Presence of Babesia odocoilei and Borrelia burgdorferi Sensu Stricto in a Tick and Dual Parasitism of Amblyomma inornatum and Ixodes scapularis on a Bird in Canada
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tick Collection
2.2. Bacteria and Piroplasm Detection
2.3. DNA Sequence Analysis
2.4. Molecular Tick Identification
3. Results
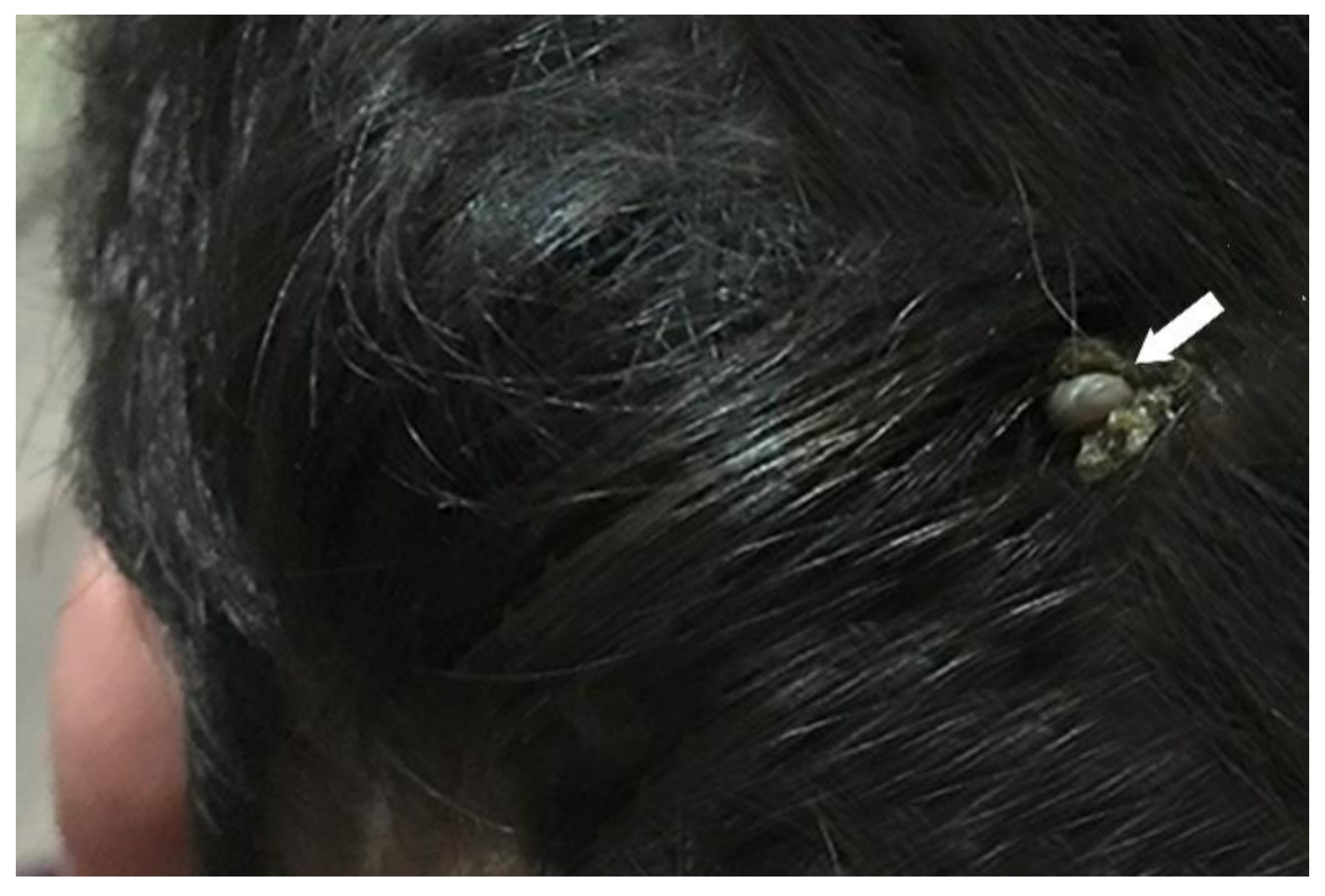
3.1. Identification of Amblyomma Tick
3.2. Identification of Tick-Borne Pathogens
3.3. Molecular Tick Assessment
4. Discussion
4.1. Identification of Amblyomma inornatum
4.2. Flight Path of Veery
4.3. Sequence of Zoonotic Infection During Flight of Veery
4.4. Tick–Host–Pathogen Dynamics
4.5. Impact of Babesia and Borrelia burgdorferi Infections on Humans
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicholson, W.A.; Sonenshine, D.E.; Noden, B.H. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press/Elsevier: London, UK, 2019; pp. 603–672. ISBN 978. [Google Scholar]
- Reed, K.D.; Meece, J.K.; Henkel, J.S.; Shukla, S.K. Birds, migration and emerging zoonoses: West Nile virus, Lyme disease, influenza A and enteropathogens. Clin. Med. Res. 2003, 1, 5–12. [Google Scholar] [CrossRef]
- Goodman, J.L.; Dennis, D.T.; Sonenshine, D.E. Tick–Borne Diseases of Humans; ASM Press: Washington, DC, USA, 2005; p. 401. ISBN 9781555812386. [Google Scholar]
- Johnson, L.; Shapiro, M.; Mankoff, J. Removing the mask of average treatment effects in chronic Lyme disease research using Big Data and subgroup analysis. Healthcare 2018, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme disease—A tick–borne spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar] [CrossRef]
- Miziara, C.S.M.G.; Serrano, V.A.G.; Yoshinari, N. Passage of Borrelia burgdorferi through diverse ixodid hard ticks caused distinct diseases: Lyme borreliosis and Baggio–Yoshinari syndrome. Clinics 2018, 73, e394. [Google Scholar] [CrossRef] [PubMed]
- Meriläinen, L.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology 2015, 161, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Fesler, M.C. Chronic Lyme disease: A working case definition. Am. J. Infect. Dis. 2018, 14, 44. [Google Scholar] [CrossRef]
- Berger, B.W. Dermatologic manifestation of Lyme disease. Rev. Infect. Dis. 1989, 11 (Suppl. 6), S1475–S1481. [Google Scholar] [CrossRef]
- Stonehouse, A.; Studdiford, J.S.; Henry, A. An update on the diagnosis and treatment of early Lyme disease: “focusing on the bull’s–eye, you may miss the mark”. J. Emerg. Med. 2010, 39, e147–e151. [Google Scholar] [CrossRef]
- Johnson, L.; Mankoff, J.; Stricker, R.B. Severity of chronic Lyme disease compared to other chronic conditions: A quality of life survey. PeerJ 2014, 2, e322. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Shein, E. The piroplasms: Life cycle and sexual stages. Adv. Parasitol. 1984, 23, 37–103. [Google Scholar]
- Kjemtrup, A.M.; Conrad, P.A. Human babesiosis: An emerging tick–borne disease. Austral. Soc. Parasitol. 2000, 30, 1323–1337. [Google Scholar] [CrossRef]
- Homer, M.J.; Aguilar–Delfin, I.; Telford, S.R., III; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef]
- Scott, J.D.; Fernando, K.; Banerjee, S.N.; Durden, L.A.; Byrne, S.K.; Banerjee, M.; Mann, R.B.; Morshed, M.G. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi–infected ticks in Canada. J. Med. Entomol. 2001, 38, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Beati, L.; Mazerolle, D.F.; Geddes, G.; Durden, L.A. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J. Parasitol. 2005, 91, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Hanincová, K.; Barker, I.K.; Bigras–Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I.; et al. Role of migratory birds in introduction and range expansion of I. scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Lee, M.-K.; Fernando, K.; Durden, L.A.; Jorgensen, D.R.; Mak, S.; Morshed, M.G. Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato, including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J. Vector Ecol. 2010, 35, 124–139. [Google Scholar] [PubMed]
- Scott, J.D.; Anderson, J.F.; Durden, L.A. Widespread dispersal of Borrelia burgdorferi–infected ticks collected from songbirds across Canada. J. Parasitol. 2012, 98, 49–59. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. New records of the Lyme disease bacterium in ticks collected from songbirds in central and eastern Canada. Int. J. Acarol. 2015, 41, 241–249. [Google Scholar] [CrossRef]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Bierman, B.C.; Durden, L.A. Far–reaching dispersal of Borrelia burgdorferi sensu lato–infected blacklegged ticks by migratory songbirds in Canada. Healthcare 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Bierman, B.C.; Durden, L.A. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare 2018, 6, 131. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. Amblyomma dissimile Koch (Acari: Ixodidae) parasitizes bird captured in Canada. Syst. Appl. Acarol. 2015, 20, 854–860. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. First record of Amblyomma rotundatum tick (Acari: Ixodidae) parasitizing a bird collected in Canada. Syst. Appl. Acarol. 2015, 20, 155–161. [Google Scholar] [CrossRef]
- Scott, J.D. Birds widely disperse pathogen–infected ticks. In Seabirds and Songbirds: Habitat Preferences, Conservation, Migratory Behavior; Mahala, G., Ed.; Nova Publishers, Inc.: New York, NY, USA, 2015; pp. 1–22. ISBN 978. [Google Scholar]
- Guglielmone, A.A.; Estrada–Peña, A.; Keirans, J.E.; Robbins, R.G. Ticks (Acari: Ixodida) of the Neotropical Zoogeographic Region; International Consortium on Ticks and Tick–borne Diseases: Atalanta, GA, USA; Houten, The Netherlands, 2003; p. 173. ISBN 987. [Google Scholar]
- Keirans, J.E.; Durden, L.A. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 1998, 35, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Guzmán–Cornejo, C.; Robbins, R.G.; Guglielmone, A.A.; Montiel–Parra, G.; Pérez, M. The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico: Identification keys, distribution and hosts. Zootaxa 2011, 2998, 16–38. [Google Scholar]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petnery, T.N.; Estrada–Pena, A.; Horak, I.G. The Hard Ticks of the World (Acari: Ixodidae); Springer: Dordrecht, The Netherlands, 2014; ISBN 978. [Google Scholar]
- Medlin, J.S.; Cohen, J.I.; Beck, D.L. Vector potential and population dynamics for Amblyomma inornatum. Ticks Tick Borne Dis. 2015, 6, 463–472. [Google Scholar] [CrossRef]
- Peñalver, E.; Arillo, A.; Delclòs, X.; Peris, D.; Grimaldi, D.A.; Anderson, S.R.; Nascimbene, P.C.; Pérez–de la Fuente, R. Ticks parasitised feathered dinosaurs as revealed by Cretaceous ambler assemblages. Nature Commun. 2018, 8, 1924. [Google Scholar] [CrossRef] [PubMed]
- Poinar, G., Jr. Fossilized mammalian erythrocytes associated with a tick reveal ancient piroplasms. J. Med. Entomol. 2017, 54, 895–900. [Google Scholar] [CrossRef]
- Poinar, G., Jr. Spirochete–like cells in a Dominican amber Amblyomma tick (Arachnida: Ixodidae). Hist. Biol. 2015, 27, 565–570. [Google Scholar] [CrossRef]
- Banerjee, S.N.; Christensen, C.I.; Scott, J.D. Isolation of Borrelia burgdorferi on mainland Ontario. Can. Com. Dis. Rep. 1995, 21, 85–86. [Google Scholar]
- Benach, J.L.; Coleman, J.L.; Habicht, G.S.; MacDonald, A.; Grunwaldt, E.; Giron, J.A. Serological evidence for simultaneous occurrences of Lyme disease and babesiosis. J. Infect. Dis. 1985, 152, 473–477. [Google Scholar] [CrossRef]
- Curcio, S.R.; Tria, L.P.; Gucwa, A.L. Seroprevalence of Babesia microti in individuals with Lyme disease. Vector Borne Zoonotic Dis. 2016, 16, 737–743. [Google Scholar] [CrossRef]
- Jones, E.K.; Clifford, C.M.; Kohls, G.M. The ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma in the Western Hemisphere; Brigham Young University Science Bulletin, Biological Series, No. 4; Brigham Young University: Provo, UT, USA, 1972; Volume 17, p. 40. [Google Scholar]
- Durden, L.A.; Keirans, J.E. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical/Veterinary Importance. Monographs; Thomas Say Publications in Entomology, Entomological Society of America: Lanham, MD, USA, 1996; p. 95. [Google Scholar]
- Clark, K.; Hendricks, A.; Burge, D. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl. Environ. Microbiol. 2005, 71, 2616–2625. [Google Scholar] [CrossRef]
- Casati, S.; Sager, H.; Gern, L.; Piffaretti, J.-C. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2016, 13, 65–70. [Google Scholar]
- McCombie, W.R.; Heiner, C.; Kelly, J.M.; Fitzgerald, M.G.; Gocayne, J.D. Rapid and reliable fluorescent cycle sequencing of double stranded templates. DNA Seq. 1992, 2, 289–296. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tools. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The ClustalX–Windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. First report of a blacklegged tick, Ixodes scapularis Say (Acari: Ixodidae), parasitizing a raptor in Canada. Syst. Appl. Acarol. 2015, 22, 208–216. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Robbins, R.G. Hard Ticks (Acari: Ixodida: Ixodidae) Parasitizing Humans, 1st ed.; Springer: New York, NY, USA, 2018; p. 314. ISBN 9783319955520. [Google Scholar]
- eBird Primary Reference. Available online: https://help.ebird.org/customer/en/portal/articles/1006835–recommended–citation (accessed on 16 March 2019).
- The Virtual Nature Trail at Penn State New Kensington Species Pages. 16 March. Available online: https://www.psu.edu/dept/nkbiology/naturetrail/speciespages/veery.html (accessed on 16 March 2019).
- Mannelli, A.; Kitron, U.; Jones, C.J.; Slajchert, T.L. Influence of season and habitat on Ixodes scapularis infestation on white–footed mice in northwestern Illinois. J. Parasitol. 1994, 80, 1038–1043. [Google Scholar] [CrossRef]
- Holman, P.J.; Waldrup, K.A.; Wagner, G.G. In vitro cultivation of a Babesia isolated from a white–tailed deer (Odocoileus virginianus). J. Parasitol. 1988, 74, 111–115. [Google Scholar] [CrossRef]
- Holman, P.J.; Madeley, J.; Craig, T.M.; Allsopp, B.A.; Allsopp, M.T.; Petrini, K.R.; Waghela, S.D.; Wagner, G.G. Antigenic, phenotypic and molecular characterization confirms Babesia odocoilei isolated from three cervids. J. Wildl. Dis. 2000, 36, 518–530. [Google Scholar] [CrossRef]
- Anderson, J.F.; Magnarelli, L.A. Avian and mammalian hosts for spirochete–infected ticks and insects in a Lyme disease focus in Connecticut. Yale J. Biol. Med. 1984, 57, 627–641. [Google Scholar]
- Anderson, J.F.; Johnson, R.C.; Magnarelli, L.A.; Hyde, F.W. Involvement of birds in the epidemiology of Lyme disease agent Borrelia burgdorferi. Infect. Immun. 1986, 51, 394–396. [Google Scholar]
- McLean, R.G.; Ubico, S.R.; Norton Hughes, C.A.; Engstrom, S.M.; Johnson, R.C. Isolation and characterization of Borrelia burdorferi from blood of a bird captured in the Saint Croix River Valley. J. Clin. Microbiol. 1993, 31, 2038–2043. [Google Scholar]
- Durden, L.A.; Oliver, J.H., Jr.; Kinsey, A.A. Ticks (Acari: Ixodidae) and spirochetes (Spirochaetaceae: Spirochaetales) recovered from birds on a Georgia barrier island. J. Med. Entomol. 2001, 38, 231–236. [Google Scholar] [CrossRef]
- Richter, D.; Spielman, A.; Komar, N.; Matuschka, F.-R. Competence of American Robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 2000, 6, 133–138. [Google Scholar] [CrossRef]
- Rollend, L.; Fish, D.; Childs, J.E. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks Tick Borne Dis. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Franke, J.; Meier, F.; Sachse, S.; Dorn, W.; Straube, E. The potential role of migratory birds in transmission cycles of Babesia spp., Anaplasma phagocytophilum, and Rickettsia spp. Ticks Tick Borne Dis. 2010, 1, 105–107. [Google Scholar] [CrossRef]
- Hersh, M.H.; Osfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; LoGiudice, K.; Schmidt, K.A.; Keesing, F. Co–infestation of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Herwaldt, B.L.; de Bruyn, G.; Pieniazek, N.J.; Homer, M.; Lofy, K.H.; Siiemenda, S.B.; Fritsche, T.R.; Persing, D.H.; Limaye, A.P. Babesia divergens–like infection, Washington State. Emerg. Infect. Dis. 2004, 10, 622–629. [Google Scholar] [CrossRef]
- Gorenflot, A.; Moubri, K.; Percigout, E.; Carey, B.; Schetters, T.P. Human babesiois. Ann. Trop. Med. Parasitol. 1998, 92, 489–501. [Google Scholar] [CrossRef]
- Shock, B.C.; Moncayo, A.; Cohen, S.; Mitchell, E.A.; Williamson, P.C.; Lopez, G.; Garrison, L.E.; Yabsley, M.J. Diversity of piroplasms detected in blood–fed and questing ticks from several states in the United States. Ticks Tick Borne Dis. 2014, 5, 373–380. [Google Scholar] [CrossRef]
- Villatoro, T.; Karp, J.K. Transfusion–transmitted babesiosis. Arch. Pathol. Lab. Med. 2019, 143, 130–134. [Google Scholar] [CrossRef]
- Cornett, J.K.; Malhotra, A.; Hart, D. Vertical transmission of babesiosis from a pregnant, splenectomized mother to her neonate. Infect. Dis. Clin. Pract. 2012, 20, 408–410. [Google Scholar] [CrossRef]
- Fox, L.M.; Winger, S.; Ahmed, A.; Arnold, A.; Chou, J.; Rhein, L.; Levy, O. Neonatal babesiosis: Case report and review of the literature. Pediatr. Infect. Dis. J. 2006, 25, 169–173. [Google Scholar] [CrossRef]
- Sherr, V.T. Human babesiosis—An unrecorded reality: Absence of formal registry undermines it detection, diagnosis and treatment, suggesting need for immediate mandatory reporting. Med. Hypotheses 2005, 63, 609–615. [Google Scholar] [CrossRef]
- Scott, J.D.; Scott, C.M. Human babesiosis caused by Babesia duncani has widespread distribution across Canada. Healthcare 2018, 6, 49. [Google Scholar] [CrossRef]
- Lloyd, V.K.; Hawkins, R.G. Under–detection of Lyme disease in Canada. Healthcare 2018, 6, 125. [Google Scholar] [CrossRef]
- Häupl, T.; Hahn, G.; Rittig, M.; Krause, A.; Schoerner, C.; Schönherr, U.; Kalden, J.R.; Burmester, G.R. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 1993, 36, 1621–1626. [Google Scholar] [CrossRef]
- Müller, M.E. Damage of collagen and elastic fibres by Borrelia burgdorferi—Known and new clinical histopathogical aspects. Open Neurol. J. 2012, 6 (Suppl. 1), S179–S186. [Google Scholar]
- Oksi, J.; Mertsola, J.; Reunanen, M. Subacute multiple–site osteomyelitis cause by Borrelia burgdorferi. Clin. Infect. Dis. 1994, 19, 891–896. [Google Scholar] [CrossRef]
- Preac–Mursic, V.; Pfister, H.W.; Spiegel, H.; Burk, R.; Wilske, B.; Reinhardt, S. First isolation of Borrelia burgdorferi from an iris biopsy. J. Clin. Neuroophthalmol. 1993, 13, 155–161. [Google Scholar]
- Oksi, J.; Kalimo, H.; Marttila, R.J.; Marjamäki, M.; Sonninen, P.; Nikoskelainen, J.; Viljanen, M.K. Inflammatory brain changes in Lyme borreliosis: A report on three patients and review of literature. Brain 1996, 119, 2143–2154. [Google Scholar] [CrossRef]
- MacDonald, A.B. Alzheimer’s neuroborreliosis with trans–synaptic spread of infection and neurofibrillary tangles derived from intraneuronal spirochetes. Med. Hypotheses 2007, 68, 822–825. [Google Scholar] [CrossRef]
- Miklossy, J. Alzheimer’s disease—A neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J. Neuroinflamm. 2011, 8, 90. [Google Scholar] [CrossRef]
- Frey, M.; Jaulhac, B.; Piemont, Y.; Marcellin, L.; Boohs, P.M.; Vautravers, P.; Jesel, M.; Kuntz, J.L.; Monteil, H.; Sibilia, J. Detection of Borrelia burgdorferi DNA in muscle of patients with chronic myalgia related to Lyme disease. Am. J. Med. 1998, 104, 591–594. [Google Scholar] [CrossRef]
- Ramesh, G.; Borda, J.T.; Dufour, J.; Kaushal, D.; Ramamoorthy, R.; Lackner, A.A.; Philipp, M.T. Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am. J. Pathol. 2008, 173, 1415–1427. [Google Scholar] [CrossRef]
- Ramesh, G.; Santana–Gould, L.; Inglis, F.M.; England, J.D.; Philipp, M.T. The Lyme disease spirochete Borrelia burgdorferi induces inflammation and apoptosis in cells from dorsal root ganglia. J. Neuroinflamm. 2013, 10, 88. [Google Scholar] [CrossRef]
- Girschick, H.J.; Huppertz, H.I.; Rüssmann, H.; Krenn, V.; Karch, H. Intracellular persistence of Borrelia burgdorferi in human synovial cells. Rheumatol. Int. 1996, 16, 125–132. [Google Scholar] [CrossRef]
- Klempner, M.S.; Noring, R.; Rogers, R.A. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 1993, 167, 1074–1081. [Google Scholar] [CrossRef]
- Middelveen, M.J.; Burke, J.; Sapi, E.; Bandoski, C.; Filush, K.R.; Wang, Y.; Franco, A.; Timmaraju, A.; Schlinger, H.A.; Mayne, P.J.; et al. Culture and identification of Borrelia spirochetes in human vaginal and seminal secretions. F1000Research 2015, 3, 309. [Google Scholar] [CrossRef]
- Embers, M.E.; Hasenkampf, N.R.; Jacobs, M.B.; Tardo, A.C.; Doyle–Meyers, A.; Philipp, M.T.; Hodzic, E. Variable manifestations, diverse seroreactivity and post–treatment persistence in non–human primates exposed to Borrelia burgdorferi by tick feeding. PLoS ONE 2017, 12, e0189071. [Google Scholar] [CrossRef]
- Bransfield, R.C. Suicide and Lyme and associated diseases. Neuropsychiatr. Dis. Treat. 2017, 13, 1575–1587. [Google Scholar] [CrossRef] [Green Version]
- Bransfield, R.C. Aggressiveness, violence, homocidality, homicide, and Lyme disease. Neuropsychiatr. Dis. Treat. 2018, 14, 693–713. [Google Scholar] [CrossRef]
- Liegner, K.B.; Duray, P.; Agricola, M.; Rosenkilde, C.; Yannuzzi, L.A.; Ziska, M.; Tilton, R.C.; Hulinska, D.; Hubbard, J.; Fallon, B.A. Lyme disease and the clinical spectrum of antibiotic responsive chronic meningoencephalomyelitides. J. Spir. Tick Borne Dis. 1997, 4, 61–73. [Google Scholar]
- Feder, H. Borrelia infections: Lyme disease. In Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant, 8th ed.; Wilson, C.B., Nizet, V., Maldonado, Y., Remington, J.S., Klein, J.O., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 544–557. ISBN 9780321472. [Google Scholar]
- Lavoie, P.E.; Lattner, B.P.; Duray, P.H.; Malawista, A.G.; Barbour, R.C. Culture positive, seronegative, transplacental Lyme borreliosis infant mortality. In Proceedings of the IV International Conference on Lyme Borreliosis, Stockholm, Sweden, 18–21 June 1990. [Google Scholar]
- MacDonald, A.B. Gestational Lyme borreliosis: Implications for the fetus. In Rheumatic Disease Clinics of North America; No., 4, Johnson, R.C., Eds.; Saunders: Philadelphia, PA, USA, 1989; Volume 15, pp. 657–677. Available online: http://www.molecularalzheimer.org/files/Gestational_Lyme_Borreliosis_–_/annotated_1989.pdf (accessed on 08 August 2017).
- Horowitz, R.I. Lyme disease and pregnancy: Implications of chronic infection, PCR testing, and prenatal treatment. In Proceedings of the 16th International Scientific Conference on Lyme Disease & Other Tick–Borne Disorders, Hartford, CT, USA, 7–8 June 2003; Lyme Disease Foundation: Hartford, CT, USA, 2003. [Google Scholar]
- Trevian, G.; Stinco, G.; Cinco, M. Neonatal skin lesions due to a spirochetal infection: A case of congenital Lyme borreliosis. Int. J. Dermatol. 1997, 36, 677–680. [Google Scholar]
- Grunwaldt, E.; Barbour, A.G.; Benach, J.L. Simultaneous occurrence of babesiosis and Lyme disease. N. Engl. J. Med. 1983, 308, 1166. [Google Scholar]
- Cameron, D.J.; Johnson, L.B.; Maloney, E.L. Evidence assessments and guideline recommendations in Lyme disease: The clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev. Anti-Infect. Ther. 2014, 12, 1103–1135. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, J.D.; Clark, K.L.; Durden, L.A. Presence of Babesia odocoilei and Borrelia burgdorferi Sensu Stricto in a Tick and Dual Parasitism of Amblyomma inornatum and Ixodes scapularis on a Bird in Canada. Healthcare 2019, 7, 46. https://doi.org/10.3390/healthcare7010046
Scott JD, Clark KL, Durden LA. Presence of Babesia odocoilei and Borrelia burgdorferi Sensu Stricto in a Tick and Dual Parasitism of Amblyomma inornatum and Ixodes scapularis on a Bird in Canada. Healthcare. 2019; 7(1):46. https://doi.org/10.3390/healthcare7010046
Chicago/Turabian StyleScott, John D., Kerry L. Clark, and Lance A. Durden. 2019. "Presence of Babesia odocoilei and Borrelia burgdorferi Sensu Stricto in a Tick and Dual Parasitism of Amblyomma inornatum and Ixodes scapularis on a Bird in Canada" Healthcare 7, no. 1: 46. https://doi.org/10.3390/healthcare7010046



