Lymphology 2004, 37, 127–133. [Google Scholar]
- De Vrieze, T.; Gebruers, N.; Tjalma, W.; Nevelsteen, I.; Thomis, S.; De Groef, A.; Dams, L.; Van der Gucht, E.; Belgrado, J.; Vandermeeren, L.; et al. What is the best method to determine excessive arm volume in patients with breast cancer-related lymphoedema in clinical practice? Reliability, time efficiency and clinical feasibility of five different methods. Clin. Rehabil. 2019, 33, 1221–1232. [Google Scholar] [CrossRef]
- Vignes, S. Lymphedema: From diagnosis to treatment. La. Rev. Med. Interne. 2017, 38, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Haley, S.M.; Fragala-Pinkham, M.A. Interpreting Change Scores of Tests and Measures Used in Physical Therapy. Phys. Ther. 2006, 86, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Jayasinghe, U.W.; Koelmeyer, L.; Ung, O.; Boyages, J. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys. Ther. 2006, 86, 205–214. [Google Scholar] [CrossRef] [PubMed]
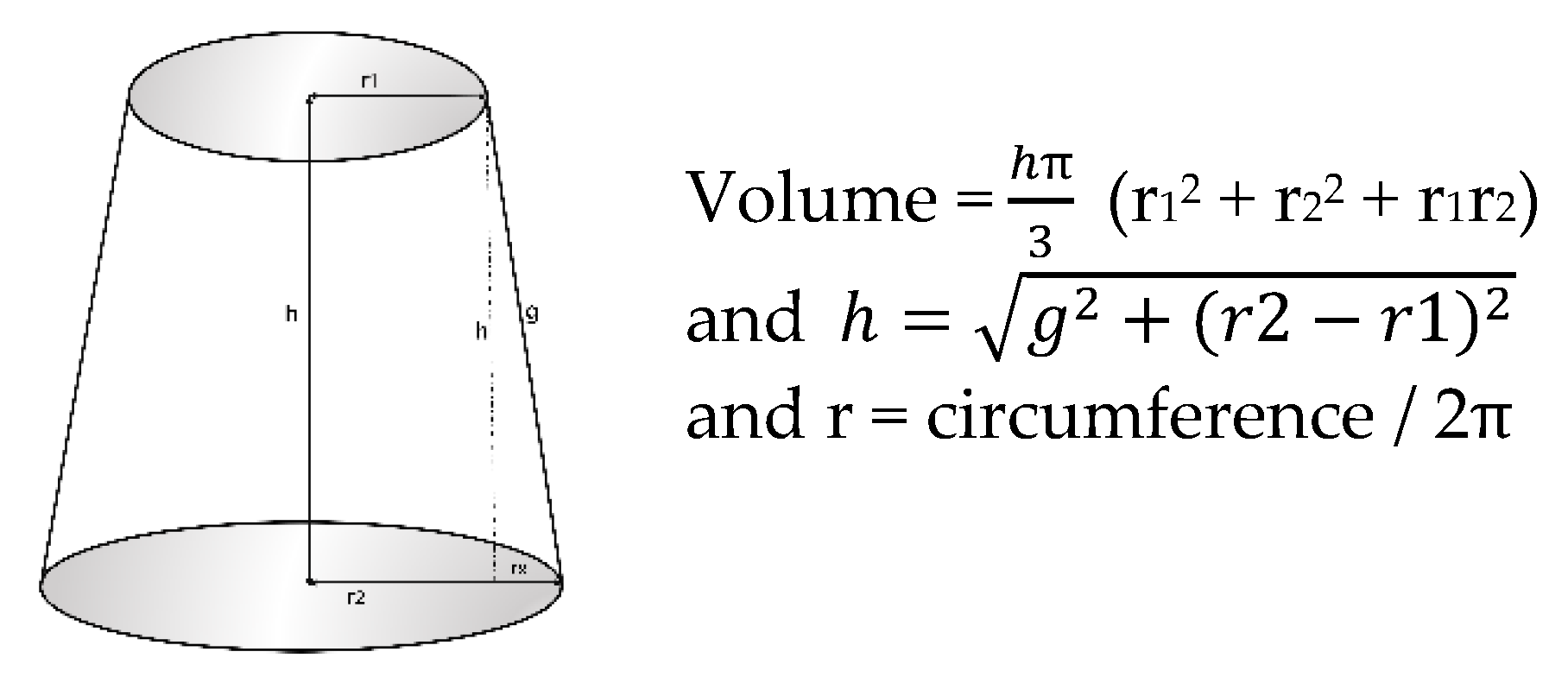

| Affected side (right/left) (n) | 11/14 | ||
| Lymphedema (yes/no) (n) | 5/20 | ||
| Mean | SD | Range | |
| Age (years) | 53.6 ± 10.7 | (79–39) | |
| Years since surgery | 5.8 ± 4,0 | (15–2) | |
| BMI (kg/m2) | 27.8 ± 4.3 | (36.6–20.3) | |
| Diff. % between arm volumes in women without lymphedema (n = 20) | 3.9 ± 2.5 | (0.5–7.9) | |
| Diff. % between arm volumes in women with lymphedema (n = 5) | 44 ± 22.7 | (11.1–60.7) | |
| Perimeter | Mean | SD | ICC | Confidence Interval 95% | CV | SD | Min. | Max. | SEM | SEM% | MCD | MCD% | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Perimeter 1a | 15.57 | 0.99 | 0.988 | (0.978 | − | 0.933) | 0.006 | 0.004 | 0.000 | 0.018 | 0.108 | 0.69 | 0.3 | 1.92 |
| Perimeter 1b | 15.58 | 1.01 | ||||||||||||
| Perimeter 2a | 16.90 | 2.28 | 0.989 | (0.981 | − | 0.994) | 0.008 | 0.010 | 0.000 | 0.052 | 0.239 | 1.41 | 0.7 | 3.91 |
| Perimeter 2b | 16.87 | 2.27 | ||||||||||||
| Perimeter 3a | 19.10 | 2.76 | 0.994 | (0.989 | − | 0.996) | 0.009 | 0.008 | 0.000 | 0.037 | 0.214 | 1.12 | 0.6 | 3.11 |
| Perimeter 3b | 19.04 | 2.85 | ||||||||||||
| Perimeter 4a | 22.16 | 3.05 | 0.990 | (0.982 | − | 0.994) | 0.009 | 0.011 | 0.000 | 0.059 | 0.305 | 1.38 | 0.8 | 3.82 |
| Perimeter 4b | 22.10 | 3.10 | ||||||||||||
| Perimeter 5a | 24.41 | 2.99 | 0.995 | (0.992 | − | 0.997) | 0.006 | 0.006 | 0.000 | 0.031 | 0.212 | 0.87 | 0.6 | 2.40 |
| Perimeter 5b | 24.31 | 2.97 | ||||||||||||
| Perimeter 6a | 25.30 | 2.90 | 0.996 | (0.993 | − | 0.998) | 0.006 | 0.004 | 0.000 | 0.026 | 0.183 | 0.72 | 0.5 | 2.01 |
| Perimeter 6b | 25.21 | 2.91 | ||||||||||||
| Perimeter 7a | 25.54 | 3.25 | 0.996 | (0.993 | − | 0.998) | 0.005 | 0.005 | 0.000 | 0.031 | 0.206 | 0.80 | 0.6 | 2.23 |
| Perimeter 7b | 25.50 | 3.14 | ||||||||||||
| Perimeter 8a | 26.80 | 3.73 | 0.997 | (0.995 | − | 0.998) | 0.006 | 0.004 | 0.000 | 0.021 | 0.204 | 0.76 | 0.6 | 2.11 |
| Perimeter 8b | 26.73 | 3.70 | ||||||||||||
| Perimeter 9a | 28.37 | 3.95 | 0.998 | (0.996 | − | 0.999) | 0.005 | 0.004 | 0.000 | 0.021 | 0.177 | 0.62 | 0.5 | 1.73 |
| Perimeter 9b | 28.26 | 3.98 | ||||||||||||
| Perimeter 10a | 29.73 | 4.17 | 0.997 | (0.994 | − | 0.998) | 0.006 | 0.005 | 0.000 | 0.025 | 0.229 | 0.77 | 0.6 | 2.13 |
| Perimeter 10b | 29.63 | 4.15 | ||||||||||||
| Perimeter 11a | 30.69 | 4.39 | 0.999 | (0.997 | − | 0.999) | 0.005 | 0.003 | 0.000 | 0.015 | 0.139 | 0.452 | 0.4 | 1.25 |
| Perimeter 11b | 30.66 | 4.46 | ||||||||||||
| Segment | Mean | SD | ICC | Confidence Interval 95% | CV | SD | Min. | Max. | SEM | SEM% | MCD | MCD% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Segment Ia | 79.16 | 11.51 | 0.993 | (0.987 − 0.996) | 0.010 | 0.007 | 0.000 | 0.028 | 0.963 | 1.22 | 2.7 | 3.37 |
| Segment Ib | 79.07 | 11.84 | ||||||||||
| Segment IIa | 95.45 | 25.78 | 0.990 | (0.983 − 0.994) | 0.015 | 0.018 | 0.002 | 0.093 | 2.578 | 2.70 | 7.1 | 7.49 |
| Segment IIb | 95.03 | 25.67 | ||||||||||
| Segment IIIa | 121.07 | 33.52 | 0.994 | (0.990 − 0.997) | 0.017 | 0.014 | 0.001 | 0.069 | 2.596 | 2.14 | 7.2 | 5.94 |
| Segment IIIb | 120.45 | 34.60 | ||||||||||
| Segment IVa | 159.91 | 43.69 | 0.990 | (0.983 − 0.994) | 0.016 | 0.020 | 0.000 | 0.110 | 4.369 | 2.73 | 12.1 | 7.57 |
| Segment IVb | 159.21 | 44.29 | ||||||||||
| Segment Va | 191.82 | 48.26 | 0.995 | (0.991 − 0.997) | 0.012 | 0.013 | 0.000 | 0.062 | 3.412 | 1.78 | 9.5 | 4.93 |
| Segment Vb | 190.21 | 47.62 | ||||||||||
| Segment VIa | 200.16 | 43.44 | 0.991 | (0.984 − 0.995) | 0.010 | 0.009 | 0.000 | 0.057 | 4.121 | 2.06 | 11.4 | 5.71 |
| Segment VIb | 198.85 | 42.90 | ||||||||||
| Segment VIIa | 208.83 | 51.85 | 0.993 | (0.988 − 0.996) | 0.011 | 0.001 | 0.000 | 0.075 | 4.338 | 2.08 | 12.0 | 5.76 |
| Segment VIIb | 207.89 | 49.22 | ||||||||||
| Segment VIIIa | 230.81 | 63.82 | 0.997 | (0.995 − 0.998) | 0.012 | 0.008 | 0.000 | 0.040 | 3.495 | 1.51 | 9.7 | 4.20 |
| Segment VIIIb | 229.54 | 62.83 | ||||||||||
| Segment IXa | 257.60 | 70.55 | 0.997 | (0.994 − 0.998) | 0.011 | 0.009 | 0.000 | 0.042 | 3.864 | 1.50 | 10.7 | 4.16 |
| Segment IXb | 256.20 | 71.79 | ||||||||||
| Segment Xa | 273.57 | 83.17 | 0.996 | (0.993 − 0.998) | 0.012 | 0.012 | 0.000 | 0.061 | 5.260 | 1.92 | 14.6 | 5.33 |
| Segment Xb | 270.68 | 81.52 | ||||||||||
| Total arm a | 1794.8 | 489.6 | 0.999 | (0.997 − 0.999) | 0.007 | 0.008 | 0.000 | 0.035 | 15.483 | 0.863 | 42.9 | 2.39 |
| Total arm b | 1782.9 | 485.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tánori-Tapia, J.M.; Romero-Pérez, E.M.; Camberos, N.A.; Horta-Gim, M.A.; Núñez-Othón, G.; Medina-Pérez, C.; de Paz, J.A. Determination of the Minimum Detectable Change in the Total and Segmental Volumes of the Upper Limb, Evaluated by Perimeter Measurements. Healthcare 2020, 8, 285. https://doi.org/10.3390/healthcare8030285
Tánori-Tapia JM, Romero-Pérez EM, Camberos NA, Horta-Gim MA, Núñez-Othón G, Medina-Pérez C, de Paz JA. Determination of the Minimum Detectable Change in the Total and Segmental Volumes of the Upper Limb, Evaluated by Perimeter Measurements. Healthcare. 2020; 8(3):285. https://doi.org/10.3390/healthcare8030285
Chicago/Turabian StyleTánori-Tapia, José Manuel, Ena Monserrat Romero-Pérez, Néstor Antonio Camberos, Mario A. Horta-Gim, Gabriel Núñez-Othón, Carlos Medina-Pérez, and José Antonio de Paz. 2020. "Determination of the Minimum Detectable Change in the Total and Segmental Volumes of the Upper Limb, Evaluated by Perimeter Measurements" Healthcare 8, no. 3: 285. https://doi.org/10.3390/healthcare8030285
APA StyleTánori-Tapia, J. M., Romero-Pérez, E. M., Camberos, N. A., Horta-Gim, M. A., Núñez-Othón, G., Medina-Pérez, C., & de Paz, J. A. (2020). Determination of the Minimum Detectable Change in the Total and Segmental Volumes of the Upper Limb, Evaluated by Perimeter Measurements. Healthcare, 8(3), 285. https://doi.org/10.3390/healthcare8030285







