Pilot Study of Immunoblots with Recombinant Borrelia burgdorferi Antigens for Laboratory Diagnosis of Lyme Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Reference Sera
2.2. Detection of IgG and IgM Serum Antibodies to B. burgdorferi by EIA
2.3. Preparation of Antigen Strips for Lyme Western Blots
2.4. Preparation of Antigen Strips for Lyme Immunoblots
2.5. Procedure for Detection of Borrelia Specific Antibodies on Lyme Immunoblots and Western Blots with Test Sera
2.6. Scoring of Positive Serological Reactions
2.7. Lyme Immunoblots with Rabbit Antisera against Lyme and TBRF Borrelia Species
2.8. Statistical Analysis
3. Results
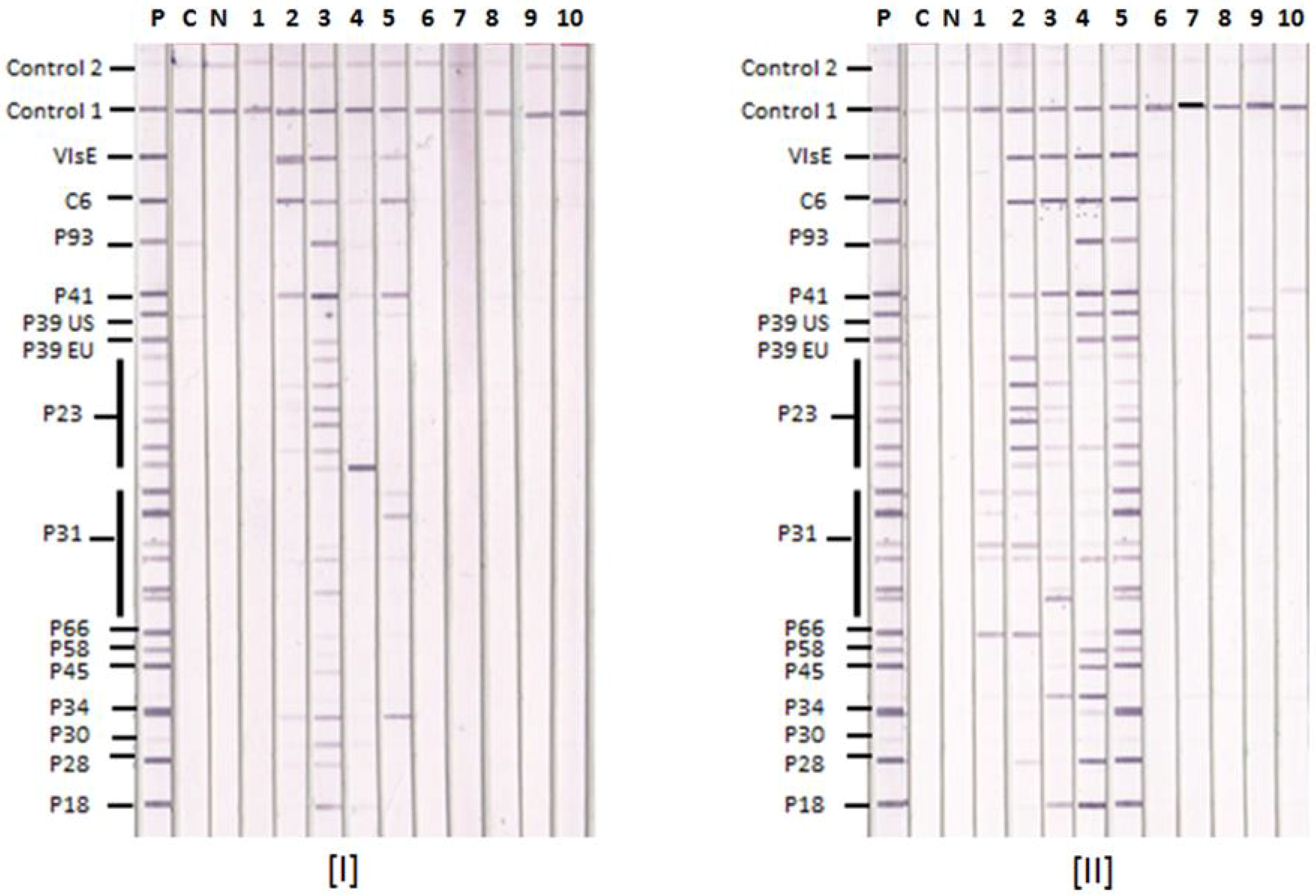
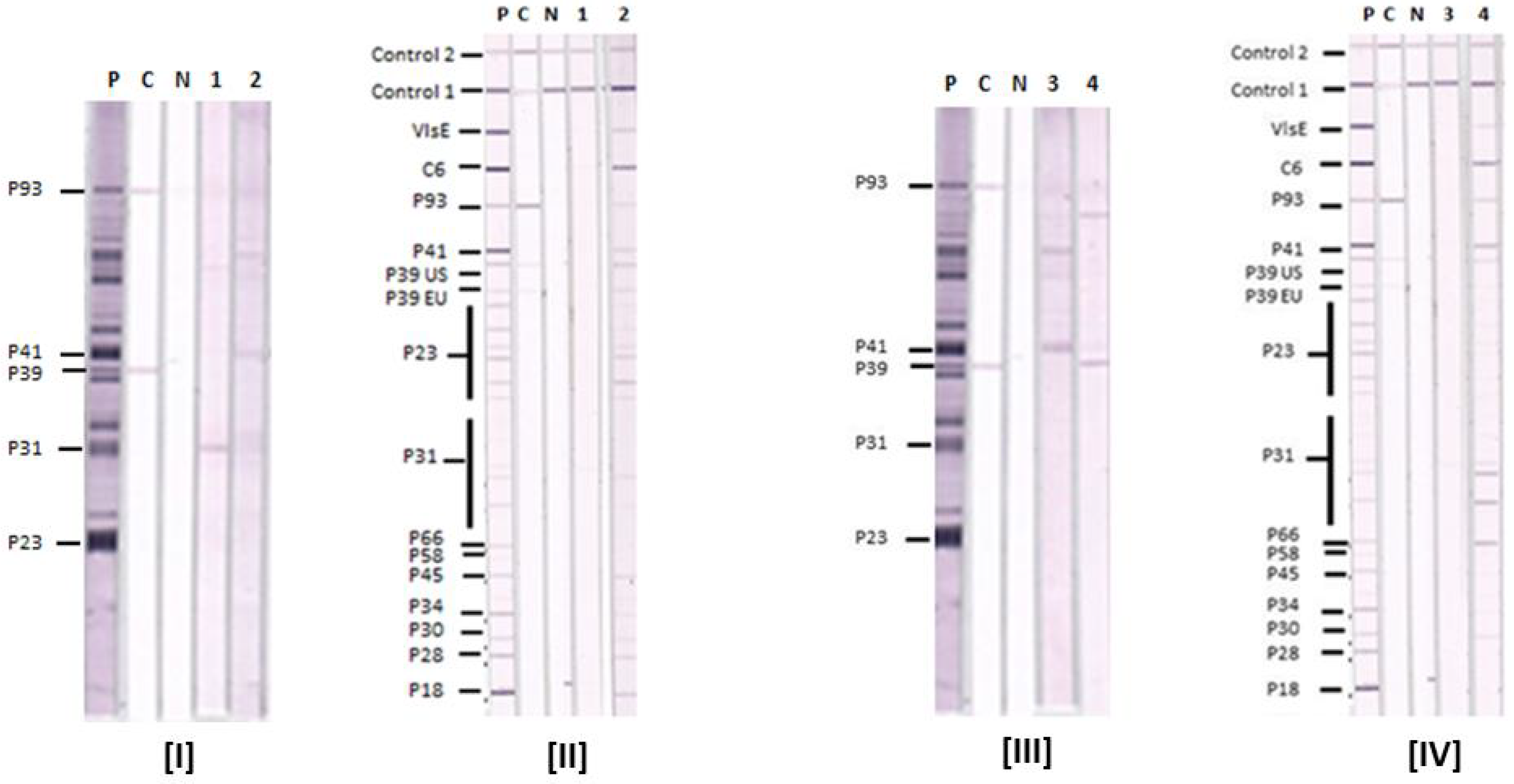
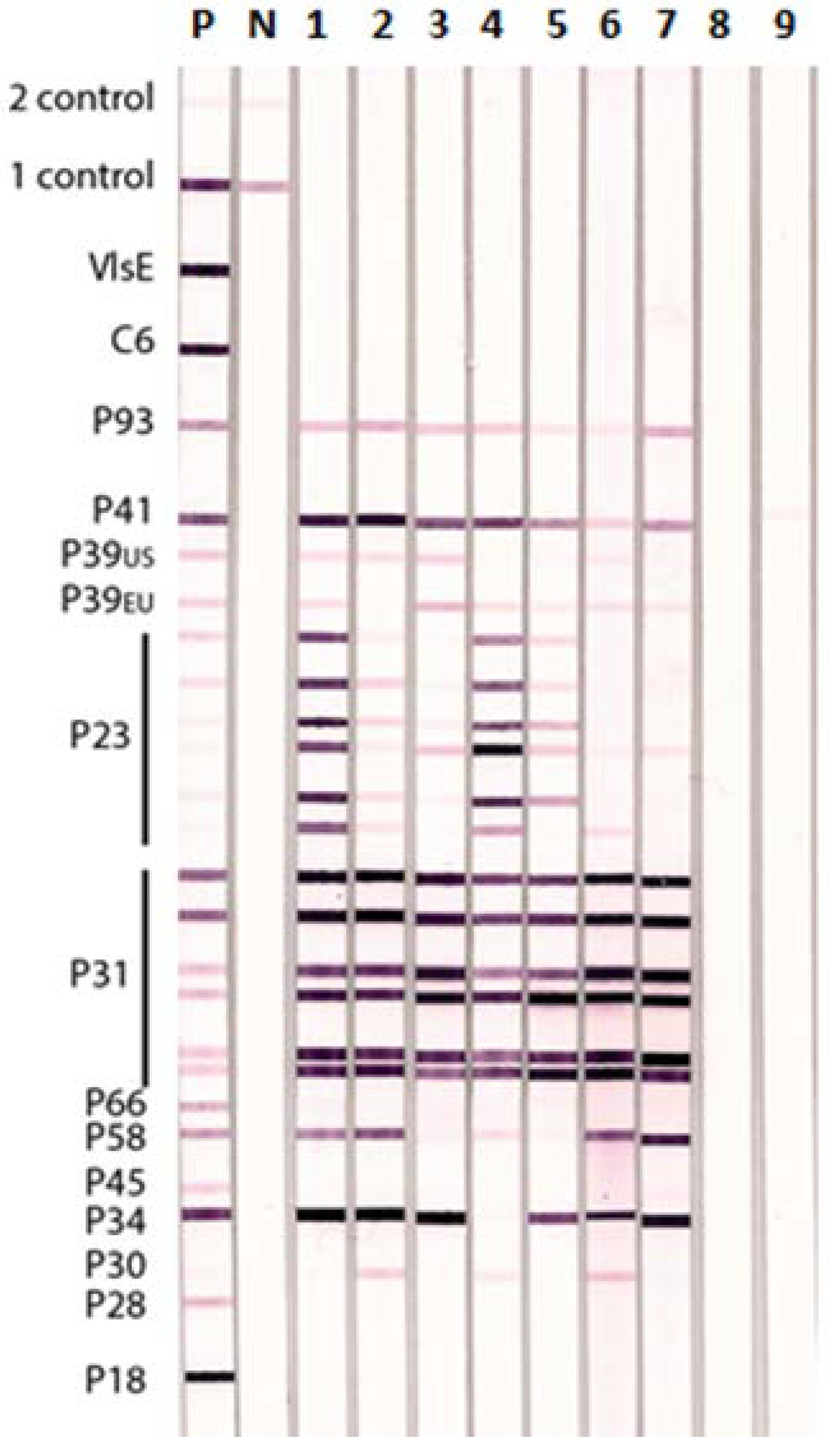
3.1. Lyme IgM and IgG Immunoblots
3.2. Comparative Reactions of Four Sera in Lyme IgM Western Blots and Lyme IgM Immunoblots
3.3. Lyme IgM and IgG Immunoblot and Western Blot Results with Known Lyme Disease-Positive Sera
3.4. Lyme IgM and IgG Immunoblots and Western Blots Detecting Different Stages of Lyme Disease
3.5. False Positive Reactions on Reference Sera in Lyme IgM and IgG Immunoblots and Western Blots
3.6. Clinical Parameters of the Lyme Immunoblot and Western Blot Tests with Reference Sera
3.7. Comparison of Performance of Lyme IBs with Two-Tiered Testing (Whole-Cell EIA Followed by Confirmation with WBs)
3.8. Antisera to TBRF Borrelia species B. hermsii and B. coriaceae Tested with Bbsl Antigens in Lyme IgG Immunoblots
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steere, A.C. Lyme disease. N. Engl. J. Med. 2001, 345, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.R. Lyme Disease: A Review. Curr. Allergy Asthma Rep. 2010, 10, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Schotthoefer, A.M.; Frost, H.M. Ecology and epidemiology of Lyme borreliosis. Clin. Lab. Med. 2015, 35, 723–766. [Google Scholar] [CrossRef] [PubMed]
- Girard, Y.A.; Fedorova, N.; Lane, R.S. Genetic Diversity of Borrelia burgdorferi and detection of B. bissettii-Like DNA in serum of North coastal California residents. J. Clin. Microbiol. 2011, 49, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.S.; Mead, P.S.; Johnson, D.K.; Neitzel, D.F.; Respicio-Kingry, L.B.; Davis, J.P.; Schiffman, E.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: A descriptive study. Lancet Infect. Dis. 2016, 16, 556–564. [Google Scholar] [CrossRef]
- Wilske, B. Diagnosis of Lyme borreliosis in Europe. Vector-Borne Zoonotic Dis. 2003, 3, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Centres for Disease Control and Prevention 2018. Available online: https://www.cdc.gov/lyme/stats/humancases.html (accessed on 28 March 2018).
- Rahn, D.W.; Malawista, S.E. Lyme disease: Recommendations for diagnosis and treatment. Ann. Intern. Med. 1991, 114, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention 2017. Lyme Disease (Borrelia burgdorferi) Case Definition. Available online: https://wwwn.cdc.gov/nndss/conditions/lyme-disease/case-definition/2017/ (accessed on 25 March 2018).
- Aguero-Rosenfeld, M.E.; Wormser, G.P. Lyme disease: Diagnostic issues and controversies. Expert Rev. Mol. Diagn. 2015, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.F.; Liu, H.W.; Hsu, T.C.; Wei, J.C.C.; Shih, C.M.; Krause, P.J.; Tsay, G.J. Serum reactivity against Borrelia burgdorferi OspA in patients with rheumatoid arthritis. Clin. Vaccine Immunol. 2007, 14, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; McKay, K.; Thompson, C.A.; Sikand, V.K.; Lentz, R.; Lepore, T.; Closter, L.; Christianson, D.; Telford, S.R.; Persing, D. Disease-specific diagnosis of co-infecting tick-borne zoonoses: Babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin. Infect. Dis. 2002, 34, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.C.; Steere, A.C.; Duray, P.H.; Anderson, A.E.; Mahoney, E.B. Fatal pancarditis in a patient with coexistent Lyme disease and babesiosis. Demonstration of spirochetes in the myocardium. Ann. Intern. Med. 1985, 103, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Fish, D.; Narasimhan, S.; Barbour, A.G. Borrelia miyamatoi infection in nature and in humans. Clin. Microbiol. Infect. 2015, 21, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J.; Rudenko, N.; Golovchenko, M. Diagnosing borreliosis. Vector-Borne Zoonotic Dis. 2017, 17, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme disease. Morb. Mortal. Wkly. Rep. 1995, 44, 590–591. [Google Scholar]
- Wilske, B.; Fingerle, V. Diagnosis of Lyme borreliosis. How to corroborate suspected borreliosis. MMW Fortschr. Med. 2000, 142, 28–31. [Google Scholar] [PubMed]
- Nowakowski, J.; Schwartz, I.; Liveris, D.; Wang, G.; Rosenfeld, M.E.A.; Girao, G.; McKenna, D.; Nadelman, R.B.; Cavaliere, L.F.; Wormser, G.P. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: A comparison of different techniques. Clin. Infect. Dis. 2001, 333, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Nelson, C.; Molins, C.; Mead, P.; Schriefer, M. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme Disease, United States. Emerg. Infect. Dis. 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Lebech, A.M.; Hansen, K.; Brandrup, F.; Clemmensen, O.; Halkier-SØrensenPa, L. Diagnostic value of PCR for detection of Borrelia burgdorferi DNA in clinical specimens from patients with erythema migrans and lyme neuroborreliosis. Mol. Diagn. 2000, 5, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Priem, S.; Rittig, M.G.; Kamradt, T.; Burmester, G.R.; Krause, A. An optimized PCR leads to rapid and highly sensitive detection of Borrelia burgdorferi in patients with Lyme borreliosis. J. Clin. Microbiol. 1997, 35, 685–690. [Google Scholar] [PubMed]
- Brettschneider, S.; Bruckhauer, H.; Klugbauer, N.; Hofmann, H. Diagnostic value for detection of Borrelia burgdorferi in skin biopsy and urine samples from patients with skin borreliosis. J. Clin. Microbiol. 1998, 36, 2658–2665. [Google Scholar] [PubMed]
- Bergmann, A.R.; Schmidt, B.L.; Derler, A.M.; Aberer, E. Importance of sample preparation for molecular diagnosis of Lyme borreliosis from urine. J. Clin. Microbiol. 2002, 40, 4581–4584. [Google Scholar] [CrossRef]
- Shah, J.S.; D’Cruz, I.; Ward, S.; Harris, N.S.; Ramasamy, R. Development of a sensitive PCR-dot blot assay to supplement serological tests for diagnosing Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. 2017. Erratum in 2018, doi:10.1007/s10096-018-3201-2. [Google Scholar] [CrossRef]
- Steere, A.C.; McHugh, G.; Damle, N.; Sikand, V.K. Prospective study of serologic tests for Lyme disease. Clin. Infect. Dis. 2008, 47, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Tracy, K.E.; Baumgarth, N. Borrelia burgdorferi manipulates innate and adaptive immunity to establish persistence in rodent reservoir hosts. Front. Immunol. 2017, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Oksi, J.; Uksila, J.; Marjamäki, M.; Nikoskelainen, J.; Viljanen, M.K. Antibodies against whole sonicated Borrelia burgdorferi spirochetes, 41-kilodalton flagellin, and P39 protein in patients with PCR- or culture-proven late Lyme borreliosis. J. Clin. Microbiol. 1995, 33, 2260–2264. [Google Scholar]
- Benach, J.L.; Coleman, J.L.; Habicht, G.S.; MacDonald, A.; Grunwaldt, E.; Giron, J.A. Serological evidence for simultaneous occurrences of Lyme disease and babesiosis. J. Infect. Dis. 1985, 152, 473–477. [Google Scholar] [CrossRef] [PubMed]
- DeMartino, S.J.; Carlyon, J.A.; Fikrig, E. Coinfections with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. N. Engl. J. Med. 2001, 345, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Nadelman, R.B.; Horowitz, H.W.; Hsieh, T.C.; Wu, J.M.; Aguero-Rosenfeld, M.E.; Schwartz, I.; Nowakowski, J.; Varde, S.; Wormser, G.P. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N. Engl. J. Med. 1997, 337, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Harris, N.S.; Yong, D.C.; Winger, E.E. Clinical and seroepidemiologic characteristics of Babesia WA-1 coinfection in patients with Lyme disease in California. J. Investig. Med. 2003, 51 (Suppl. 1), S145. [Google Scholar]
- Reed, K.D. Laboratory testing for Lyme disease: Possibilities and practicalities. J. Clin. Microbiol. 2002, 40, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Du Cruz, I.; Narciso, W.; Lo, W.; Harris, N.S. Improved sensitivity of Lyme disease western blots prepared with a mixture of Borrelia burgdorferi strains 297 and B31. Chronic Dis. Int. 2014, 1, 7. [Google Scholar]
- Mavin, S.R.; Milner, M.; Evans, R.; Chatterton, J.M.W.; Joss, A.W.L.; Ho-Yen, D.O. The use of local isolates in Western blots improves serological diagnosis of Lyme disease in Scotland. J. Med. Microbiol. 2007, 56, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Merljak Skocir, L.; Ruzić-Sabljić, E.; Maraspin-Carman, V.; Lotrič-Furlan, S.; Logar, M.; Strle, F. Comparison of different Borrelia burgdorferi sensu lato strains for detection of immune response in patients with erythema migrans. Int. J. Med. Microbiol. 2008, 298, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Dessau, R.B.; Møller, J.K.; Kolmos, B.; Henningsson, A.J. Multiplex assay (Mikrogen recomBead) for detection of serum IgG and IgM antibodies to 13 recombinant antigens of Borrelia burgdorferi sensu lato in patients with neuroborreliosis: The more the better? Med. Microbiol. 2015, 64, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Branda, J.A.; Strle, F.; Strle, K.; Sikand, N.; Ferraro, M.J.; Steere, A.C. Performance of United States serologic assays in the diagnosis of Lyme borreliosis acquired in Europe. Clin. Infect. Dis. 2013, 57, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.T.; Steere, A.C.; Marques, A.R.; Johnson, B.J.; Miller, J.N.; Philipp, M.T. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 1999, 37, 3990–3996. [Google Scholar] [PubMed]
- VassarStats Website. Available online: http://vassarstats.net/index.html (accessed on 28 March 2018).
- Ang, C.W.; Notermans, D.W.; Hommes, M.; Simoons-Smit, A.M.; Herremans, T. Large differences between test strategies for the detection of anti-Borrelia antibodies are revealed by comparing eight ELISAs and five immunoblots. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.J.; Puri, B.K. Commercial test kits for detection of Lyme borreliosis: A meta-analysis of test accuracy. Int. J. Gen. Med. 2016, 9, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Waddell, L.A.; Greig, J.; Mascarenhas, M.; Harding, S.; Lindsay, R.; Ogden, N. The accuracy of diagnostic tests for Lyme disease in humans, a systematic review and meta-analysis of North American research. PLoS ONE 2016, 11, e0168613. [Google Scholar] [CrossRef] [PubMed]
- Craft, J.F.; Ficher, D.K.; Shimamoto, G.T.; Steere, A.C. Antigens of Borrelia burgdorferi recognized during Lyme disease: Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J. Clin. Investig. 1997, 78, 934–939. [Google Scholar] [CrossRef] [PubMed]
| Source | Samples | Number of Samples | Expected Results for Lyme Disease | |
|---|---|---|---|---|
| Positive | Negative | |||
| CDC | CDC—Set 1 | 10 | 5 | 5 |
| CDC | CDC—Set 2 | 32 | 12 | 20 |
| CAP and NYSH | Proficiency test (PT) samples | 20 | 9 | 11 |
| CAP and NYSH | Autoimmune diseases (22 with RA) | 42 | 0 | 42 |
| NYB | Virus infections | 46 | 0 | 46 |
| NYB | RPR + ve | 28 | 0 | 28 |
| Total | 178 | 26 | 152 | |
| Samples | Number of Known Positive Sera | EIA (+) | Positive | 2-Tiered (CDC) | Positive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lyme WB (In-House) | Lyme WB (CDC) | Lyme IB (In-House) | Lyme IB (CDC) | ||||||||||||
| IgM | IgG | IgG or IgM | IgM | IgG | IgG or IgM | IgM | IgG | IgG or IgM | IgM | IgG | IgG or IgM | ||||
| CDC-Set 1 * | 5 | 4 | 2 | 4 | 4 | 2 | 3 | 4 | 4 | 3 | 5 | 5 | 2 | 4 | 4 |
| CDC-Set 2 * | 12 | 8 | 7 | 8 | 9 | 7 | 5 | 9 | 8 | 11 | 10 | 12 | 9 | 4 | 10 |
| PT Samples | 9 | 9 | 9 | 6 | 9 | 9 | 6 | 9 | 9 | 9 | 6 | 9 | 9 | 6 | 9 |
| Total Positive | 26 | 21 | 18 | 18 | 22 | 18 | 14 | 22 | 21 | 23 | 21 | 26 | 20 | 14 | 23 |
| Samples | Number of Known Positive Sera | EIA (+) | Positive | 2-Tiered (CDC) | Positive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lyme WB (In-House) | Lyme WB (CDC) | Lyme IB (In-House) | Lyme IB (CDC) | ||||||||||||
| IgM | IgG | IgG or IgM | IgM | IgG | IgG or IgM | IgM | IgG | IgG or IgM | IgM | IgG | IgG or IgM | ||||
| Early Lyme | 10 | 6 | 6 | 5 | 6 | 5 | 1 | 6 | 5 | 8 | 8 | 10 | 7 | 1 | 7 |
| Lyme Arthritis | 4 | 4 | 0 | 4 | 4 | 0 | 4 | 4 | 4 | 3 | 4 | 4 | 2 | 4 | 4 |
| Lyme Neuroborreliosis | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Total Positive | 17 | 13 | 9 | 12 | 13 | 8 | 8 | 13 | 12 | 14 | 15 | 17 | 12 | 8 | 14 |
| Samples | Number of Known Positive Sera | EIA (+) | Positive | 2-Tiered (CDC) | Positive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lyme WB (In-House) | Lyme WB (CDC) | Lyme IB (In-House) | Lyme IB (CDC) | ||||||||||||
| IgM | IgG | IgG or IgM | IgM | IgG | IgG or IgM | IgM | IgG | IgG or IgM | IgM | IgG | IgG or IgM | ||||
| CDC—Set 1 * | 5 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CDC—Set 2 * | 20 | 4 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| PT Samples | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Autoimmune disease | 42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Viral Infections | 46 | 3 | 10 | 5 | 15 | 2 | 0 | 2 | 2 | 0 | 1 | 1 | 0 | 0 | 0 |
| RPR + | 28 | 11 | 6 | 3 | 7 | 1 | 0 | 1 | 1 | 0 | 2 | 2 | 0 | 1 | 1 |
| Total False Positive | 18 | 16 | 10 | 24 | 3 | 0 | 3 | 3 | 0 | 4 | 4 | 0 | 1 | 1 | |
| Total True Negative | 152 | 134 | 136 | 142 | 128 | 149 | 152 | 149 | 149 | 152 | 148 | 148 | 152 | 151 | 151 |
| WB In-House | WB CDC | 2-Tiered CDC | IB In-House | IB CDC | |
|---|---|---|---|---|---|
| Sensitivity IgG or IgM | 84.6 (64.3–95.0) | 84.6 (64.3–95.0) | 80.8 (60.0–92.7) | 100.0 (84.0–100) | 88.5 (68.7–97.0) |
| Sensitivity IgG | 69.2 (48.1–84.9) | 53.8 (33.7–72.9) | 80.8 (60.0–93.7) | 57.7 (37.2–76.0) | |
| Sensitivity IgM | 75.0 (52.9–89.4) | 69.2 (48.1–84.9) | 88.5 (68.7–97.0) | 73.1 (51.9–87.6) | |
| Specificity IgG or IgM | 84.2 (77.2–89.4) | 98.0 (93.9–99.5) | 98.0 (93.9–99.5) | 96.7 (92.1–98.8) | 99.3 (95.8–100) |
| Specificity IgG | 93.4 (87.9–96.6) | 100.0 (96.9–100) | 97.4 (93.0–99.2) | 99.3 (95.8–100) | |
| Specificity IgM | 89.5 (83.2–93.7) | 98.0 (93.9–99.5) | 100.0 (96.9–100) | 100 (96.9–100) | |
| PPV IgG or IgM | 47.8 (33.1–62.9) | 88.0 (67.7–96.8) | 87.5 (66.6–96.7) | 83.9 (65.5–93.9) | 95.8 (76.9–99.8) |
| PPV IgG | 64.3 (44.1–80.7) | 100.0 (73.2–100) | 84.0 (63.1–94.7) | 93.8 (67.7–99.7) | |
| PPV IgM | 52.9 (35.4–69.8) | 85.7 (62.6–96.2) | 100.0 (82.2–100) | 100.0 (79.1–100) | |
| NPV IgG or IgM | 97.0 (91.9–99.0) | 97.4 (93.0–99.2) | 96.8 (92.2–98.7) | 100.0 (96.8–100) | 98.1 (94.0–99.5) |
| NPV IgG | 94.7 (89.4–97.5) | 92.7 (87.3–96.0) | 96.7 (92.1–98.8) | 93.2 (87.9–96.4) | |
| NPV IgM | 95.8 (90.6–98.3) | 94.9 (89.9–97.6) | 98.1 (94.0–99.5) | 95.6 (90.8–98.1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Cruz, I.D.; Ramos, C.C.; Taleon, P.; Ramasamy, R.; Shah, J. Pilot Study of Immunoblots with Recombinant Borrelia burgdorferi Antigens for Laboratory Diagnosis of Lyme Disease. Healthcare 2018, 6, 99. https://doi.org/10.3390/healthcare6030099
Liu S, Cruz ID, Ramos CC, Taleon P, Ramasamy R, Shah J. Pilot Study of Immunoblots with Recombinant Borrelia burgdorferi Antigens for Laboratory Diagnosis of Lyme Disease. Healthcare. 2018; 6(3):99. https://doi.org/10.3390/healthcare6030099
Chicago/Turabian StyleLiu, Song, Iris Du Cruz, Catherine Calalo Ramos, Paula Taleon, Ranjan Ramasamy, and Jyotsna Shah. 2018. "Pilot Study of Immunoblots with Recombinant Borrelia burgdorferi Antigens for Laboratory Diagnosis of Lyme Disease" Healthcare 6, no. 3: 99. https://doi.org/10.3390/healthcare6030099
APA StyleLiu, S., Cruz, I. D., Ramos, C. C., Taleon, P., Ramasamy, R., & Shah, J. (2018). Pilot Study of Immunoblots with Recombinant Borrelia burgdorferi Antigens for Laboratory Diagnosis of Lyme Disease. Healthcare, 6(3), 99. https://doi.org/10.3390/healthcare6030099






