Characterization and Adsorption Behavior of Strontium from Aqueous Solutions onto Chitosan-Fuller’s Earth Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan-Fuller’s Earth (CF) Beads
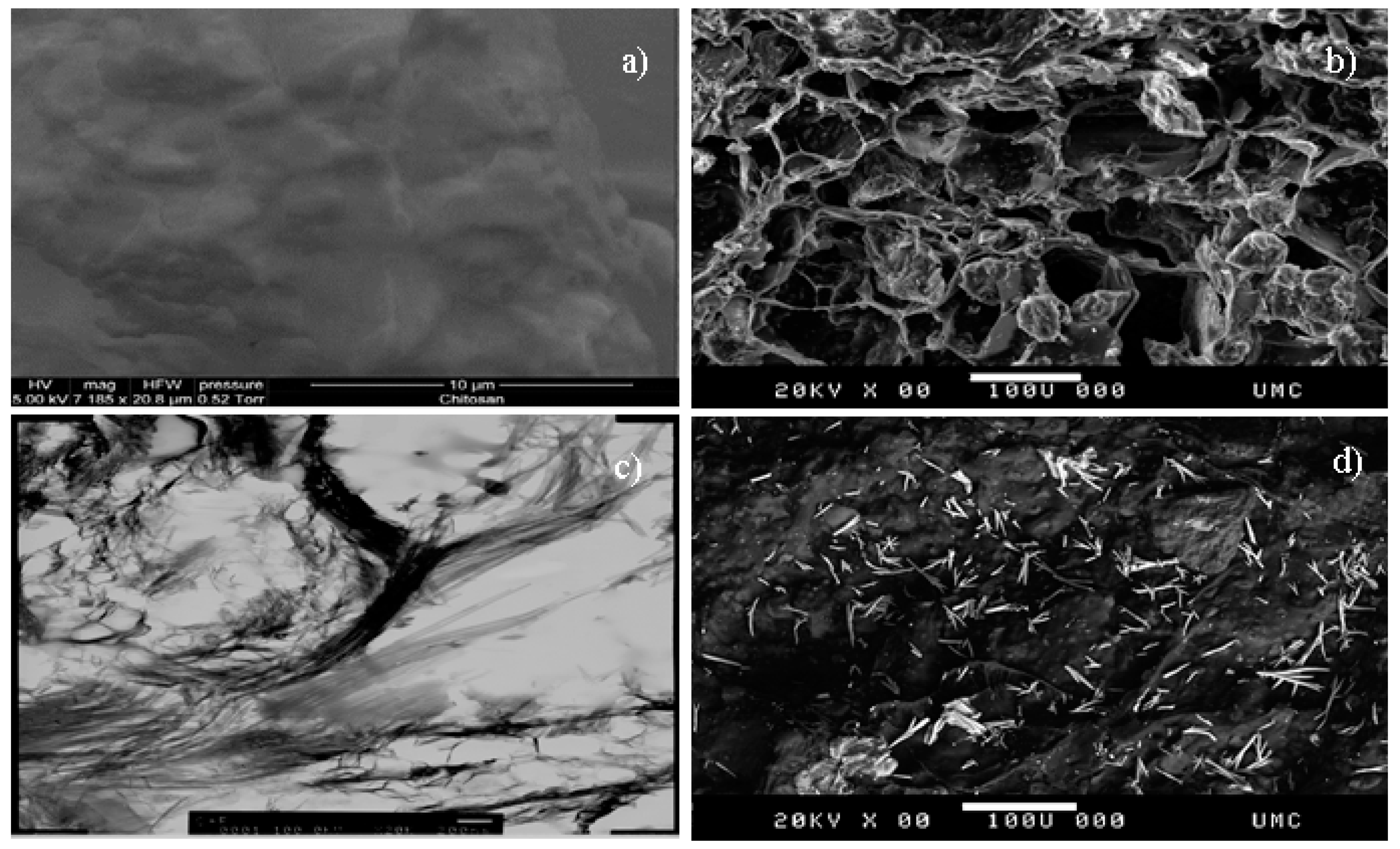
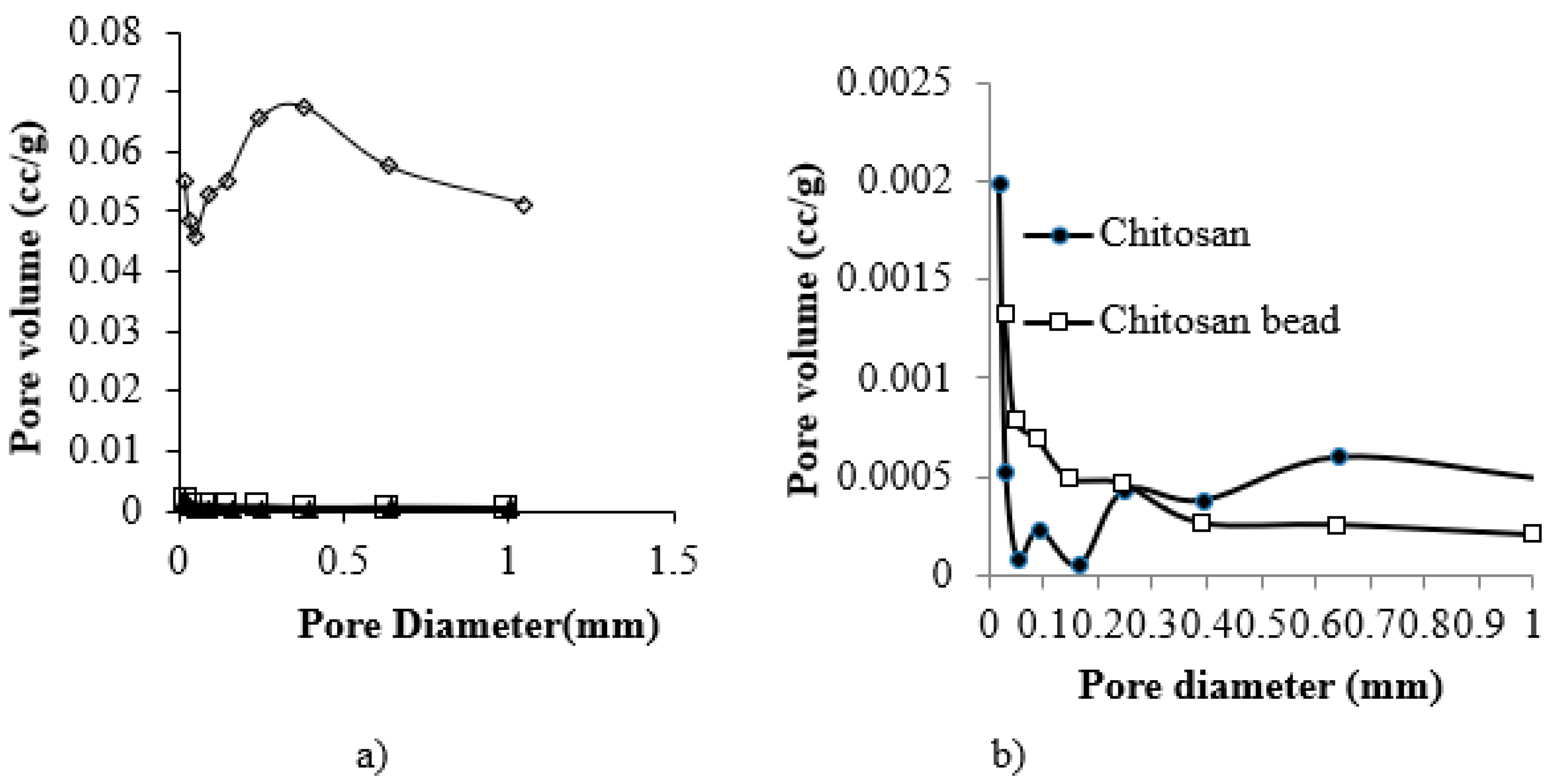
2.3. Characterization
2.4. Experimental Procedure
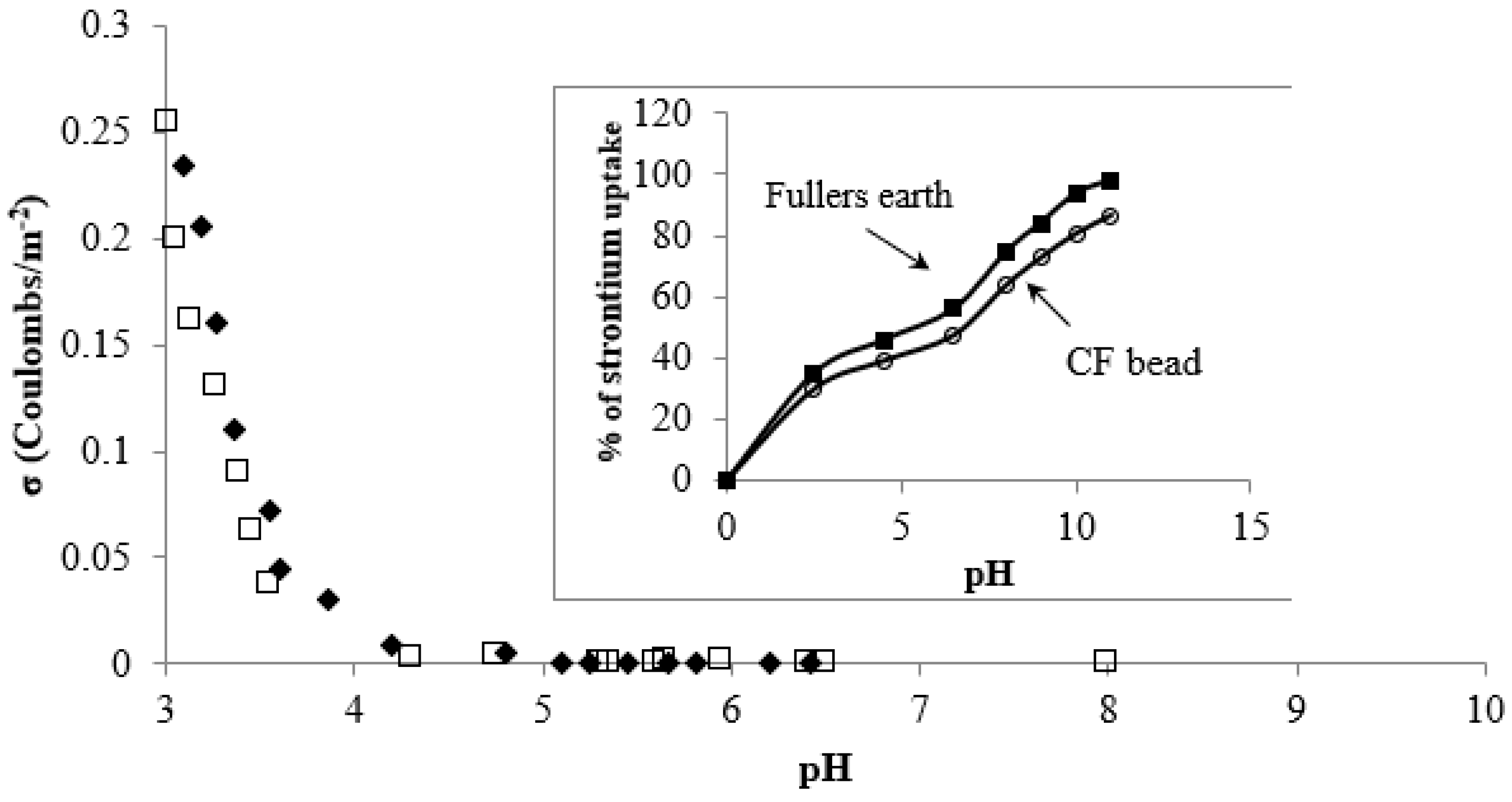
2.4.1. Effect of pH on Strontium Adsorption onto CF Beads
2.4.2. Batch Sorption Experiments of CF Beads
3. Results and Discussion
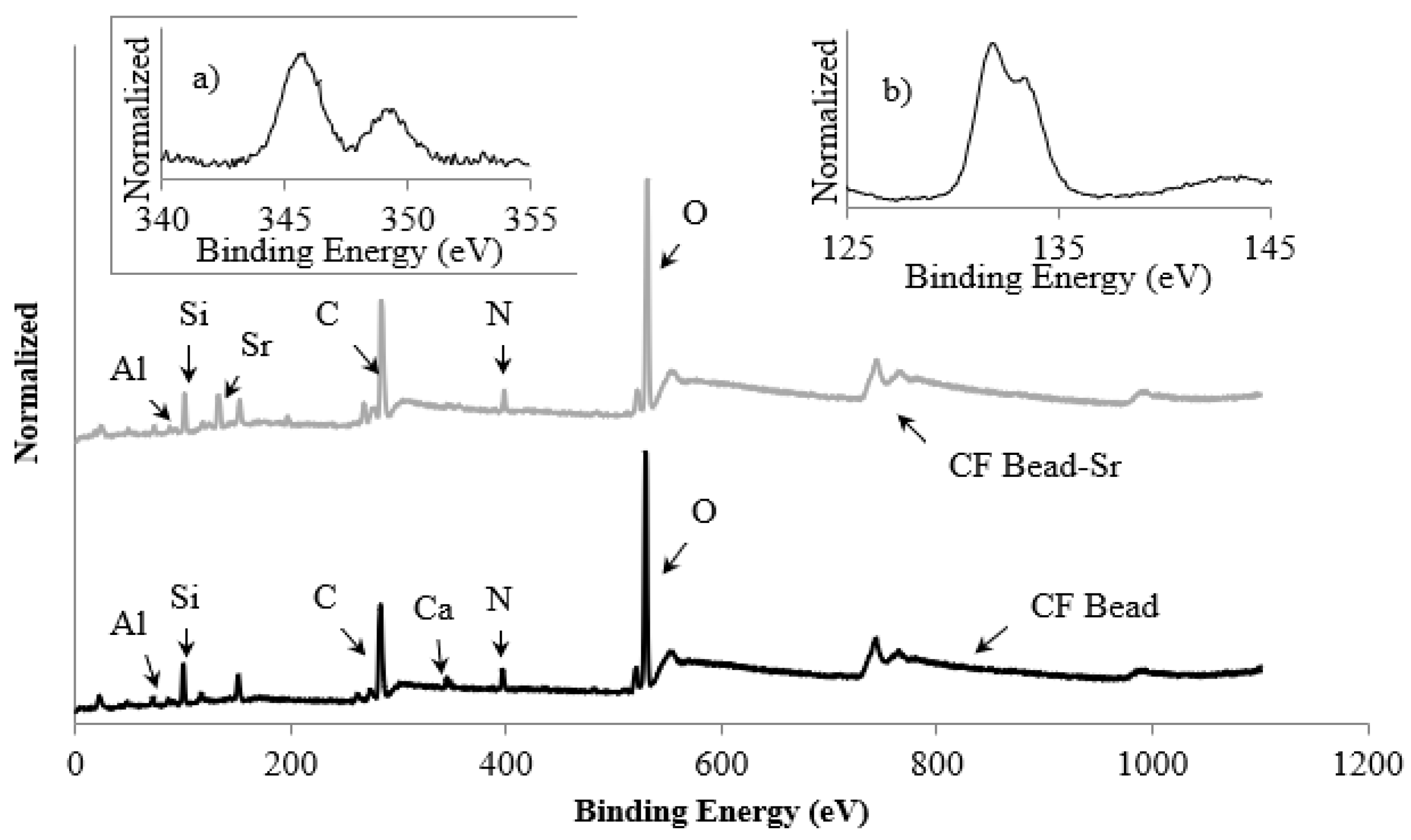
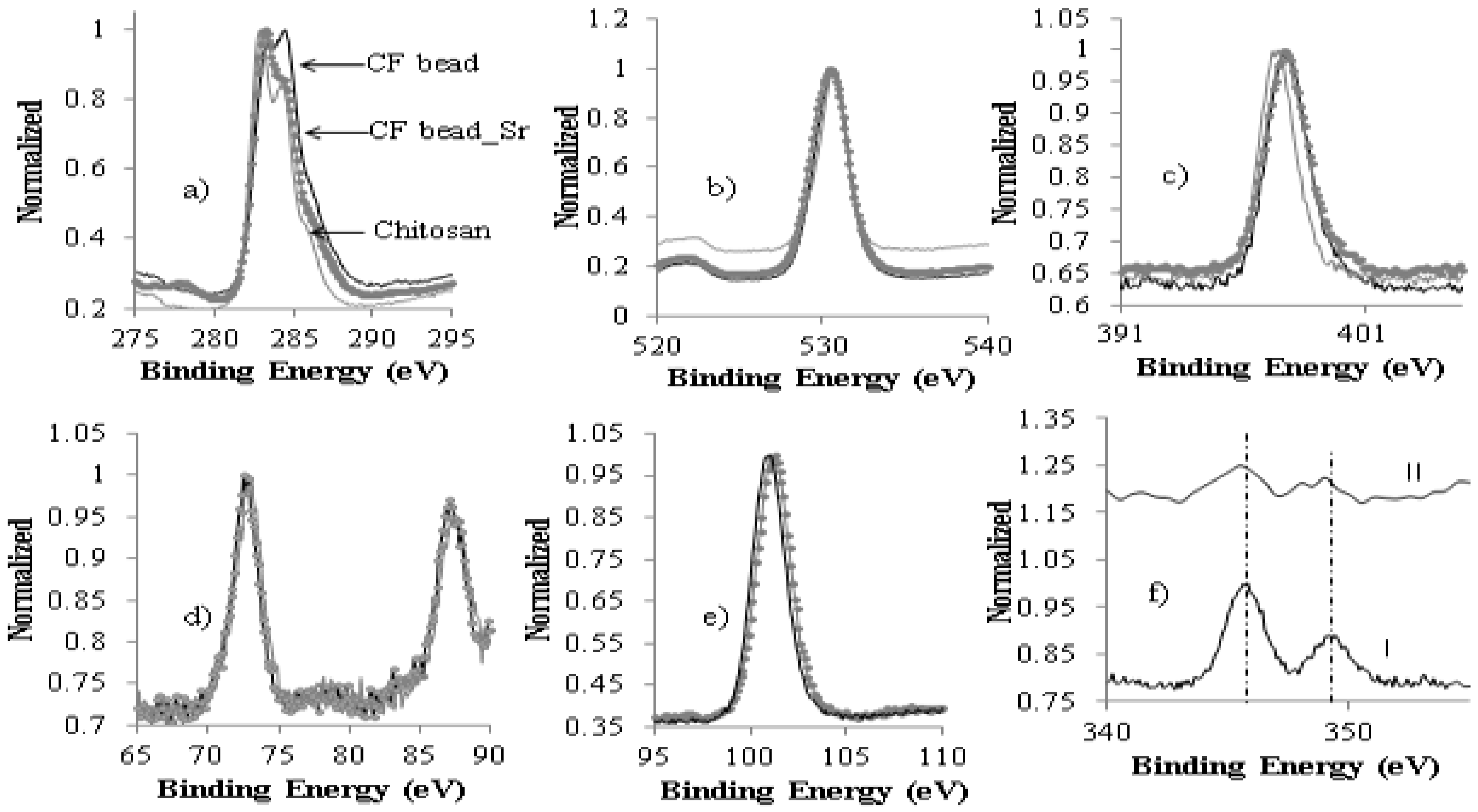
3.1. Characterization of CF Beads
3.2. pH and Surface Charge Effects on Strontium Uptake by CF Beads
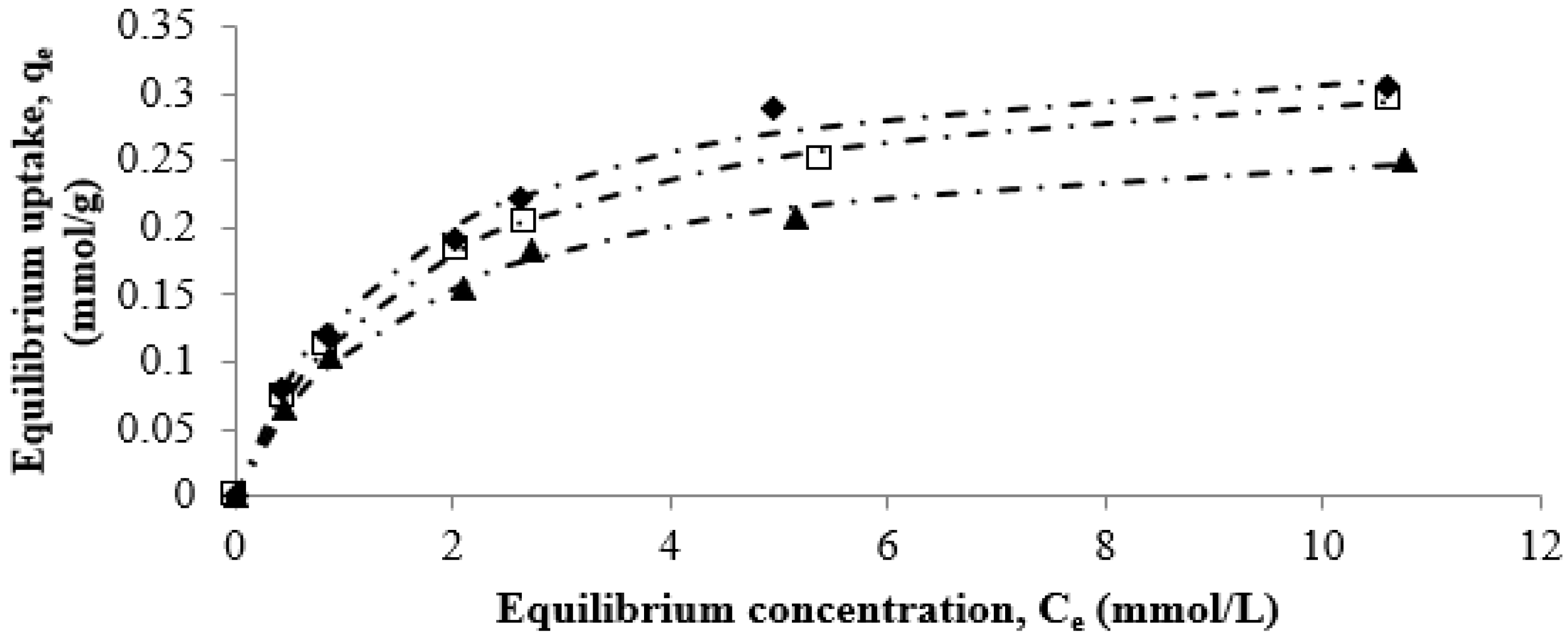
3.3. Equilibrium Adsorption Studies
3.4. Mechanism of Strontium Adsorption on CF Bead
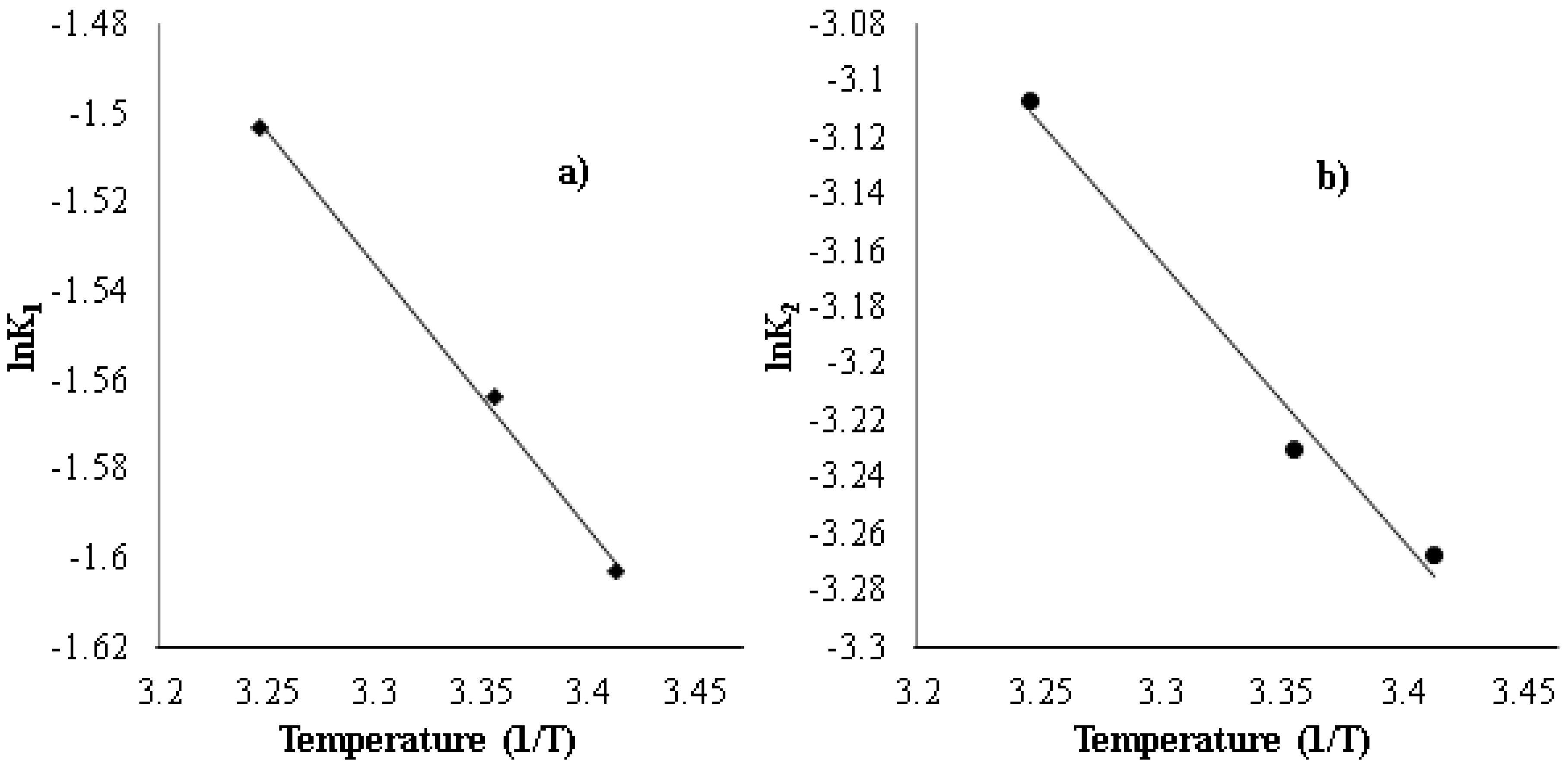
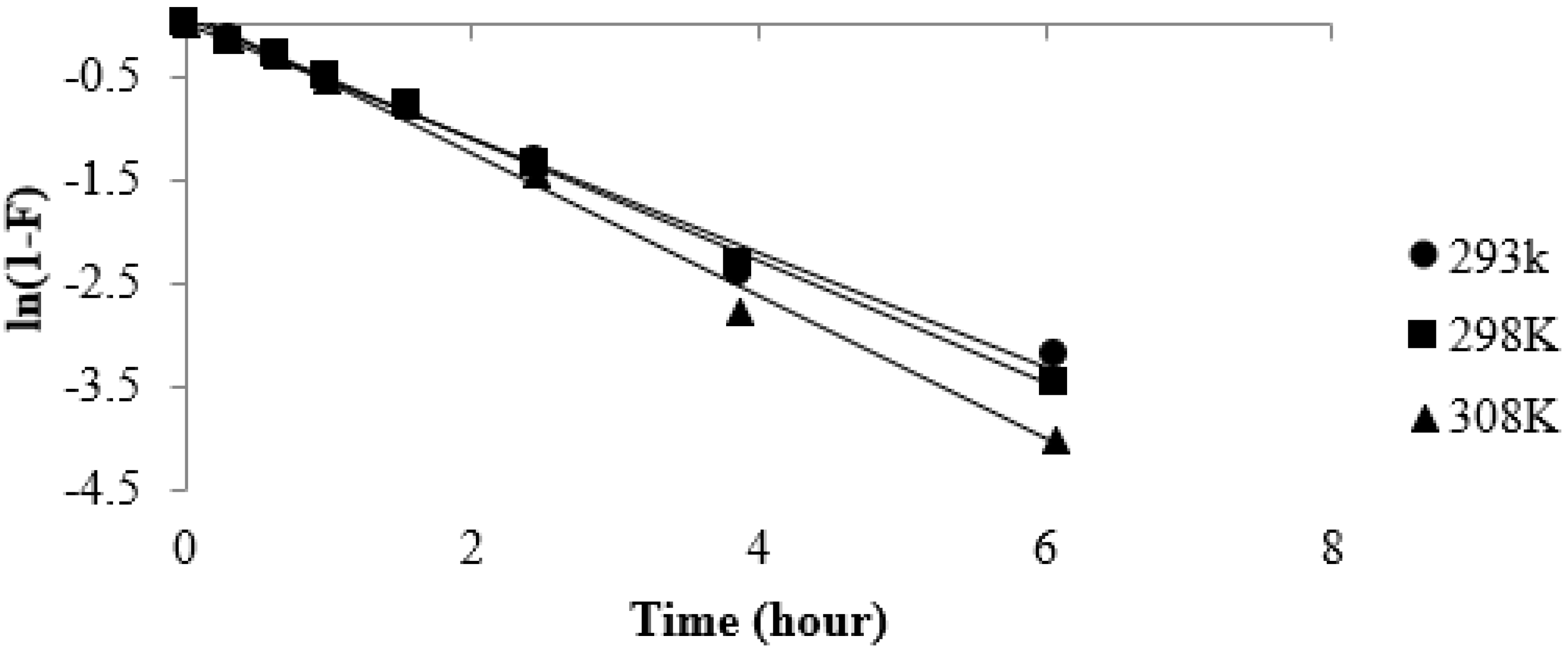
3.5. Effect of Temperature on Strontium Uptake
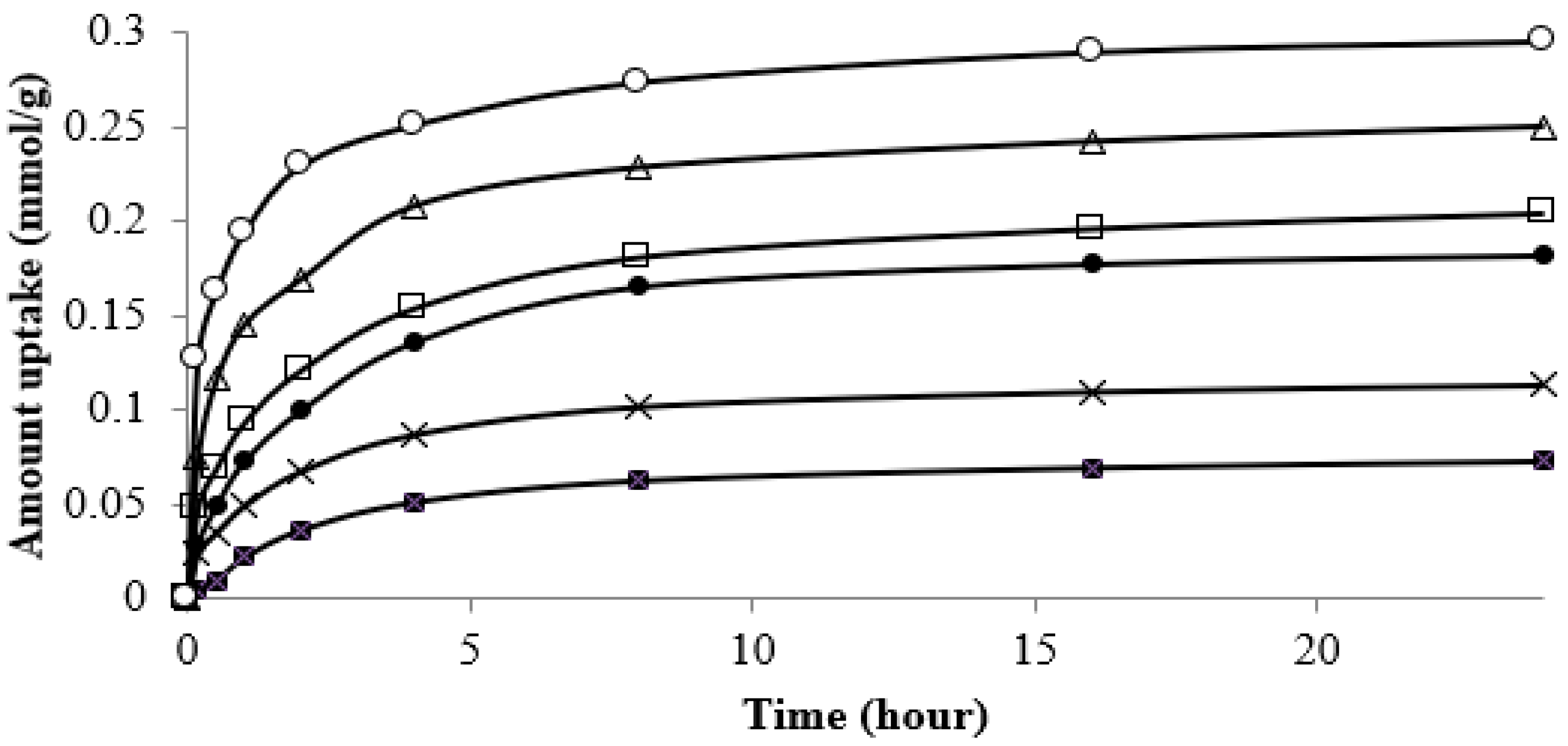
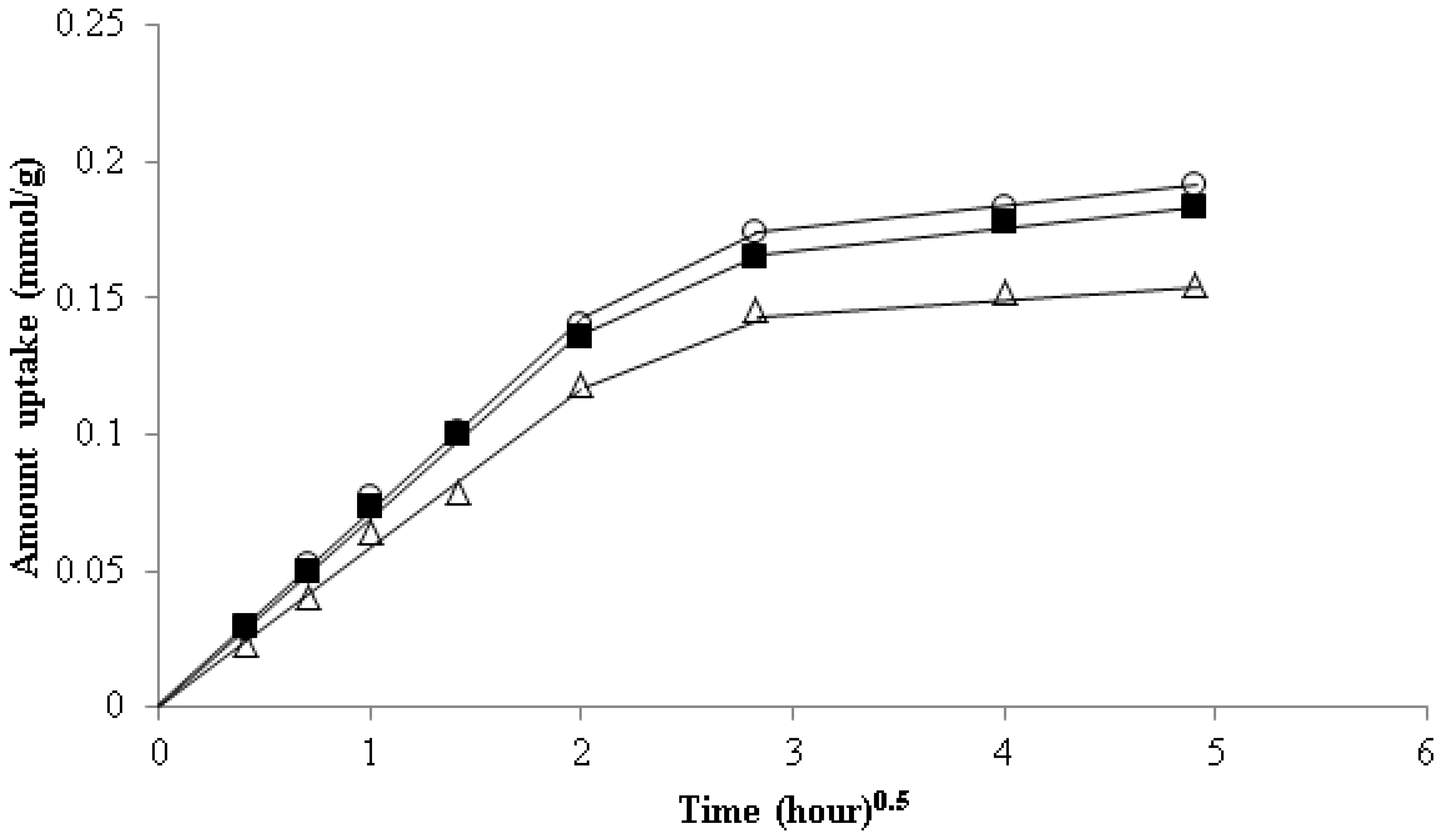
3.6. Kinetics Study of Strontium Adsorption
Elovich Equation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pathak, P. An assessment of strontium sorption onto bentonite buffer material in waste repository. Environ. Sci. Pollut. Res. 2017, 24, 8825–8836. [Google Scholar] [CrossRef]
- Sureda, R.; Martinez-Llado, X.; Rovira, M.; Pablo, J.D.; Casas, I.; Gimenez, J. Sorption of strontium on uranyl peroxide: Implications for a high-level nuclear waste repository. J. Hazard. Mater. 2010, 181, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.H.; Shaw, S.; Morris, K.; Small, J.S.; Fuller, A.J.; Burke, A.T. Effect of groundwater pH and ionic strength on strontium sorption in aquifer sediments: Implications for 90Sr mobility at contaminated nuclear sites. Appl. Geochem. 2012, 27, 1482–1491. [Google Scholar] [CrossRef]
- Yusan, S.; Erenturk, S. Adsorption characterization of strontium on PAN/Zeolite composite adsorbent. World J. Nuclear Sci. Technol. 2011, 1, 6. [Google Scholar] [CrossRef]
- Esfandian, H.; Fakhraee, H.; Azizi, A. Removal of Strontium Ions by Synthetic Nano Sodalite Zeolite from Aqueous Solution. Int. J. Eng. Trans. B Appl. 2016, 29, 160–169. [Google Scholar]
- Glasstone, S.; Dolan, P.J. XII: Biological effects. In The Effects of Nuclear Weapons; Department of Defense: Fort Lee, VA, USA, 1977; p. 605. [Google Scholar]
- Tu, Y.-Z.; You, C.-F.; Zhang, Z.; Duan, Y.; Fu, J.; Xu, D. Strontium removal in seawater by means of composite magnetic nanoparticles derived from industrial sludge. Water 2016, 8, 357. [Google Scholar] [CrossRef]
- Ogata, F.; Kawasaki, N. Adsorption of calcined Gibbsite for V, Sr, and Mo from a complex solution system. J. Water Environ. Technol. 2016, 14, 362–371. [Google Scholar] [CrossRef]
- Lateef, I.M.; Huckman, M.E.; Anthony, R.G. Modelling cesium ion-exchange on fixed bed columns of crystalline silicotitanate granules. Ind. Eng. Chem. Res. 2000, 39, 1356–1363. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; Ibrahium, H.A.; Hung, Y.-T. Liquid radioactive wastes treatment: A review. Water 2011, 3, 551–565. [Google Scholar] [CrossRef]
- Lehto, J.; Brodkin, L.; Harjula, R. Separation of radioactive strontium from alkaline nuclear waste solution with highly effective ion-exchanger SrTreat. Nuclear Technol. 1999, 127, 81–87. [Google Scholar] [CrossRef]
- Manos, M.J.; Ding, N.; Kanatzidis, M.G. Layered metal sulfides: Exceptionally selective agents for radioactive strontium removal. Proc. Natl. Acad. Sci. USA 2008, 105, 3696–3699. [Google Scholar] [CrossRef] [PubMed]
- Behrens, E.A.; Sylvester, P.; Clearfield, A. Assessment of a sodium nonatitanate and pharmacosiderite type ion exchangers for strontium and cesium removal from DOE waste simulants. Environ. Sci. Technol. 1998, 32, 101–107. [Google Scholar] [CrossRef]
- Rousseau, S.; Vijayan, S. Technology presrectives on the removal of radiostrontium from large volumes of groundwater. In Proceedings of the WM’04 Conference, Tucson, AZ, USA, 29 February–4 March 2004. [Google Scholar]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Kaygun, K.; Earl, M.; Erenturk, S.A. Removal of cesium and strontium using natural attapulgite: Evaluation of adsorption isotherm and thermodynamic data. J. Radioanal. Nuclear Chem. 2017, 311, 1459–1464. [Google Scholar] [CrossRef]
- Song, D.; Park, S.-J.; Kang, H.W.; Park, S.B.; Han, J.-I. Recovery of Lithium(I), Strontium (II) and Lanthanum (III) using Ca-alginate beads. J. Chem. Eng. Data 2013, 58, 2455–2464. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Zabihi, M.; Tahmasbi, M.; Bastami, T.R. Effect of adsorbents and chemical treatments on the removal of strontium from aqueous solutions. J. Hazard. Mater. 2010, 182, 552–556. [Google Scholar] [CrossRef]
- Inan, S.; Altas, Y. Preparation of zirconium-manganese oxide/polyacrylonitrile (Zr-Mn oxide/PAN) composite spheres and the investigation of Sr(II) sorption by experimental design. Chem. Eng. J. 2011, 168, 1263–1271. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J. Removal of radionuclides Sr2+ ions from aqueous solution using synthesized magnetic beads. Nuclear Eng. Des. 2012, 242, 445–451. [Google Scholar] [CrossRef]
- Marinović, S.S.; Ajduković, M.J.; Jović-Jovičić, N.J.; Mudrinić, T.M.; Nedić-Vasiljević, B.N.; Banković, P.T.; Milutinović-Nikolić, A.D. Adsorption of strontium on different sodium-enriched bentonites. J. Serb. Chem. Soc. 2017, 82, 449–463. [Google Scholar] [CrossRef]
- Yang, D.; Zheng, Z.; Liu, H.; Zhu, H.; Ke, X.; Xu, Y.; Sun, Y. Layered titanate nanofibers as efficient adsorbents for removal of toxic radioactive and heavy metal ions from water. J. Phys. Chem. C 2008, 112, 16275–16280. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J.; Yang, X.; Li, W. Removal of strontium ions by immobilized Saccharomyces Crevisiae in magnetic chitosan microsphere. Nuclear Eng. Technol. 2017, 49, 172–177. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Feng, Q.; Dong, F. Preparation of magnetic clinoptilolite/CoFe2O4 composites for removal of Sr2+ from aqueous solutions: Kinetic, equilibrium, and hermodynamic studies. J. Saudi Chem. Soc. 2017, 21, 58–66. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Jiang, L.; Luo, M.; Chi, C.; Ma, J. Adsorption of strontium from aqueous solution by silica mesoporous SBA-15. J. Radioanal. Nuclear Chem. 2015, 303, 1671–1677. [Google Scholar] [CrossRef]
- Yang, J.; Guo, N.; Wang, J.; Chen, H. Adsorption of uranium, strontium and cesium in radioactive wastewater by modified attapulgite. In Proceedings of the 7th International Conference on Environmental Science and Technology, Houston, TX, USA, 9–13 June 2014. [Google Scholar]
- Hasan, S. Development of Materials for the Removal of Metal Ions from Radioactive and Nonradioactive Waste Streams. Ph.D. Thesis, University of Missouri-Columbia, Columbia, MO, USA, 2005. [Google Scholar]
- Hasan, S.; Ghosh, T.K.; Viswanath, D.S.; Boddu, V.M. Dispersion of chitosan on perlite for enhancement of copper(II) adsorption capacity. J. Hazard. Mater. 2008, 152, 826–837. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH Diagrams; Geological Survey of Japan Open File Report No. 419; National Institute of Advanced Industrial Science and Technology: Tokyo, Japan, 2005; p. 245. [Google Scholar]
- Apak, R.; Atun, G.; Guclu, K.; Tutem, E. Sorptive removal of Cesium-137 and Strontium-90 from water by conventional sorbents. J. Nuclear Sci. Technol. 1996, 33, 396–402. [Google Scholar] [CrossRef]
- Shahwan, T.; Erten, H.N. Characterization of Sr2+ uptake on natural minerals of kaolinite and magnesite using XRPD, SEM/EDS, XPS, and DRIFT. Radiochim. Acta 2005, 93, 225–232. [Google Scholar] [CrossRef]
- Van der Heide, P.A.W. Systematic X-ray Photoelectron Spectroscopic Study of La1-xSrx-based Perovskite-type oxides. Surf. Interface Anal. 2002, 33, 414–425. [Google Scholar] [CrossRef]
- Dambis, L.; Vincent, T.; Guibal, E. Treatment of arsenic containing solutions using chitosan derrivatives: Uptake mechanism and sorption performances. Water Res. 2002, 36, 3699–3710. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Trans. IChemE 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Bilbao, L.; Ortueta, M.; Mijangos, F. Effect of concentration and temperature on mass transfer in metal ion exchange. Ind. Eng. Chem. Res. 2016, 55, 7287–7295. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S. Insight into Adsorption Thermodynamics, Thermodynamics; Tadashi, E., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-544-0. Available online: http://www.intechopen.com/books/thermodynamics/insight-into-adsorption-thermodynamics (accessed on 21 November 2018).
- Horsfall, M., Jr.; Spiff, A.I. Effects of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by Caladium bicolor (Wild Cocoyam) biomass. Electron. J. Biotechnol. 2005, 8, 162–169. [Google Scholar] [CrossRef]
- Venkatesan, K.A.; Selvam, G.P.; Rao, P.R.V. Sorption of strontium on hydrous zirconium oxide. Sep. Sci. Technol. 2000, 35, 2343–2357. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A. Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Desalin. Water Treat. 2012, 39, 149–157. [Google Scholar] [CrossRef]
- Yu, Z.; Qi, T.; Qu, J.; Wang, L.; Chu, J. Removal of Ca(II) and Mg(II) from potassium chromate solution on Amberlite IRC 748 synthetic resin by ion exhcnage. J. Hazard. Mater. 2009, 167, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Guo, H. Behavior and mechanism of arsenate adsorption on activated natural siderite: Evidences from FTIR and XANES analysis. Environ. Sci. Pollut. Res. 2014, 21, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- McLintock, I.S. The Elovich equation in chemisorption kinetics. Nature 1967, 216, 1204–1205. [Google Scholar] [CrossRef]
- Wen, T.; Wu, X.; Liu, M.; Xing, Z.; Wang, X.; Xu, A.W. Efficient capture of strontium from aqueous solutions using graphene oxide-hydroxyapatite nanocomposites. Dalton Trans. 2014, 43, 7464–7472. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhang, G.-H.; Gu, P. Adsorption kinetics and equilibrium modeling of cesium on copper ferrocyanide. J. Radioanal. Nuclear Chem. 2013, 295, 369–377. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Min, L.L.; Zhong, L.B.; Zheng, Y.M.; Liu, Q.; Yuan, Z.H.; Yang, L.M. Functionalized chitosan electrospun nanofiber for effective removal of trace arsenate from water. Sci. Rep. 2016, 6, 32480. [Google Scholar] [CrossRef]
- Milenković, D.D.; Milosavljević, M.M.; Marinković, A.D.; Đokić, V.R.; Mitrović, J.Z.; Bojić, A.L. Removal of copper(II) ion from aqueous solution by high-porosity activated carbon. Water SA 2013, 39, 515–522. [Google Scholar] [CrossRef]
- Fierro, V.; Torne-Fernandez, V.; Montane, D.; Celzard, A. Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater. 2008, 111, 276–284. [Google Scholar] [CrossRef]
- Yaneva, Z.L.; Koumanova, B.K.; Allen, S.J. Applicability comparison of different kinetic/diffusion models for 4-nitrophenol sorption on Rhizopusoryzae dead biomass. Bulg. Chem. Commun. 2013, 45, 161–168. [Google Scholar]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford Press: London, UK, 1957; pp. 89–96. [Google Scholar]
- Chen, H.-L.; Viswanath, D.S. A generalized model for leaching of chlorine from Illinois coal and the effect of particle size. Fuel 1989, 68, 1184–1188. [Google Scholar] [CrossRef]
| Adsorbent | Initial Concentration (mg/L) | pH | Exposure Time (h) | Temp. (K) | Uptake (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Natural attapulgite | 5–300 | 4.8 | 5 | 303 | 8.11 | [16] |
| Ca-alginate | 10–500 | Neutral | − | RT | 6.7 | [17] |
| PAN-Zeolite | 25–175 | 5 | − | 298 | 0.011 | [4] |
| Modified gibbsite | 5–50 | 11.1 | 24 | 298 | 64.72 | [8] |
| Almond shell-AC | 45–102 | Neutral | − | 298 | 116.3 | [18] |
| Composite sludge | − | 10.25 | 2 | 318 | 23.04 | [7] |
| Modified bentonite | 25–100 | 4–8.5 | 3 | 298 | 46.1 | [21] |
| Zr-MnO2/PAN | 20–200 | 8 | 4 | 333 | 21.37 | [19] |
| Natural clinoptilolite | − | 7 | − | − | 9.8 | [22] |
| Magnetic chitosan microsphere | 5–300 | 8 | 5 | 303 | 81.96 | [23] |
| Clinoptilolite/CoFe2O4 | 20–400 | 4 | 24 | 298 | 20.58 | [24] |
| SBA-15 | 0–80 | 6 | 5 | − | 17.67 | [25] |
| Chitosan-Fuller’s earth bead | 20–1000 | 6.5 | 24 | 298 | 30.58 | This study |
| Properties | Chitosan Fuller’s Earth Adsorbent |
|---|---|
| Particle diameter dp (m) | 2.2 × 10−3 |
| Particle density ρ (kg/m3) | 1395 |
| Particle porosity (εp) | 2.2 × 10−3 |
| Shape | spherical |
| Chitosan content (wt%) | 32 |
| Surface area (m2/g) | 48.5 |
| Pore volume (m3/kg) | 1.03 × 10−4 |
| Average pore diameter (m) | 8.26 × 10−9 |
| Model | Parameters | Temperature | ||
|---|---|---|---|---|
| 293 K | 298 K | 308 K | ||
| Langmuir isotherm | (mg/g) | 31.15 | 30.03 | 25.13 |
| b (L/mg) | 0.0074 | 0.0065 | 0.0069 | |
| Absolute error (%) | −0.37 | 0.52 | 0.601 | |
| Maximum (+) error | 6.39 | 5.25 | 4.28 | |
| Maximum (−) error | 5.18 | 3.06 | 4.56 | |
| Temperature (K) | Kc | −∆G (kJ/mol) | ∆H (kJ/mol) | ∆S (kJ/mol·K) |
|---|---|---|---|---|
| 293 | 2.72 | 2.44 | 8.2831 | 0.0365 |
| 298 | 2.833 | 2.58 | ||
| 308 | 3.2 | 2.98 |
| Model | Parameters | Temperature (K) | ||
|---|---|---|---|---|
| 293 | 298 | 308 | ||
| 1st-order | (min−1) | 0.196 | 0.21 | 0.253 |
| 95 | 97.4 | 99.5 | ||
| 2nd-order | (g/mg·min) | 3.71 | 3.9 | 4.64 |
| 99.9 | 99.9 | 99.9 | ||
| Elovich | (mg/g·min) | 32.43 | 31.864 | 26.123 |
| (g/mg) | 0.3153 | 0.33 | 0.38 | |
| 98 | 98.12 | 96.92 | ||
| Weber–Morris | (mg/g·min1/2) | 5.313 | 5.032 | 4.51 |
| (mg/g) | 0.9753 | 1.085 | 0.613 | |
| 98.43 | 98.03 | 98.29 | ||
| Model | Parameters | Initial Concentration of Sr2+ in Solution (mg/L) at 298 K and pH 6.5 | |||||
|---|---|---|---|---|---|---|---|
| 55 | 100 | 220 | 280 | 500 | 1000 | ||
| 1st-order | (mg/g) | 6.4 | 6.46 | 16.1 | 18 | 22 | 26 |
| (mg/g) | 5.14 | 5.91 | 11.57 | 11.48 | 11.22 | 10.96 | |
| (min−1) | 0.189 | 0.22 | 0.21 | 0.184 | 0.191 | 0.203 | |
| 97.04 | 98 | 97.4 | 97 | 94 | 97 | ||
| 2nd-order | (mg/g) | 7.14 | 7.09 | 17.1 | 18.73 | 22.52 | 26.5 |
| (g/mg·min) | 5.2 | 5.6 | 3.9 | 4.4 | 5.8 | 7.03 | |
| (mg/g·min) | 0.044 | 0.05 | 0.19 | 0.26 | 0.493 | 0.82 | |
| 99.8 | 99.3 | 99.9 | 99.9 | 99.94 | 99.96 | ||
| Elovich | (mg/g·min) | 4.95 | 15.72 | 31.864 | 57.01 | 165.56 | 755.40 |
| (g/mg) | 0.698 | 0.519 | 0.33 | 0.3312 | 0.31 | 0.321 | |
| 94 | 97.34 | 98.12 | 98.68 | 98.66 | 98.34 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, S.; Iasir, A.R.M.; Ghosh, T.K.; Sen Gupta, B.; Prelas, M.A. Characterization and Adsorption Behavior of Strontium from Aqueous Solutions onto Chitosan-Fuller’s Earth Beads. Healthcare 2019, 7, 52. https://doi.org/10.3390/healthcare7010052
Hasan S, Iasir ARM, Ghosh TK, Sen Gupta B, Prelas MA. Characterization and Adsorption Behavior of Strontium from Aqueous Solutions onto Chitosan-Fuller’s Earth Beads. Healthcare. 2019; 7(1):52. https://doi.org/10.3390/healthcare7010052
Chicago/Turabian StyleHasan, Shameem, A. Rafi M. Iasir, Tushar K. Ghosh, Bhaskar Sen Gupta, and Mark A. Prelas. 2019. "Characterization and Adsorption Behavior of Strontium from Aqueous Solutions onto Chitosan-Fuller’s Earth Beads" Healthcare 7, no. 1: 52. https://doi.org/10.3390/healthcare7010052
APA StyleHasan, S., Iasir, A. R. M., Ghosh, T. K., Sen Gupta, B., & Prelas, M. A. (2019). Characterization and Adsorption Behavior of Strontium from Aqueous Solutions onto Chitosan-Fuller’s Earth Beads. Healthcare, 7(1), 52. https://doi.org/10.3390/healthcare7010052





