The New Diagnosis-Related Group Reimbursement System and Laboratory Test Quality in Korea: Analysis of External Quality Assessment Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Data Source
2.3. Performance of General Chemistry Tests
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. General Characteristics of Study Subjects
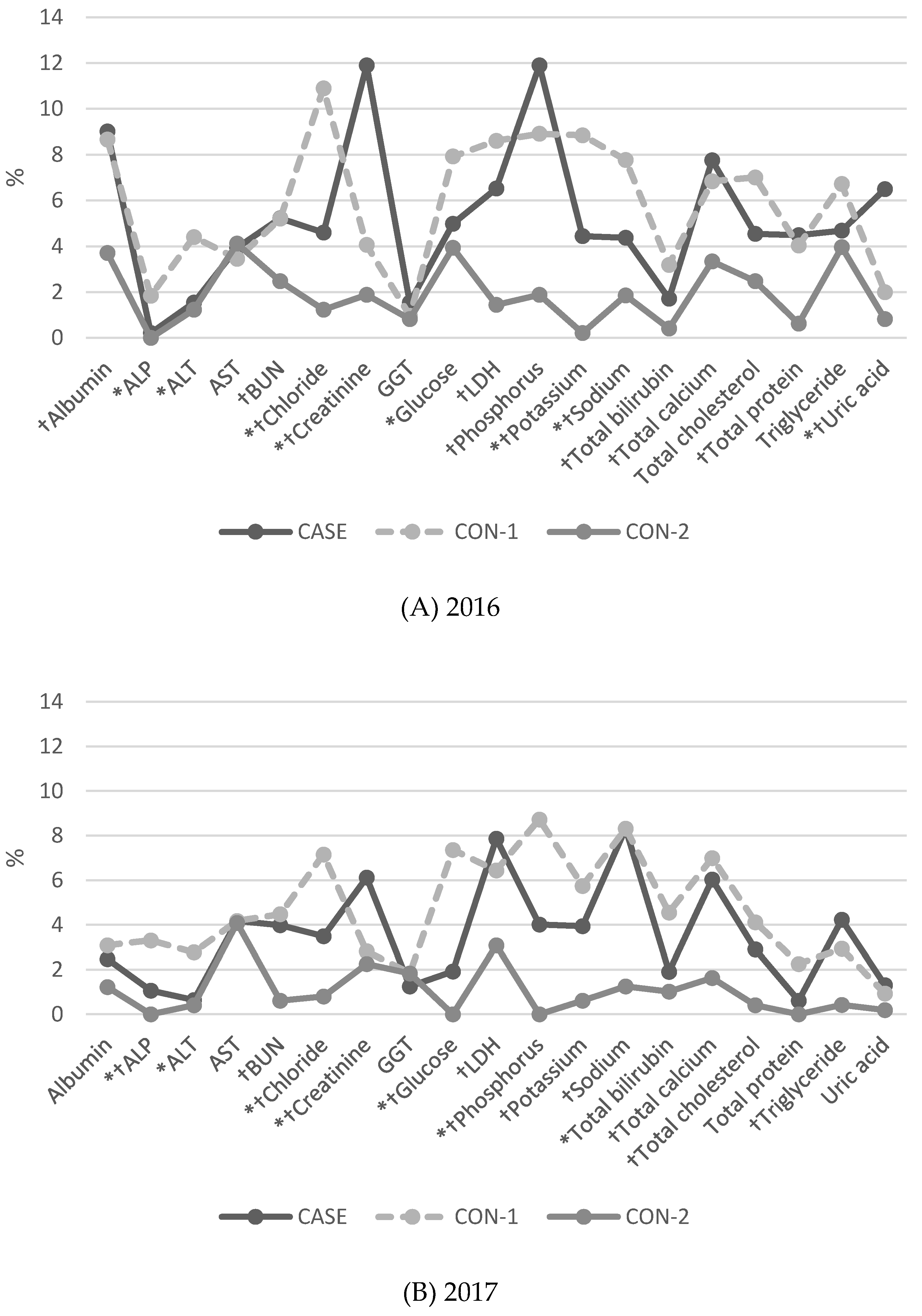
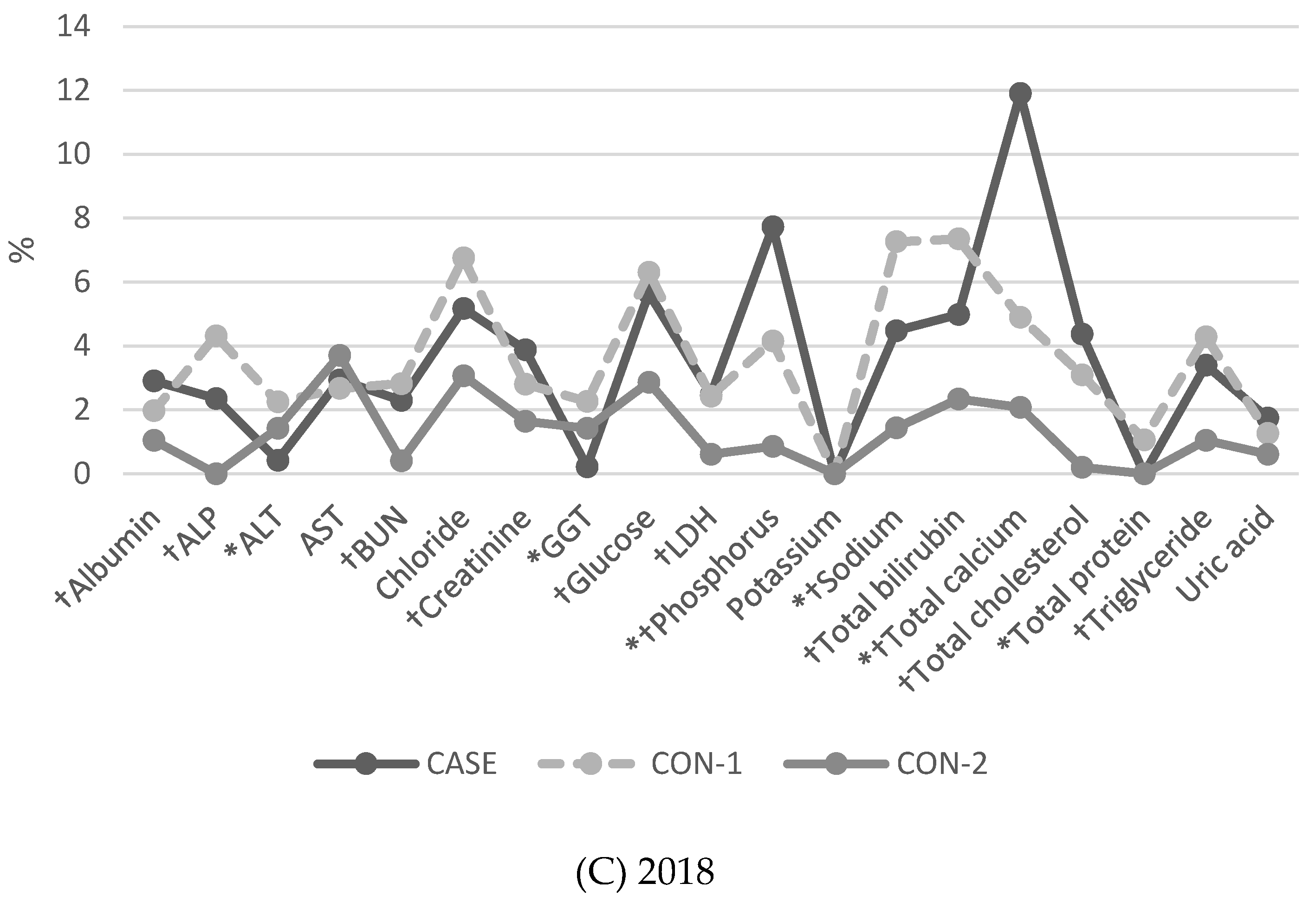
3.2. Proportion of Results of More than 2 SDI (BLQM) in General Chemistry Tests
3.3. Differences in Mean SDIs for General Chemistry Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, K.H.; Lee, S.C.; Lee, S.K.; Choi, B.J.; Jeong, W.; Kim, S.J. Does Korea’s current diagnosis-related group-based reimbursement system appropriately classify appendectomy patients? Ann. Surg. Treat. Res. 2016, 91, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecellio, E.; Li, L.; Xiong, J.; Georgiou, A.; Eigenstetter, A.; Gibson-Roy, C.; Cobain, T.; Golding, M.; Wilson, R.; Lindeman, R.; et al. Examination of Variation in Hospital Pathology Investigations by Diagnosis-Related Groups and Associations with Outcomes and Costs; Centre for Health Systems and Safety Research, Macquarie University: Sydney, Australia, 2015. [Google Scholar]
- Geissler, A.; Scheller-Kreinsen, D.; Quentin, W.; Euro, D.R.G.G. Do diagnosis-related groups appropriately explain variations in costs and length of stay of hip replacement? A comparative assessment of DRG systems across 10 European countries. Health Econ. 2012, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassler, M.; Wild, V.; Clarinval, C.; Tschopp, A.; Faehnrich, J.A.; Biller-Andorno, N. Impact of the DRG-based reimbursement system on patient care and professional practise: Perspectives of Swiss hospital physicians. Swiss Med. Wkly. 2015, 145, w14080. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, S.J.; Park, H.K.; Jang, S.I.; Kim, T.H.; Park, E.C. Effects of a mandatory DRG payment system in South Korea: Analysis of multi-year nationwide hospital claims data. BMC Health Serv Res. 2019, 19, 776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.Y.; Yeh, C.F.; Shiao, A.S.; Tu, T.Y. Effects of diagnosis-related group payment on health-care provider behaviors: A consecutive three-period study. J. Chin. Med. Assoc. 2015, 78, 678–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.W.; Pak, H.; Lee, I.; Kim, E.H. The Effect of Diagnosis-Related Group Payment System on Quality of Care in the Field of Obstetrics and Gynecology among Korean Tertiary Hospitals. Yonsei Med. J. 2018, 59, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Han, K.T.; Kim, W.; Kim, S.J.; Park, E.C. Early Impact on Outpatients of Mandatory Adoption of the Diagnosis-Related Group-Based Reimbursement System in Korea on Use of Outpatient Care: Differences in Medical Utilization and Presurgery Examination. Health Serv Res. 2018, 53, 2064–2083. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Kim, S.H.; Shin, D.G.; Kang, J.G. The Effect of Reform of New-Diagnosis Related Groups (KDRGs) on Accuracy of Payment. Health Policy Manag. 2017, 27, 211–218. [Google Scholar] [CrossRef]
- Kim, M. Status and Future Challenges of the New DRG system. In Policy Trends; Health Insurance Review & Assessment Service: Wonju-si, Korea, 2017; Volume 11, pp. 7–13. [Google Scholar]
- Kim, G.-D.; Park, J.H. Effects of New Diagnosis-Related Group-Based Payment on Efficiency of Public Hospitals. Korean J. Public Adm. 2018, 56, 33–57. [Google Scholar] [CrossRef]
- Ngo, A.; Gandhi, P.; Miller, W.G. Frequency that Laboratory Tests Influence Medical Decisions. J. Appl. Lab. Med. 2017, 1, 410–414. [Google Scholar] [CrossRef] [Green Version]
- World Health Organizsation. WHO Manual for Organizing a Nati Onal External Quality Assessment Programme for Health Laboratories and Other Testing Sites; WHO Press: Geneva, Switzerland, 2016. [Google Scholar]
- Korean Ministry of Health and Welfare. Details on the application criteria and methods of healthcare benefit. In Korean Ministry of Health and Welfare Notification 2017-111; Korean Ministry of Health and Welfare: Sejong-si, Korea, 2017. [Google Scholar]
- Korean Medical Service Act. Available online: https://elaw.klri.re.kr/ (accessed on 1 April 2020).
- Krleza, J.L.; Celap, I.; Tanaskovic, J.V. External Quality Assessment in Croatia: Problems, challenges, and specific circumstances. Biochem Med. 2017, 27, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Yun, Y.M.; Um, T.H.; Chang, J.; Lee, K.S.; Chun, S.; Cho, K.D.; Han, T.H. Status of Quality Control for Laboratory Tests of Medical Institutions in Korea: Analysis of 10 Years of Data on External Quality Assessment Participation. Healthcare 2020, 8, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.A.; Yoon, Y.A.; Song, J.; Kim, J.H.; Min, W.K.; Lee, J.S.; Lee, Y.W.; Lee, Y.K. Effect of Accreditation on Accuracy of Diagnostic Tests in Medical Laboratories. Ann. Lab. Med. 2017, 37, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takemura, Y.; Beck, J.R. The effects of a fixed-fee reimbursement system introduced by the Federal Government on laboratory testing in the United States. Rinsho Byori 1999, 47, 1–10. [Google Scholar] [PubMed]
- Shimetani, N. Medical reimbursement for diagnostic tests. Expert Rev. Pharm. Outcomes Res. 2004, 4, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Department of Medical Insurance Benefits Development. Details of Health Insurance Care Benefits Expenses; Health Insurance Review and Assessment Service: Seoul, Korea, 2003. [Google Scholar]
- Jun, J.K.; Sung, N.Y.; Song, S.H.; Hong, S.; Jang, M.A.; Song, J.; Kim, J.H.; Min, W.K.; Lee, Y.K. Budget Impact of the Accreditation Program for Clinical Laboratories on Colorectal Cancer Screening via Fecal Immunochemical Testing: Results from the National Cancer Screening Program in Korea. Ann. Lab. Med. 2018, 38, 249–254. [Google Scholar] [CrossRef] [PubMed]
| CASE | CON-1 | CON-2 | ||||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Total number | 42 | 100 | 84 | 100 | 42 | 100 |
| NDRG participation time | ||||||
| at Sep 2012 | 36 | NA | NA | |||
| after Sep 2012 | 6 | NA | NA | |||
| Type of medical institution | ||||||
| Small-to-medium hospital | 9 | 21.4 | 11 | 13.1 | 0 | 0.0 |
| General hospital | 33 | 78.6 | 72 | 85.7 | 0 | 0.0 |
| Tertiary hospital | 0 | 0.0 | 1 | 1.2 | 42 | 100.0 |
| Number of beds in institution | ||||||
| <100 | 4 | 9.5 | 8 | 9.5 | 0 | 0.0 |
| 100–500 | 33 | 78.6 | 66 | 78.6 | 0 | 0.0 |
| ≥500 | 5 | 11.9 | 10 | 11.9 | 42 | 100.0 |
| LMF accreditation † | ||||||
| 2016 | 17 | 40.5 | 58 | 69.0 | 42 | 100.0 |
| 2017 | 18 | 42.9 | 58 | 69.0 | 42 | 100.0 |
| 2018 | 18 | 42.9 | 58 | 69.0 | 42 | 100.0 |
| Test Item | Year | SDI (95% CI) | p-Value (ANOVA) | Multiple Comparison (Duncan) | ||
|---|---|---|---|---|---|---|
| CASE | CON-1 | CON-2 | ||||
| Albumin | 2016 | 0.59 (0.53–0.65) | 0.69 (0.64–0.73) | 0.62 (0.57–0.68) | 0.0161 | (CASE, CON-1) * |
| 2017 | 0.50 (0.45–0.56) | 0.46 (0.42–0.50) | 0.50 (0.45–0.55) | 0.3529 | ||
| 2018 | 0.44 (0.37–0.51) | 0.40 (0.36–0.44) | 0.42 (0.37–0.49) | 0.5677 | ||
| ALP | 2016 | 0.61 (0.56–0.67) | 0.59 (0.55–0.63) | 0.80 (0.74–0.86) | <0.0001 | (CASE, CON-2) (CON-1, CON-2) |
| 2017 | 0.62 (0.57–0.68) | 0.58 (0.54–0.63) | 0.77 (0.71–0.84) | <0.0001 | (CASE, CON-2) (CON-1, CON-2) | |
| 2018 | 0.72 (0.66–0.79) | 0.63 (0.59–0.68) | 0.86 (0.79–0.94) | <0.0001 | (CASE, CON-1) (CASE, CON-2) (CON-1, CON-2) | |
| ALT | 2016 | 0.41 (0.37–0.46) | 0.47 (0.43–0.50) | 0.52 (0.47–0.57) | 0.01 | (CASE, CON-2) |
| 2017 | 0.31 (0.27–0.36) | 0.33 (0.30–0.37) | 0.32 (0.28–0.37) | 0.7598 | ||
| 2018 | 0.43 (0.38–0.48) | 0.46 (0.43–0.50) | 0.50 (0.45–0.55) | 0.1296 | (CASE, CON-2) | |
| AST | 2016 | 0.50 (0.45–0.55) | 0.55 (0.52–0.59) | 0.65 (0.59–0.71) | 0.0004 | (CASE, CON-2) (CON-1, CON-2) |
| 2017 | 0.34 (0.29–0.40) | 0.41 (0.38–0.46) | 0.47 (0.41–0.54) | 0.0079 | (CASE, CON-1) (CASE, CON-2) | |
| 2018 | 0.41 (0.36–0.47) | 0.45 (0.42–0.49) | 0.47 (0.41–0.53) | 0.3206 | ||
| BUN | 2016 | 0.51 (0.47–0.56) | 0.50 (0.47–0.54) | 0.38 (0.34–0.42) | <0.0001 | (CASE, CON-2) (CON-1, CON-2) |
| 2017 | 0.41 (0.36–0.46) | 0.42 (0.39–0.46) | 0.31 (0.28–0.35) | 0.0002 | (CASE, CON-2) (CON-1, CON-2) | |
| 2018 | 0.42 (0.37–0.46) | 0.43 (0.40–0.47) | 0.40 (0.36–0.44) | 0.4678 | ||
| Chloride | 2016 | 0.54 (0.50–0.59) | 0.63 (0.59–0.67) | 0.45 (0.42–0.49) | <0.0001 | (CASE, CON-1) (CASE, CON-2) (CON-1, CON-2) |
| 2017 | 0.51 (0.45–0.57) | 0.51 (0.47–0.56) | 0.52 (0.47–0.58) | 0.9161 | ||
| 2018 | 0.38 (0.33–0.45) | 0.47 (0.42–0.52) | 0.43 (0.38–0.49) | 0.099 | ||
| Creatinine | 2016 | 0.62 (0.55–0.69) | 0.50 (0.46–0.53) | 0.38 (0.34–0.42) | <0.0001 | (CASE, CON-1) (CASE, CON-2) (CON-1, CON-2) |
| 2017 | 0.50 (0.45–0.55) | 0.40 (0.38–0.43) | 0.36 (0.33–0.41) | 0.0002 | (CASE, CON-1) (CASE, CON-2) | |
| 2018 | 0.45 (0.41–0.50) | 0.41 (0.39–0.45) | 0.38 (0.34–0.43) | 0.1 | ||
| GGT | 2016 | 0.52 (0.47–0.58) | 0.53 (0.49–0.57) | 0.44 (0.39–0.49) | 0.0126 | (CASE, CON-2) (CON-1, CON-2) |
| 2017 | 0.41 (0.36–0.47) | 0.46 (0.42–0.50) | 0.48 (0.43–0.53) | 0.1426 | ||
| 2018 | 0.42 (0.37–0.47) | 0.43 (0.39–0.47) | 0.42 (0.36–0.48) | 0.9065 | ||
| Glucose | 2016 | 0.38 (0.34–0.43) | 0.51 (0.48–0.56) | 0.40 (0.36–0.44) | <0.0001 | (CASE, CON-1) (CON-1, CON-2) |
| 2017 | 0.36 (0.32–0.42) | 0.49 (0.45–0.54) | 0.33 (0.29–0.38) | <0.0001 | (CASE, CON-1) (CON-1, CON-2) | |
| 2018 | 0.36 (0.31–0.41) | 0.38 (0.34–0.43) | 0.30 (0.25–0.35) | 0.0268 | (CON-1, CON-2) | |
| LDH | 2016 | 0.50 (0.45–0.56) | 0.55 (0.50–0.59) | 0.37 (0.33–0.42) | <0.0001 | (CASE, CON-2) (CON-1, CON-2) |
| 2017 | 0.45 (0.38–0.52) | 0.40 (0.35–0.45) | 0.37 (0.32–0.43) | 0.2316 | ||
| 2018 | 0.58 (0.52–0.66) | 0.59 (0.54–0.64) | 0.57 (0.52–0.62) | 0.9349 | ||
| Phosphorus | 2016 | 0.56 (0.49–0.64) | 0.45 (0.41–0.50) | 0.34 (0.29–0.39) | <0.0001 | (CASE, CON-1) (CASE, CON-2) (CON-1, CON-2) |
| 2017 | 0.33 (0.27–0.40) | 0.41 (0.36–0.46) | 0.34 (0.30–0.40) | 0.0641 | ||
| 2018 | 0.42 (0.36–0.51) | 0.35 (0.31–0.40) | 0.33 (0.28–0.38) | 0.0828 | ||
| Potassium | 2016 | 0.52 (0.47–0.57) | 0.57 (0.52–0.62) | 0.50 (0.46–0.54) | 0.0605 | |
| 2017 | 0.19 (0.16–0.24) | 0.22 (0.19–0.25) | 0.16 (0.13–0.20) | 0.0652 | ||
| 2018 | 0.31 (0.26–0.38) | 0.34 (0.30–0.39) | 0.31 (0.26–0.36) | 0.5434 | ||
| Sodium | 2016 | 0.51 (0.45–0.57) | 0.54 (0.50–0.59) | 0.44 (0.40–0.49) | 0.0252 | (CASE, CON-2) |
| 2017 | 0.38 (0.32–0.46) | 0.44 (0.39–0.49) | 0.28 (0.23–0.33) | <0.0001 | (CASE, CON-2) | |
| 2018 | 0.27 (0.23–0.32) | 0.37 (0.33–0.42) | 0.37 (0.32–0.43) | 0.008 | (CASE, CON-1) | |
| Total bilirubin | 2016 | 0.56 (0.51–0.60) | 0.68 (0.64–0.72) | 0.60 (0.55–0.65) | 0.0004 | (CASE, CON-1) (CON-1, CON-2) |
| 2017 | 0.63 (0.58–0.69) | 0.61 (0.57–0.65) | 0.56 (0.51–0.62) | 0.1957 | ||
| 2018 | 0.61 (0.56–0.68) | 0.59 (0.55–0.63) | 0.67 (0.62–0.73) | 0.0998 | ||
| Total calcium | 2016 | 0.59 (0.53–0.66) | 0.46 (0.42–0.50) | 0.42 (0.37–0.47) | <0.0001 | (CASE, CON-1) (CASE, CON-2) |
| 2017 | 0.46 (0.36–0.53) | 0.40 (0.36–0.44) | 0.27 (0.23–0.32) | <0.0001 | (CASE, CON-2) (CON-1, CON-2) | |
| 2018 | 0.52 (0.46–0.59) | 0.40 (0.37–0.44) | 0.33 (0.29–0.38) | <0.0001 | (CASE, CON-1) (CASE, CON-2) (CON-1, CON-2) | |
| Total cholesterol | 2016 | 0.42 (0.37–0.47) | 0.53 (0.49–0.58) | 0.39 (0.35–0.44) | <0.0001 | (CASE, CON-1) (CON-1, CON-2) |
| 2017 | 0.39 (0.34–0.45) | 0.49 (0.45–0.53) | 0.39 (0.35–0.44) | 0.0009 | (CASE, CON-1) (CON-1, CON-2) | |
| 2018 | 0.44 (0.40–0.50) | 0.47 (0.44–0.51) | 0.46 (0.41–0.51) | 0.6273 | ||
| Total protein | 2016 | 0.35 (0.30-0.42) | 0.32 (0.29-0.37) | 0.24 (0.20–0.29) | 0.0062 | (CASE, CON-2) (CON-1, CON-2) |
| 2017 | 0.37 (0.33–0.42) | 0.45 (0.41–0.48) | 0.33 (0.29–0.37) | <0.0001 | (CASE, CON-1) (CON-1, CON-2) | |
| 2018 | 0.31 (0.27–0.36) | 0.34 (0.31–0.38) | 0.24 (0.20–0.28) | 0.0006 | (CASE, CON-2) | |
| Triglyceride | 2016 | 0.44 (0.39–0.48) | 0.51 (0.48–0.55) | 0.39 (0.35–0.44) | 0.0001 | (CASE, CON-1) (CON-1, CON-2) |
| 2017 | 0.40 (0.35–0.46) | 0.38 (0.34–0.42) | 0.31 (0.27–0.35) | 0.0193 | (CASE, CON-2) (CON-1, CON-2) | |
| 2018 | 0.38 (0.33–0.43) | 0.42 (0.38–0.46) | 0.33 (0.29–0.37) | 0.0057 | (CON-1, CON-2) | |
| Uric acid | 2016 | 0.49 (0.43–0.55) | 0.45 (0.41–0.49) | 0.45 (0.39–0.51) | 0.6028 | |
| 2017 | 0.35 (0.31–0.41) | 0.42 (0.38–0.46) | 0.37 (0.33–0.42) | 0.0916 | ||
| 2018 | 0.43 (0.37–0.49) | 0.32 (0.28–0.36) | 0.38 (0.33–0.44) | 0.0056 | (CASE, CON-1) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Yun, Y.-M.; Kim, H.; Um, T.-H.; Chang, J.; Jeong, H.; Lee, K.S.; Chun, S.; Choi, Y.-J.; Heo, J.-H.; et al. The New Diagnosis-Related Group Reimbursement System and Laboratory Test Quality in Korea: Analysis of External Quality Assessment Results. Healthcare 2020, 8, 127. https://doi.org/10.3390/healthcare8020127
Kim S, Yun Y-M, Kim H, Um T-H, Chang J, Jeong H, Lee KS, Chun S, Choi Y-J, Heo J-H, et al. The New Diagnosis-Related Group Reimbursement System and Laboratory Test Quality in Korea: Analysis of External Quality Assessment Results. Healthcare. 2020; 8(2):127. https://doi.org/10.3390/healthcare8020127
Chicago/Turabian StyleKim, Sollip, Yeo-Min Yun, Hyeongsu Kim, Tae-Hyun Um, Jeonghyun Chang, Hojin Jeong, Kun Sei Lee, Sail Chun, Yong-Jun Choi, Jae-Hyeok Heo, and et al. 2020. "The New Diagnosis-Related Group Reimbursement System and Laboratory Test Quality in Korea: Analysis of External Quality Assessment Results" Healthcare 8, no. 2: 127. https://doi.org/10.3390/healthcare8020127
APA StyleKim, S., Yun, Y.-M., Kim, H., Um, T.-H., Chang, J., Jeong, H., Lee, K. S., Chun, S., Choi, Y.-J., Heo, J.-H., & Han, T.-H. (2020). The New Diagnosis-Related Group Reimbursement System and Laboratory Test Quality in Korea: Analysis of External Quality Assessment Results. Healthcare, 8(2), 127. https://doi.org/10.3390/healthcare8020127






