Feasibility of Using a Risk Assessment Tool to Predict Hospital Transfers or Death for Older People in Australian Residential Aged Care. A Retrospective Cohort Study
Abstract
1. Introduction
1.1. Aim
1.2. Objectives
- To examine the feasibility of identifying RACF residents who are in the last 6–12 months of life using modified CriSTAL tool criteria.
- To better understand the independent predictors of hospital transfers and death (after initial assessment).
- To examine predictors of a composite outcome of hospital transfer and/or death (after initial assessment).
1.3. Outcomes
2. Materials and Methods
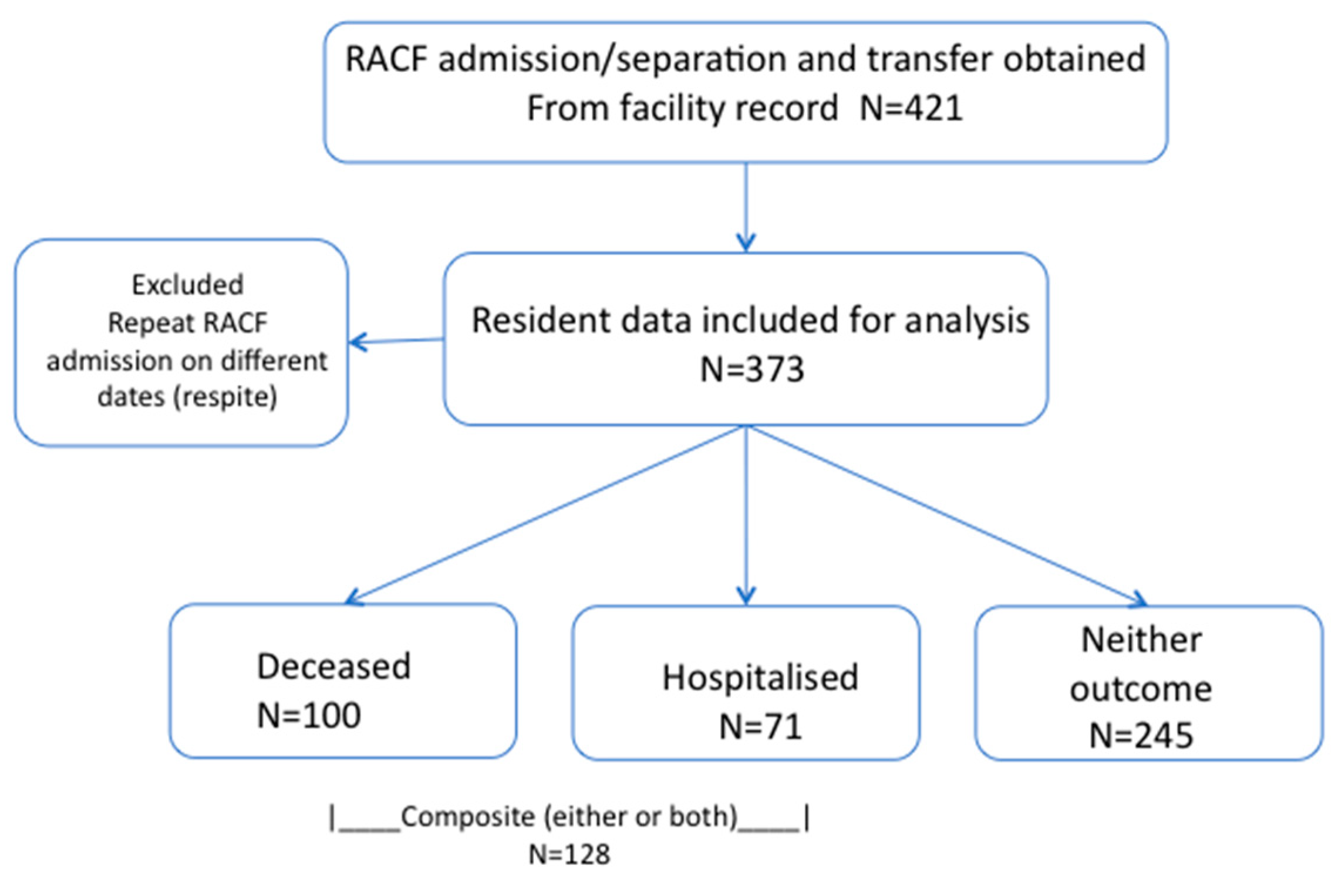
2.1. Participants
2.2. Data Collection
2.3. Ethical Considerations
2.4. Statistical Analysis
3. Results
3.1. Participant Profile
3.2. Poor Composite Outcome
4. Discussion
4.1. Comparison with Other Studies
4.2. Limitations
4.3. Implications of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- AIHW. Older Australia at a Glance. Available online: https://www.aihw.gov.au/getmedia/2cb104f4-c6d1-4728-9be3-a418840588de/Older-Australia-at-a-glance.pdf.aspx?inline=true: (accessed on 13 July 2020).
- Borowski, A. Longevity and Social Change in Australia; UNSW Press: Sydney, Australia, 2007. [Google Scholar]
- Ingarfield, S.; Finn, J.; Jacobs, I.; Gibson, N.; Holman, C.; Jelinek, G. Use of emergency departments by older people from residential aged care: A population based study. Age Ageing 2009, 38, 314–318. [Google Scholar] [CrossRef]
- Lalic, S.; Slugget, J.; Ilomaki, J.; Wimmer, B.; Tan, E.; Robson, L. Polypharmacy and Medication REgimen Complexity as Risk Factors for Hospitalisation Among Residents of Long Term Care Facilities: A Prospective Cohort Study. J. Am. Med. Direct. Assoc. 2016, 17, 1607.e1–1607.e6. [Google Scholar] [CrossRef]
- Arendts, G.; Dickson, C.; Howard, K.; Quine, S. Transfer from residential aged care to emergency departments an analysis of patient outcomes. Intern. Med. J. 2012, 42, 75–82. [Google Scholar] [CrossRef]
- O’Connell, B.; Hawkins, M.; Considine, J.; Au, C. Referrals to hospital emergency departments from residential aged care facilities: Stuck in a time warp. Contemp. Nurse 2013, 45, 228–233. [Google Scholar] [CrossRef]
- Kinley, J.; Hockley, J.; Stone, L.; Dewey, M.; Hansford, P.; Stewart, R.; McCrone, P.; Begum, A.; Sykes, N. The provision of care for residents dying in UK nursing care homes. Age Ageing 2013, 43, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.; Zordan, R.D.; Weiland, T.J.; Philip, J. Hospitalisation of high-care residents of aged care facilities: Are goals of care discussed? Intern. Med. J. 2013, 43, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, R.; Gabbe, B.; Stoelwinder, J.U.; Lowthian, J. A systematic review of outcomes following emergency transfer to hospital for residents of aged care facilities. Age Ageing 2014, 43, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Hillman, K.M.; Cardona-Morrell, M. The ten barriers to appropriate management of patients at the end of their life. Intensive Care Med. 2015, 41, 1700–1702. [Google Scholar] [CrossRef] [PubMed]
- Graverholt, B.; Forsetlund, L.; Jamtvedt, G. Reducing hospital admissions from nursing homes: A systematic review. BMC Health Serv. Res. 2014, 14, 36. [Google Scholar] [CrossRef]
- Cardona-Morrell, M.; Hillman, K. Development of a tool for defining and identifying the dying patient in hospital: Criteria for Screening and Triaging to Appropriate aLternative care (CriSTAL). BMJ Support. Palliat. Care 2015, 5, 78–90. [Google Scholar] [CrossRef]
- Cardona, M.; Lewis, E.T.; Kristensen, M.R.; Skjot-Arkil, H.; Ekmann, A.A.; Nygaard, H.H.; Jensen, J.J.; Jensen, R.O.; Pedersen, J.L.; Turner, R.M.; et al. Predictive validity of the CriSTAL tool for short-term mortality in older people presenting at Emergency Departments: A prospective study. Eur. Geriatr. Med. 2018, 9, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Cardona, M.; O’Sullivan, M.; Lewis, E.T.; Turner, R.M.; Garden, F.; Alkhouri, H.; Asha, S.; Mackenzie, J.; Perkins, M.; Suri, S.; et al. Prospective Validation of a Checklist to Predict Short-term Death in Older Patients After Emergency Department Admission in Australia and Ireland. Acad. Emerg. Med. 2018, 26, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, J.H.; Perry, H.M., III; Lynchard, G.S.; Morley, J.E. Polypharmacy and Hospitalization Among Older Home Care Patients. J. Gerontol. Ser. A 2000, 55, M554–M559. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.; Arthur, A.; Savva, G.M. How do potentially inappropriate medications and polypharmacy affect mortality in frail and non-frail cognitively impaired older adults? A cohort study. BMJ Open 2019, 9, e026171. [Google Scholar] [CrossRef]
- Paul, S.; Harvey, L.; Carroll, T.; Li, Q.; Boufous, S.; Priddis, A.; Tiedemann, A.; Clemson, L.; Lord, S.; Muecke, S.; et al. Trends in fall-related ambulance use and hospitalisation among older adults in NSW, 2006–2013: A retrospective, population-based study. Public Health Res. Pract. 2017, 27, e27341701. [Google Scholar] [CrossRef]
- Falcone, M.; Russo, A.; Gentiloni Silverj, F.; Marzorati, D.; Bagarolo, R.; Monti, M.; Velleca, R.; D’Angelo, R.; Frustaglia, A.; Zuccarelli, G.C.; et al. Predictors of mortality in nursing-home residents with pneumonia: A multicentre study. Clin. Microbiol. Infect. 2018, 24, 72–77. [Google Scholar] [CrossRef]
- Kamo, T.; Takayama, K.; Ishii, H.; Suzuki, K.; Eguchi, K.; Nishida, Y. Coexisting severe frailty and malnutrition predict mortality among the oldest old in nursing homes: A 1-year prospective study. Arch. Gerontol. Geriatr. 2017, 70, 99–104. [Google Scholar] [CrossRef]
- Teno, J.; Branco, K.; Mor, V.; Philips, C.; Hawes, C.; Morris, J.; Fries, B. Barriers to Advance Care Planning at the End of Life: An Explanatory Systematic Review of Implementation Studies. J. Am. Geriatr. Soc. 2015, 10, e0116629. [Google Scholar]
- Cardona-Morrell, M.; Kim, J.; Turner, R.M.; Anstey, M.; Mitchell, I.A.; Hillman, K. Non-beneficial treatments in hospital at the end of life: A systematic review on extent of the problem. Int. J. Qual. Health Care 2016, 28, 456–469. [Google Scholar] [CrossRef]
- Australian Government Department of Health. Cognitive Impairment Scale (PAS). Available online: https://www.health.gov.au/resources/publications/cognitive-impairment-scale-pas (accessed on 17 August 2020).
- Heinze, G.; Dunkler, D. Five myths about variable selection. Transpl. Int. 2017, 30, 6–10. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Kada, O.; Janig, H.; Likar, R.; Cernic, K.; Pinter, G. Reducing Avoidable Hospital Transfers From Nursing Homes in Austria: Project Outline and Baseline Results. Gerontol. Geriatr. Med. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Christian, R.; Baker, K. Effectiveness of Nurse Practitioners in nursing homes: A systematic review. JBI Libr. Syst. Rev. 2009, 7, 1333–1352. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lou, V.W.Q.; Li, Y.; Chi, I. Development and Validation of a Prognostic Tool for Identifying Residents at Increased Risk of Death in Long-Term Care Facilities. J. Palliat. Med. 2019, 22, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.; Wilson, A. Polypharmacy and falls in the elderly: A literature review. Nurs. Midwifery Stud. 2013, 2, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Leong, L.J.P.; Crawford, G.B. Residential aged care residents and components of end of life care in an Australian hospital. BMC Palliat. Care 2018, 17, 84. [Google Scholar] [CrossRef]
- Wester, P.; Angus, R.; Easlea, D.; Lin, M.; Chen, B.; Bisset, L. Use of the malnutrition screening tool by non-dietitians to identify at-risk patients in a rehabilitation setting: A validation study. Nutr. Diet. 2018, 75, 324–330. [Google Scholar] [CrossRef]
- Morphet, J.; Innes, K.; Griffiths, D.L.; Crawford, K.; Williams, A. Resident transfers from aged care facilities to emergency departments: Can they be avoided? Emerg. Med. Australas. 2015, 27, 412–418. [Google Scholar] [CrossRef]
- Lemoyne, S.E.; Herbots, H.H.; De Blick, D.; Remmen, R.; Monsieurs, K.G.; Van Bogaert, P. Appropriateness of transferring nursing home residents to emergency departments: A systematic review. BMC Geriatr. 2019, 19, 17. [Google Scholar] [CrossRef]
- Street, M.; Ottmann, G.; Johnstone, M.J.; Considine, J.; Livingston, P.M. Advance care planning for older people in Australia presenting to the emergency department from the community or residential aged care facilities. Health Soc. Care Community 2015, 23, 513–522. [Google Scholar] [CrossRef]
- Dwyer, R.; Stoelwinder, J.; Gabbe, B.; Lowthian, J. Unplanned Transfer to Emergency Departments for Frail Elderly Residents of Aged Care Facilities: A Review of Patient and Organizational Factors. J. Am. Med. Direct. Assoc. 2015, 16, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Karam, G.; Radden, Z.; Berall, L.E.; Cheng, C.; Gruneir, A. Efficacy of emergency department-based interventions designed to reduce repeat visits and other adverse outcomes for older patients after discharge: A systematic review. Geriatr. Gerontol. Int. 2015, 15, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Attree, M.; Jones, I.; Al Gamal, E.; Garbutt, D. Diagnosis, prognosis and awareness of dying in nursing homes: Towards the Gold Standard? Int. J. Older People Nurs. 2014, 9, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Dubucs, X.; de Souto Barreto, P.; Rolland, Y. Organizational and environmental factors associated with transfers of nursing home residents to emergency departments. Eur. Geriatr. Med. 2018, 9, 339–346. [Google Scholar] [CrossRef]
| Variable | n = 373 | Percentage (%) |
|---|---|---|
| Frailty | ||
| Not Frail | 11 | 2.9 |
| Mild/Moderately Frail | 181 | 48.5 |
| Severely Frail | 181 | 48.5 |
| Chronic Conditions | ||
| Advanced Malignancy ǂ | 7 | 1.9 |
| Chronic Kidney Disease | 3 | 0.8 |
| Congestive Heart Failure | 15 | 4.0 |
| Chronic Obstructive Pulmonary Disease | 41 | 11.0 |
| Cerebrovascular Disease | 64 | 17.2 |
| Myocardial Infarction | 14 | 3.8 |
| Liver Disease | 1 | 0.3 |
| ≥1 chronic condition | 129 | 34.6 |
| ≥2 chronic conditions | 16 | 4.3 |
| Cognitive Impairment | ||
| Dementia | 217 | 58.2 |
| Long Term Mental illness (Anxiety/Depression) | 141 | 37.8 |
| Behavioural Alterations | 7 | 1.9 |
| ≥1 Cognitive Impairment ψ | 277 | 74.3 |
| Nutritional Vulnerability | ||
| Sarcopenia | 1 | 0.3 |
| Malnutrition ∞ | 50 | 13.4 |
| Unintentional Weight Loss (>3 kg) | 20 | 5.4 |
| Feeding Tube | 3 | 0.8 |
| Feeding Dependency Ω | 22 | 5.9 |
| Modified Diet Types | 84 | 22.5 |
| ≥1 Nutritional Vulnerability Criteria § | 141 | 37.8 |
| ≥2 Falls in 6 Months | 98 | 26.4 |
| Unadjusted Poor Composite Outcomea | Fully Adjusted Poor Composite Outcome a | Unadjusted Death Outcome | Fully Adjusted Death Outcome | Unadjusted Hospital Admission | Fully Adjusted Hospital Admission | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age (continuous) | 1.02 (0.99–1.04) | 1.01 (0.99–1.04) | 1.03 (1.00–1.06) * | 1.03 (0.99–1.06) | 1.00 (0.97–1.04) | 1.00 (0.97–1.04) |
| Male sex | 1.34 (0.94–1.90) | 1.43 (0.98–2.07) | 1.23 (0.823–1.83) | 1.12 (0.72–1.74) | 1.68 (1.05–2.70) * | 1.72 (1.05–2.82) * |
| Severe Frailty (as per CFS) b | 1.45 (1.02–2.07) * | 1.26 (0.86–1.84) | 1.58 (1.05–2.38) * | 1.26 (0.81–1.96) | 1.26 (0.792–2.02) | 1.25 (0.75-)2.08 |
| Chronic heart failure | 1.46 (0.68–3.14) | 1.61 (0.73–3.55) | 1.62 (0.71–3.72) | 1.31 (0.54–3.17) | 1.81 (0.73–4.52) | 2.06 (0.79–5.36) |
| COPD c | 2.28 (1.40–3.73) ** | 2.88 (1.71–4.85) ** | 2.35 (1.35–4.08) ** | 3.14 (1.72–5.74) *** | 1.59 (0.76–3.32) | 1.72 (0.79–3.73) |
| Stroke | 0.89 (0.57–1.40) | 0.98 (0.62–1.56) | 0.90 (0.54–1.50) | 0.83 (0.49–1.42) | 1.11 (0.63–1.98) | 1.23 (0.67–2.23) |
| Myocardial infarction | 0.45 (0.11–1.80) | 0.42 (0.10–1.71) | 0.57 (0.14–2.33) | 0.75 (0.18–3.16) | 0.39 (0.06–2.83) | 0.36 (0.05–2.68) |
| Cognitive Impairment d | 1.50 (0.92–2.42) | 1.56 (0.95–2.56) | 1.85 (1.02–3.32) * | 2.22 (1.18–4.16) * | 1.21 (0.66–2.20) | 1.30 (0.70–2.41) |
| Nutritional Vulnerability e | 1.10 (0.77–1.56) | 1.20 (0.82–1.75) | 1.17 (0.79–1.76) | 1.38 (0.89–2.12) | 0.87(0.54–1.43) | 0.87 (0.52–1.47) |
| Falls in the past 6 months f | 2.18 (1.48–3.20) *** | 2.22 (1.47–3.34) *** | 2.03 (1.34–3.08) *** | 1.93 (1.23–3.01) ** | 1.65 (0.99–2.73) | 1.65 (0.98–2.79) |
| Hospitalisation for ≥1 night | – | – | 2.99 (2.00–4.47) *** | 2.96 (1.93–4.55) *** | – | – |
| Adjusted Predictors of Poor Follow-Up Outcome | Poor Composite Outcome § | Death | Hospital Admission | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.02 (0.99–1.05) | 0.192 | 1.03 (1.00–1.06) | 0.071 | 1.01 (0.98–1.05) | 0.568 |
| Male | 1.41 (0.97–2.05) | 0.070 | 1.11 (0.72–2.73) | 0.628 | 1.78 (1.09–2.91) | 0.022 |
| COPD | 2.64 (1.59–4.39) | <0.001 | 3.22 (1.78–5.81) | 0.0001 | ||
| Cognitive impairment | 1.49 (0.91–2.42) | 0.111 | 2.22 (1.18–4.15) | 0.013 | ||
| Falls in past 6 months | 2.14 (1.46–3.12) | <0.001 | 2.02 (1.31–3.14) | 0.002 | 1.64 (0.98–2.75) | 0.059 |
| Hospital admission | 2.99 (1.97–4.53) | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooi, M.; Lewis, E.T.; Brisbane, J.; Tubb, E.; McClean, T.; Assareh, H.; Hillman, K.; Achat, H.; Cardona, M. Feasibility of Using a Risk Assessment Tool to Predict Hospital Transfers or Death for Older People in Australian Residential Aged Care. A Retrospective Cohort Study. Healthcare 2020, 8, 284. https://doi.org/10.3390/healthcare8030284
Ooi M, Lewis ET, Brisbane J, Tubb E, McClean T, Assareh H, Hillman K, Achat H, Cardona M. Feasibility of Using a Risk Assessment Tool to Predict Hospital Transfers or Death for Older People in Australian Residential Aged Care. A Retrospective Cohort Study. Healthcare. 2020; 8(3):284. https://doi.org/10.3390/healthcare8030284
Chicago/Turabian StyleOoi, Meidelynn, Ebony T Lewis, Julianne Brisbane, Evalynne Tubb, Tom McClean, Hassan Assareh, Ken Hillman, Helen Achat, and Magnolia Cardona. 2020. "Feasibility of Using a Risk Assessment Tool to Predict Hospital Transfers or Death for Older People in Australian Residential Aged Care. A Retrospective Cohort Study" Healthcare 8, no. 3: 284. https://doi.org/10.3390/healthcare8030284
APA StyleOoi, M., Lewis, E. T., Brisbane, J., Tubb, E., McClean, T., Assareh, H., Hillman, K., Achat, H., & Cardona, M. (2020). Feasibility of Using a Risk Assessment Tool to Predict Hospital Transfers or Death for Older People in Australian Residential Aged Care. A Retrospective Cohort Study. Healthcare, 8(3), 284. https://doi.org/10.3390/healthcare8030284






