Pilot Randomized Controlled Study on the Effectiveness of a Virtual Reality-Based Dementia Prevention Program Using Self-Regulated Learning Strategies Among Older Adults with Mild Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
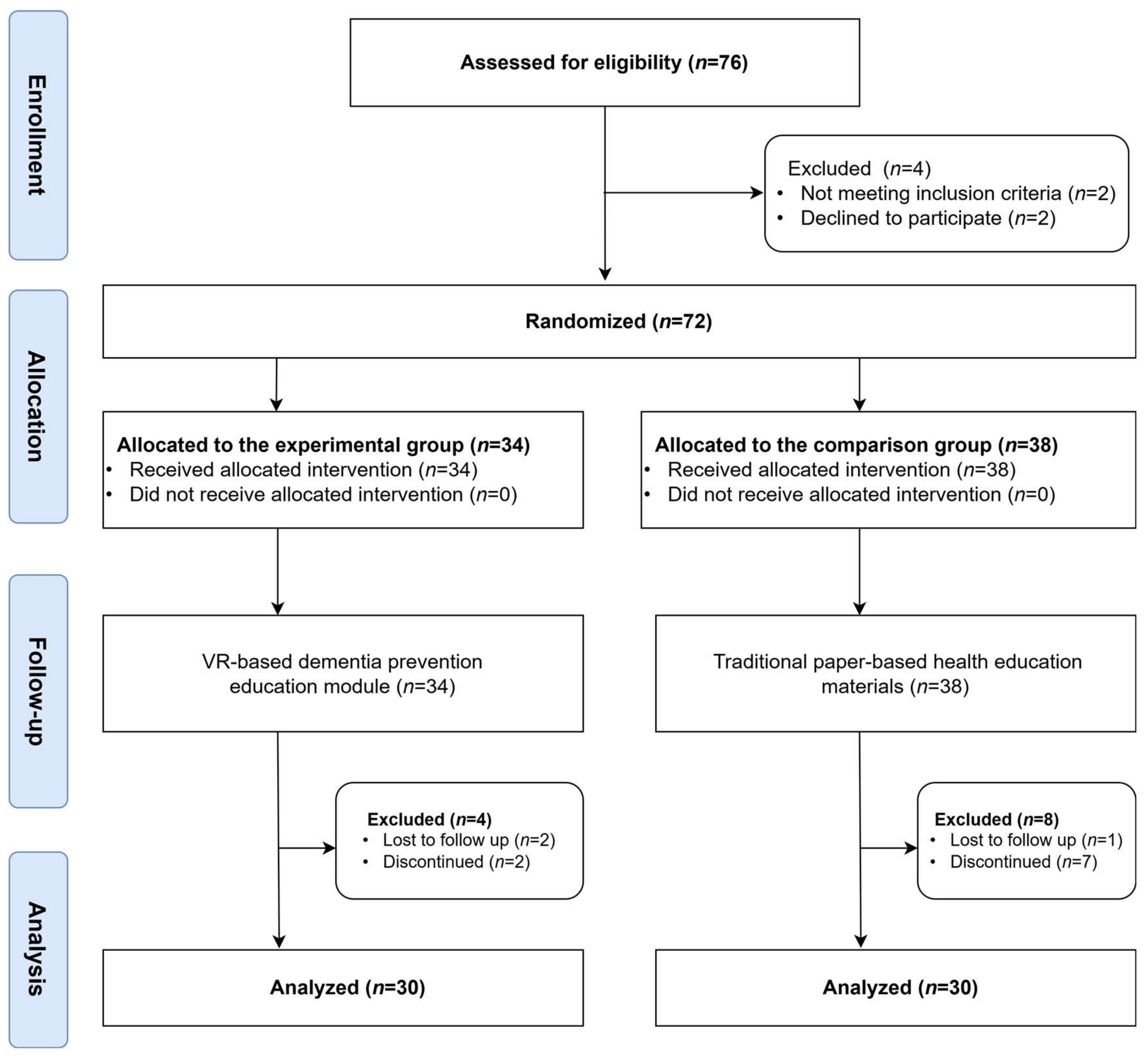
2.2. Participants
2.3. Study Procedure
2.4. Intervention Development
2.5. Measurements
2.5.1. Dementia-Related Knowledge
2.5.2. Dementia-Related Health Literacy
2.5.3. Self-Efficacy
2.6. Data Collection and Analysis
2.7. Ethical Considerations
3. Results
3.1. Demographic Characteristics
3.2. Intervention Effects on Dementia Knowledge, Health Literacy, and Self-Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
AI Disclosure Statement
Abbreviations
| MCI | Mild cognitive impairment |
| SRL | Self-regulated learning |
References
- World Health Organizations. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 28 December 2024).
- Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef] [PubMed]
- Rabins, P.V.; Mace, N.L.; Lucas, M.J. The impact of dementia on the family. JAMA 1982, 248, 333–335. [Google Scholar] [CrossRef]
- Bradfield, N.I. Mild cognitive impairment: Diagnosis and subtypes. Clin. EEG Neurosci. 2023, 54, 4–11. [Google Scholar] [CrossRef]
- Ganguli, M.; Jia, Y.; Hughes, T.F.; Snitz, B.E.; Chang, C.C.H.; Berman, S.B.; Sullivan, K.J.; Kamboh, M.I. Mild cognitive impairment that does not progress to dementia: A population-based study. J. Am. Geriatr. Soc. 2019, 67, 232–238. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, C.; Zhang, S.; Wang, Y. A bibliometric analysis on the health behaviors related to mild cognitive impairment. Front. Aging Neurosci. 2024, 16, 1402347. [Google Scholar] [CrossRef]
- Perry, M.; Drašković, I.; Lucassen, P.; Vernooij-Dassen, M.; van Achterberg, T.; Rikkert, M.O. Effects of educational interventions on primary dementia care: A systematic review. Int. J. Geriatr. Psychiatry 2011, 26, 1–11. [Google Scholar] [CrossRef]
- D’Cruz, K.; Meikle, L.; White, M.; Herrmann, A.; McCallum, C.; Romero, L. Tailoring education of adults with cognitive impairment in the inpatient hospital setting: A scoping review. Aust. Occup. Ther. J. 2021, 68, 90–102. [Google Scholar] [CrossRef]
- Pintrich, P.R. A conceptual framework for assessing motivation and self-regulated learning in college students. Educ. Psychol. Rev. 2004, 16, 385–407. [Google Scholar] [CrossRef]
- Zimmerman, B.J. Self-regulated learning and academic achievement: An overview. Educ. Psychol. 1990, 25, 3–17. [Google Scholar] [CrossRef]
- Robinson, J.D.; Persky, A.M. Developing self-directed learners. Am. J. Pharm. Educ. 2020, 84, 847512. [Google Scholar] [CrossRef]
- Schlomann, A.; Even, C.; Hammann, T. How older adults learn ICT—Guided and self-regulated learning in individuals with and without disabilities. Front. Comput. Sci. 2022, 3, 803740. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, S.; Lan, Y. The research on the self-regulation strategies support for virtual interaction. Multimed. Tools Appl. 2024, 83, 49723–49747. [Google Scholar] [CrossRef]
- An, J.; Oh, J.; Park, K. Self-Regulated Learning Strategies for Nursing Students: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 9058. [Google Scholar] [CrossRef]
- D’Cunha, N.M.; Nguyen, D.; Naumovski, N.; McKune, A.J.; Kellett, J.; Georgousopoulou, E.N.; Frost, J.; Isbel, S. A Mini-Review of Virtual Reality-Based Interventions to Promote Well-Being for People Living with Dementia and Mild Cognitive Impairment. Gerontology 2019, 65, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.C.M.; Andringa, G. The Potential of Immersive Virtual Reality for Cognitive Training in Elderly. Gerontology 2020, 66, 614–623. [Google Scholar] [CrossRef]
- Mandal, K.; Morrison, A.M.; Bag, S. Developing virtual reality tourism for people with dementia based on meeting psychological and behavioural needs. Tour. Manag. 2025, 108, 105100. [Google Scholar] [CrossRef]
- Porras-Garcia, B.; Rojas-Rincón, J.; Adams, A.; Garolera, M.; Chang, R. Immersive Virtual Reality Cognitive Training for Improving Cognition and Depressive Symptoms Among Older Adults. Current Evidence and Future Recommendations. A Systematic Review. Cyberpsychology Behav. Soc. Netw. 2024, 27, 692–703. [Google Scholar] [CrossRef]
- Liu, J.Y.W.; Yin, Y.-H.; Kor, P.P.K.; Cheung, D.S.K.; Zhao, I.Y.; Wang, S.; Su, J.J.; Christensen, M.; Tyrovolas, S.; Leung, A.Y. The effects of immersive virtual reality applications on enhancing the learning outcomes of undergraduate health care students: Systematic review with meta-synthesis. J. Med. Internet Res. 2023, 25, e39989. [Google Scholar] [CrossRef]
- Oliveira, J.; Gamito, P.; Souto, T.; Conde, R.; Ferreira, M.; Corotnean, T.; Fernandes, A.; Silva, H.; Neto, T. Virtual reality-based cognitive stimulation on people with mild to moderate dementia due to Alzheimer’s disease: A pilot randomized controlled trial. Int. J. Environ. Res. Public Health 2021, 18, 5290. [Google Scholar] [CrossRef]
- Appel, L.; Appel, E.; Kisonas, E.; Lewis-Fung, S.; Pardini, S.; Rosenberg, J.; Appel, J.; Smith, C. Evaluating the impact of virtual reality on the behavioral and psychological symptoms of dementia and quality of life of inpatients with dementia in acute care: Randomized controlled trial (VRCT). J. Med. Internet Res. 2024, 26, e51758. [Google Scholar] [CrossRef]
- Kim, O.; Pang, Y.; Kim, J.-H. The effectiveness of virtual reality for people with mild cognitive impairment or dementia: A meta-analysis. BMC Psychiatry 2019, 19, 219. [Google Scholar] [CrossRef]
- Manera, V.; Chapoulie, E.; Bourgeois, J.; Guerchouche, R.; David, R.; Ondrej, J.; Drettakis, G.; Robert, P. A feasibility study with image-based rendered virtual reality in patients with mild cognitive impairment and dementia. PLoS ONE 2016, 11, e0151487. [Google Scholar] [CrossRef]
- Thompson, M.; Yu, Z.; Odum, S. Randomization Strategies. In Introduction to Surgical Trials; Lyman, S., Ayeni, O.R., Koh, J.L., Nakamura, N., Karlsson, J., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 13–19. [Google Scholar]
- Coban, M.; Bolat, Y.I.; Goksu, I. The potential of immersive virtual reality to enhance learning: A meta-analysis. Educ. Res. Rev. 2022, 36, 100452. [Google Scholar] [CrossRef]
- Hendry, K.; Green, C.; McShane, R.; Noel-Storr, A.H.; Stott, D.J.; Anwer, S.; Sutton, A.J.; Burton, J.K.; Quinn, T.J. AD-8 for detection of dementia across a variety of healthcare settings. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Cines, S.; Farrell, M.; Steffener, J.; Sullo, L.; Huey, E.; Karlawish, J.; Cosentino, S. Examining the pathways between self-awareness and well-being in mild to moderate Alzheimer disease. Am. J. Geriatr. Psychiatry 2015, 23, 1297–1306. [Google Scholar] [CrossRef]
- Annear, M.J.; Toye, C.; Elliott, K.-E.J.; McInerney, F.; Eccleston, C.; Robinson, A. Dementia knowledge assessment scale (DKAS): Confirmatory factor analysis and comparative subscale scores among an international cohort. BMC Geriatr. 2017, 17, 168. [Google Scholar] [CrossRef]
- Kimzey, M.; Howe, C.J.; Martin, C.; McLarty, J.; Baucham, R. Development of health literacy in persons and caregivers living with dementia: A qualitative directed content analysis. Dementia 2022, 21, 540–555. [Google Scholar] [CrossRef]
- Ishimaru, M.; Nagata, A.; Sato, T.; Sakai, A.; Suzuki, S.; Kubota, K. Developing an Instructional Design-Based Dementia Education Program. Gerontol. Geriatr. Med. 2022, 8, 23337214221134874. [Google Scholar] [CrossRef]
- Tordet, C.; Fernandez, J.; Jamet, E. The effects of embedded quizzes on self-regulated processes and learning performance during a multimedia lesson. J. Comput. Assist. Learn. 2025, 41, e13083. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Wang, J.; Ma, X. The Impact of Prompts and Feedback on the Performance during Multi-Session Self-Regulated Learning in the Hypermedia Environment. J. Intell. 2023, 11, 131. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hwang, G.-J. A structured reflection-based graphic organizer approach for professional training: A technology-supported AQSR approach. Comput. Educ. 2022, 183, 104502. [Google Scholar] [CrossRef]
- Kuo, L.-H.; Chang, C.-H.; Huang, S.-F.; Chien, H.-C.; Huang, C.-M.; Guo, J.-L. User Experience Evaluation of a Spherical Video-based Virtual Reality Dementia Educational Program among Older Adults with Mild Cognitive Impairment. In Proceedings of the 2024 International Conference on Consumer Electronics-Taiwan (ICCE-Taiwan), Taichung, Taiwan, 9–11 July 2024; pp. 27–28. [Google Scholar]
- DeVellis, R.F.; Thorpe, C.T. Scale Development: Theory and Applications; Sage Publications: London, UK, 2021. [Google Scholar]
- Oliveira, D.; Bosco, A.; di Lorito, C. Is poor health literacy a risk factor for dementia in older adults? Systematic literature review of prospective cohort studies. Maturitas 2019, 124, 8–14. [Google Scholar] [CrossRef]
- Nutbeam, D. Health literacy as a public health goal: A challenge for contemporary health education and communication strategies into the 21st century. Health Promot. Int. 2000, 15, 259–267. [Google Scholar] [CrossRef]
- Lin, L.-C.; Huang, C.-M.; Hsu, H.-P.; Liao, J.-Y.; Lin, C.-Y.; Guo, J.-L. Integrating health literacy into a theory-based drug-use prevention program: A quasi-experimental study among junior high students in Taiwan. BMC Public Health 2021, 21, 1768. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Huang, C.-M.; Sheu, J.-J.; Liao, J.-Y.; Hsu, H.-P.; Wang, S.-W.; Guo, J.-L. Examining the effectiveness of 3D virtual reality training on problem-solving, self-efficacy, and teamwork among inexperienced volunteers helping with drug use prevention: Randomized controlled trial. J. Med. Internet Res. 2021, 23, e29862. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Q.; Liu, H.; Wang, G.; Lu, A. Effects of immersive and non-immersive virtual reality-based rehabilitation training on cognition, motor function, and daily functioning in patients with mild cognitive impairment or dementia: A systematic review and meta-analysis. Clin. Rehabil. 2024, 38, 305–321. [Google Scholar] [CrossRef]
- Van der Kruk, S.R.; Zielinski, R.; MacDougall, H.; Hughes-Barton, D.; Gunn, K.M. Virtual reality as a patient education tool in healthcare: A scoping review. Patient Educ. Couns. 2022, 105, 1928–1942. [Google Scholar] [CrossRef]
- Fang, J.-W.; Li-Yuan, H.; Gwo-Jen, H.; Xiu-Wei, Z.; Chu-Nu, B.; Fu, Q.-K. A concept mapping-based self-regulated learning approach to promoting students’ learning achievement and self-regulation in STEM activities. Interact. Learn. Environ. 2023, 31, 7159–7181. [Google Scholar] [CrossRef]
- Dinh, T.T.H.; Bonner, A. Exploring the relationships between health literacy, social support, self-efficacy and self-management in adults with multiple chronic diseases. BMC Health Serv. Res. 2023, 23, 923. [Google Scholar] [CrossRef]
- Lee, J.; Cho, E.; Kim, H.; Lee, K.H.; Kim, E.; Ye, B.S. The development and evaluation of a self-efficacy enhancement program for older adults with mild cognitive impairment. Appl. Nurs. Res. 2023, 73, 151726. [Google Scholar] [CrossRef]
- Chen, J. The effectiveness of self-regulated learning (SRL) interventions on L2 learning achievement, strategy employment and self-efficacy: A meta-analytic study. Front. Psychol. 2022, 13, 1021101. [Google Scholar] [CrossRef]
- Follmer, D.J. Implementing a Simple, Scalable Self-Regulated Learning Intervention to Promote Graduate Learners’ Statistics Self-Efficacy and Concept Knowledge. J. Stat. Data Sci. Educ. 2023, 31, 80–90. [Google Scholar] [CrossRef]
- Niu, Z.; Willoughby, J.; Zhou, R. Associations of Health Literacy, Social Media Use, and Self-Efficacy With Health Information–Seeking Intentions Among Social Media Users in China: Cross-sectional Survey. J. Med. Internet Res. 2021, 23, e19134. [Google Scholar] [CrossRef]
- Park, S.; Shin, H.J.; Kwak, H.; Lee, H.J. Effects of immersive technology–based education for undergraduate nursing students: Systematic review and meta-analysis using the grading of recommendations, assessment, development, and evaluation (GRADE) approach. J. Med. Internet Res. 2024, 26, e57566. [Google Scholar] [CrossRef]
- Skidmore, N.; Ryan, C.; Mankelow, J.; Bradford, C.; Graham, A.; Martin, D. Exploring the potential of virtual reality for the self-management of chronic pain–a scoping review of its use to address health literacy. Musculoskelet. Sci. Pract. 2024, 72, 102962. [Google Scholar] [CrossRef]
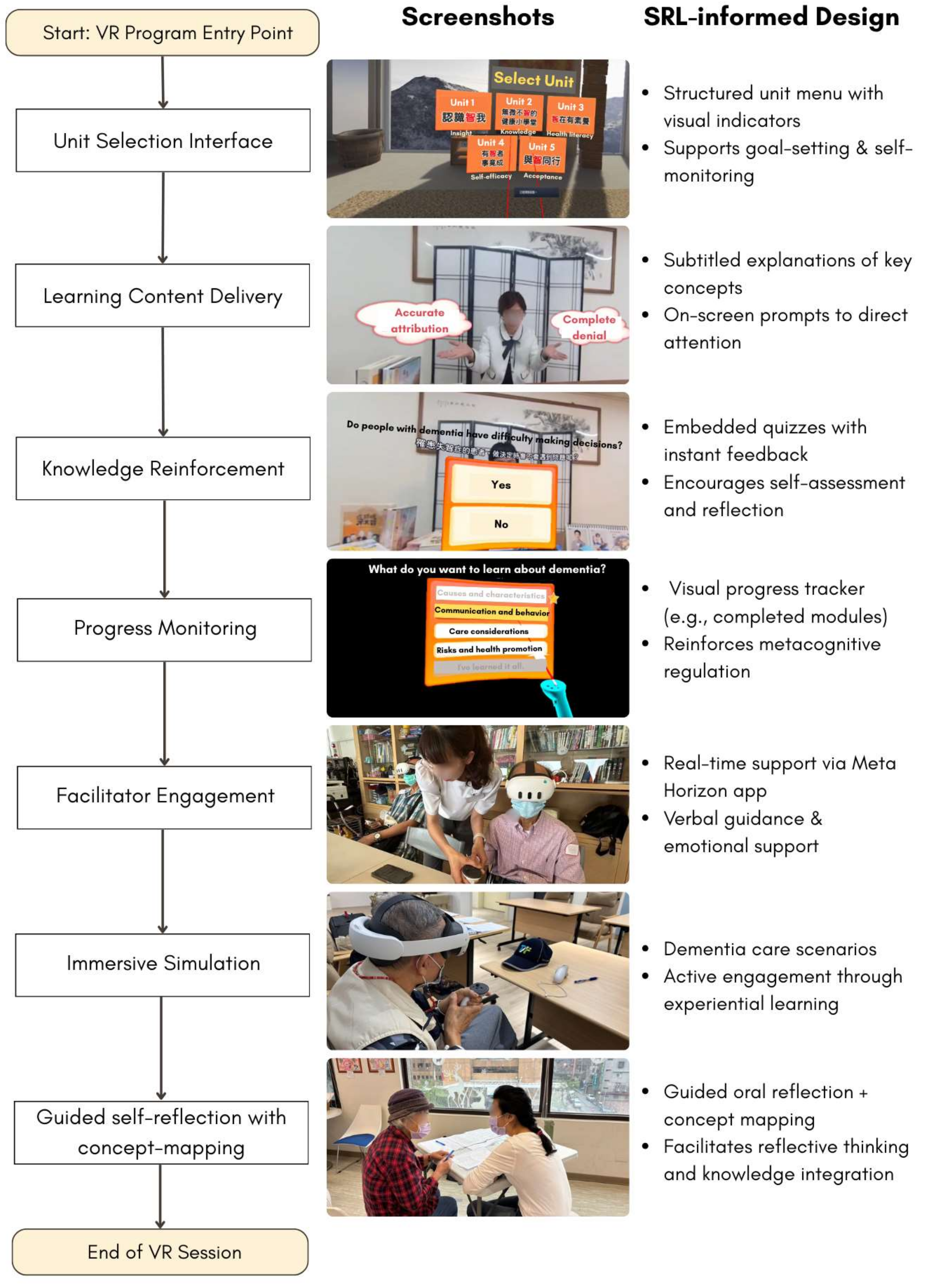
| Unit | Learning Objective | Outcome Variable |
|---|---|---|
| 1 |
| Insight |
| 2 | Acquire knowledge about dementia, encompassing its causes, characteristics, communication and behavioral aspects, care considerations, risks, and preventive methods. | Dementia-related knowledge |
| 3 |
| Health literacy |
| 4 | Foster confidence and a sense of capability in accomplishing specific tasks or goals. | Self-efficacy |
| 5 |
| Acceptance of dementia |
| Variables | Experimental Group (n = 30, 50%) | Comparison Group (n = 30, 50%) | χ2/t | p-Value |
|---|---|---|---|---|
| Age (years), mean (SD) | 76.83 (6.70) | 76.67 (7.01) | −0.094 | 0.925 |
| Gender, n (%) | 0.000 | 1.000 | ||
| Male | 6 (20.00) | 6 (20.00) | ||
| Female | 24 (80.00) | 24 (80.00) | ||
| Marital status, n (%) | 2.072 | 0.558 | ||
| Unmarried | 0 (0.00) | 2 (6.67) | ||
| Married | 13 (43.33) | 12 (40.00) | ||
| Divorced or separated | 1 (3.33) | 1 (3.33) | ||
| Widowed | 16 (53.33) | 15 (50.00) | ||
| Educational level, n (%) | 0.328 | 0.988 | ||
| Elementary school | 8 (27.59) | 9 (32.14) | ||
| Middle school | 4 (13.79) | 4 (14.29) | ||
| High school | 9 (31.03) | 9 (32.14) | ||
| University and above | 8 (27.59) | 6 (21.43) | ||
| Past experience with dementia-related health education, n (%) | 1.014 | 0.314 | ||
| Yes | 12 (40.0) | 8 (27.59) | ||
| No | 18 (60.0) | 21 (72.41) | ||
| Relatives diagnosed with dementia, n (%) | 4.109 | 0.128 | ||
| Yes | 2 (6.67) | 7 (24.14) | ||
| No | 23 (76.67) | 16 (55.17) | ||
| Not sure | 5 (16.67) | 6 (20.69) | ||
| Variables | Coefficient (β) | SE | Wald χ2 | p-Value |
|---|---|---|---|---|
| Dementia-related Knowledge (0–25 points) | ||||
| Group (Experimental group) b | −0.400 | 1.229 | 0.106 | 0.745 |
| Time (Post-test) c | 0.700 | 0.640 | 1.197 | 0.274 |
| Group (Experimental) Time (Post-test) d | 5.333 | 1.359 | 15.391 | <0.001 *** |
| Causes and Characteristics (0–7 points) | ||||
| Group (Experimental group) b | −0.333 | 0.428 | 0.606 | 0.436 |
| Time (Post-test) c | 0.567 | 0.244 | 5.415 | 0.020 * |
| Group (Experimental) Time (Post-test) d | 1.767 | 0.503 | 12.318 | <0.001 *** |
| Communication and Behavior (0–6 points) | ||||
| Group (Experimental group) b | −0.367 | 0.384 | 0.910 | 0.340 |
| Time (Post-test) c | −0.033 | 0.186 | 0.032 | 0.857 |
| Group (Experimental) Time (Post-test) d | 1.167 | 0.443 | 6.943 | 0.008 ** |
| Care Consideration (0–6 points) | ||||
| Group (Experimental group) b | −0.033 | 0.415 | 0.006 | 0.936 |
| Time (Post-test) c | −0.067 | 0.320 | 0.044 | 0.835 |
| Group (Experimental) Time (Post-test) d | 1.400 | 0.450 | 9.664 | 0.002 ** |
| Risks and Health Promotion (0–6 points) | ||||
| Group (Experimental group) b | 0.333 | 0.390 | 0.730 | 0.393 |
| Time (Post-test) c | 0.233 | 0.187 | 1.562 | 0.211 |
| Group (Experimental) Time (Post-test) d | 1.000 | 0.360 | 7.710 | 0.005 ** |
| Health Literacy (20–100 points) | ||||
| Group (Experimental group) b | 0.333 | 4.944 | 0.005 | 0.946 |
| Time (Post-test) c | −0.867 | 2.398 | 0.131 | 0.718 |
| Group (Experimental) Time (Post-test) d | 6.700 | 4.518 | 2.199 | 0.138 |
| Functional Health Literacy (8–40 points) | ||||
| Group (Experimental group) b | 0.367 | 1.971 | 0.035 | 0.852 |
| Time (Post-test) c | −0.533 | 0.947 | 0.317 | 0.573 |
| Group (Experimental) Time (Post-test) d | 0.933 | 1.695 | 0.303 | 0.582 |
| Critical Health Literacy (7–35 points) | ||||
| Group (Experimental group) b | 0.067 | 1.902 | 0.001 | 0.972 |
| Time (Post-test) c | −0.600 | 0.953 | 0.396 | 0.529 |
| Group (Experimental) Time (Post-test) d | 3.700 | 1.728 | 4.584 | 0.032 * |
| Interactive Health Literacy (5–25 points) | ||||
| Group (Experimental group) b | −0.100 | 1.458 | 0.005 | 0.945 |
| Time (Post-test) c | 0.267 | 1.052 | 0.064 | 0.800 |
| Group (Experimental) Time (Post-test) d | 2.067 | 1.734 | 1.421 | 0.233 |
| Self-efficacy (6–30 points) | ||||
| Group (Experimental group) b | −1.267 | 1.633 | 0.602 | 0.438 |
| Time (Post-test) c | −0.033 | 0.947 | 0.001 | 0.972 |
| Group (Experimental) Time (Post-test) d | 4.200 | 1.588 | 6.997 | 0.008 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-H.; Huang, K.-Y.; Kuo, L.-H.; Cheng, Y.-W.; Huang, S.-F.; Chuang, T.-H.; Huang, C.-M.; Guo, J.-L. Pilot Randomized Controlled Study on the Effectiveness of a Virtual Reality-Based Dementia Prevention Program Using Self-Regulated Learning Strategies Among Older Adults with Mild Cognitive Impairment. Healthcare 2025, 13, 1082. https://doi.org/10.3390/healthcare13091082
Chang C-H, Huang K-Y, Kuo L-H, Cheng Y-W, Huang S-F, Chuang T-H, Huang C-M, Guo J-L. Pilot Randomized Controlled Study on the Effectiveness of a Virtual Reality-Based Dementia Prevention Program Using Self-Regulated Learning Strategies Among Older Adults with Mild Cognitive Impairment. Healthcare. 2025; 13(9):1082. https://doi.org/10.3390/healthcare13091082
Chicago/Turabian StyleChang, Ching-Hao, Kuei-Yu Huang, Lou-Hui Kuo, Ya-Wen Cheng, Su-Fei Huang, Tien-Hsi Chuang, Chiu-Mieh Huang, and Jong-Long Guo. 2025. "Pilot Randomized Controlled Study on the Effectiveness of a Virtual Reality-Based Dementia Prevention Program Using Self-Regulated Learning Strategies Among Older Adults with Mild Cognitive Impairment" Healthcare 13, no. 9: 1082. https://doi.org/10.3390/healthcare13091082
APA StyleChang, C.-H., Huang, K.-Y., Kuo, L.-H., Cheng, Y.-W., Huang, S.-F., Chuang, T.-H., Huang, C.-M., & Guo, J.-L. (2025). Pilot Randomized Controlled Study on the Effectiveness of a Virtual Reality-Based Dementia Prevention Program Using Self-Regulated Learning Strategies Among Older Adults with Mild Cognitive Impairment. Healthcare, 13(9), 1082. https://doi.org/10.3390/healthcare13091082










