Modulation Effect of Physical Activity on Sleep Quality and Mental Hyperactivity in Higher-Education Students
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Instruments and Variables
2.3. Procedure
2.4. Data Analysis
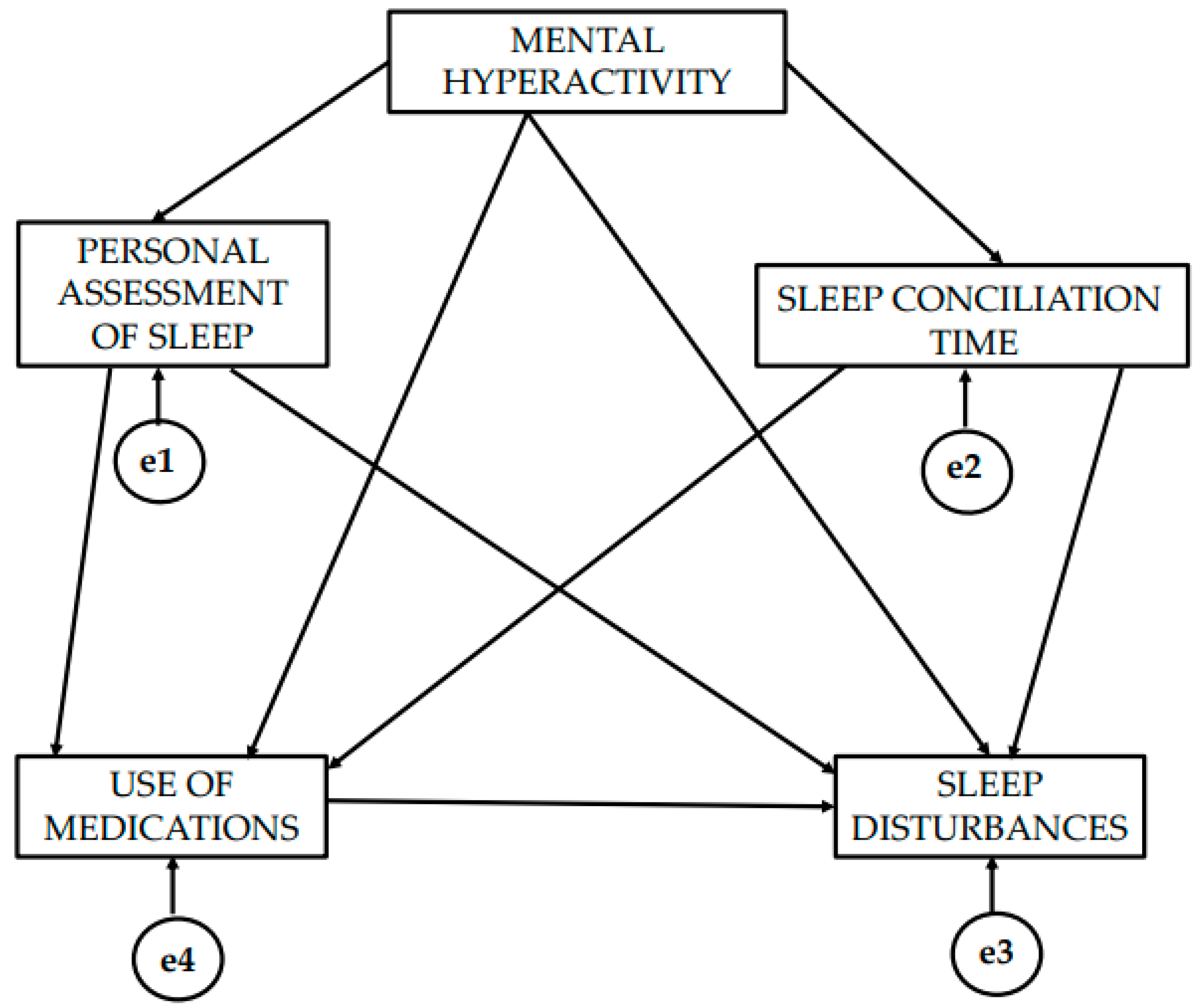
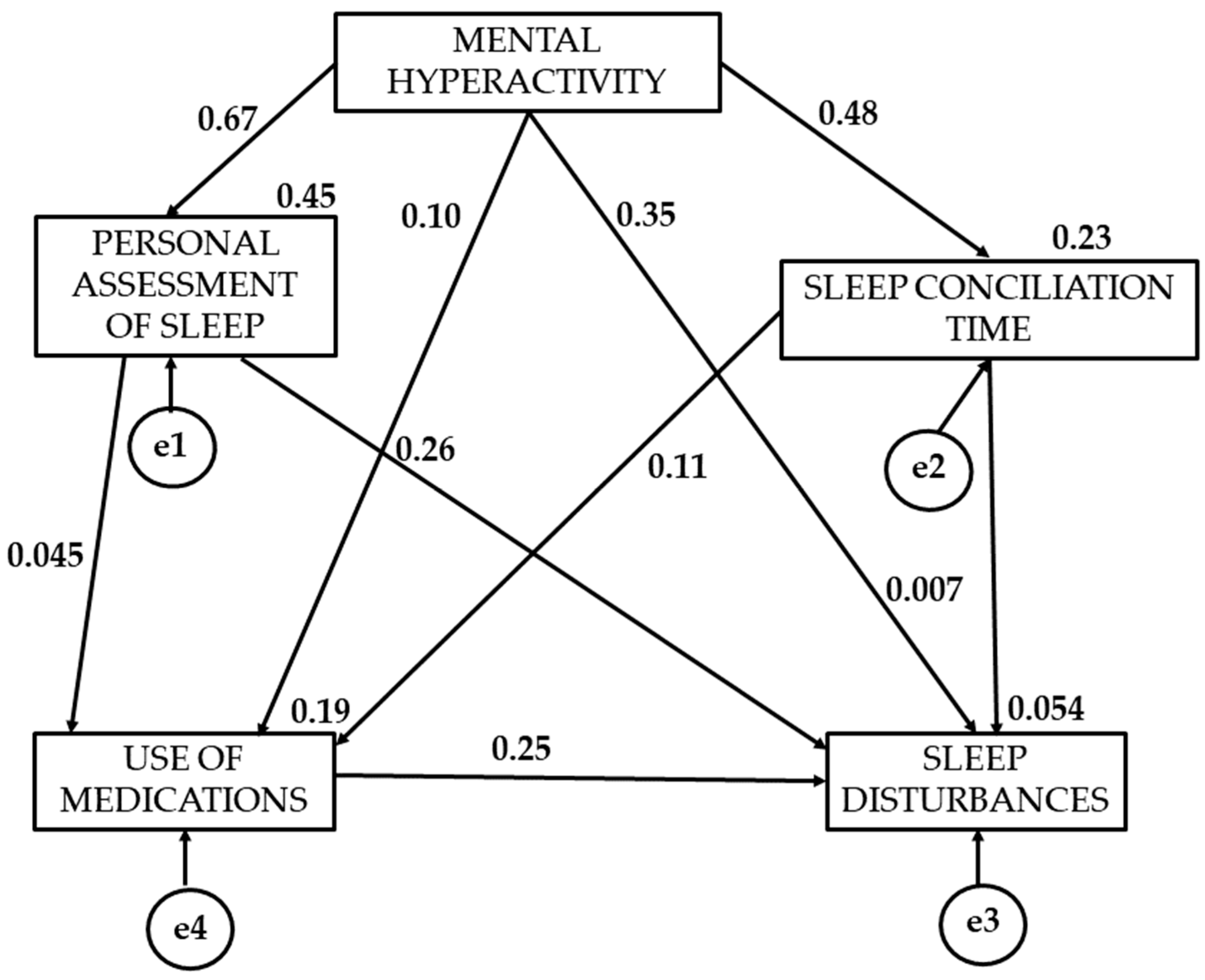
3. Results
4. Discussion
5. Limitations and Perspective Futures
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dempsey, P.C.; Matthews, C.E.; Dashti, S.G.; Doherty, A.R.; Bergouignan, A.; van Roekel, E.H.; Dunstan, D.W.; Wareham, N.J.; Yates, T.E.; Wijndaele, K.; et al. Sedentary behavior and chronic disease: Mechanisms and future directions. J. Phys. Act. Health 2020, 17, 52–61. [Google Scholar] [CrossRef]
- López-Valenciano, A.; Mayo, X.; Liguori, G. Changes in sedentary behaviour in European Union adults between 2002 and 2017. BMC Public Health 2020, 20, 1206. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Oh, P.I.; Faulkner, G.E. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S. World Health Organization 2020 guidelines on physical activity and sedentary behavior. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- An, H.-Y.; Chen, W.; Wang, C.-W. The relationships between physical activity and life satisfaction and happiness among young, middle-aged, and older adults. Int. J. Environ. Res. Public Health 2020, 17, 4817. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef]
- Pearce, M.; Garcia, L.; Abbas, A. Association between physical activity and risk of depression: A systematic review and meta-analysis. JAMA Psychiatry 2022, 79, 550. [Google Scholar] [CrossRef]
- Wu, X.Y.; Han, L.H.; Zhang, J.H. The influence of physical activity, sedentary behavior on health-related quality of life among the general population of children and adolescents: A systematic review. PLoS ONE 2017, 12, e0187668. [Google Scholar] [CrossRef]
- Rogerson, M.; Brown, D.K.; Sandercock, G. A comparison of four typical green exercise environments and prediction of psychological health outcomes. Perspect. Public Health 2016, 136, 171–180. [Google Scholar] [CrossRef]
- Gascon, M.; Zijlema, W.; Vert, C. Outdoor blue spaces, human health and well-being: A systematic review of quantitative studies. Int. J. Hyg. Environ. Health 2017, 220, 1207–1221. [Google Scholar] [CrossRef]
- van Sluijs, E.M.F.; Ekelund, U.; Crochemore-Silva, I.; Guthold, R.; Ha, A.; Lubans, D.; Oyeyemi, A.L.; Ding, D.; Katzmarzyk, P.T. Physical activity behaviours in adolescence: Current evidence and opportunities for intervention. Lancet 2021, 398, 429–442. [Google Scholar] [CrossRef]
- Bauman, A.E.; Reis, R.S.; Sallis, J.F.; Wells, J.C.; Loos, R.J.F.; Martin, B.W. Correlates of physical activity: Why are some people physically active and others not? Lancet 2012, 380, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.D.B.C.P.; Fermino, R.C.; Seabra, A.; Garganta, R.; Maia, J.A.R. Padrão de atividade física em crianças e jovens: Um breve resumo do estado do conhecimento. Braz. J. Kinanthropometry Hum. Perform. 2009, 12, 68–77. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Global trends in insufficient physical activity among adolescents: A pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child Adolesc. Health 2020, 4, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Strahler, J.; Nater, U.M. The effects of chronic fatigue and chronic stress on alterations in immune cell responses to acute psychosocial stress. Brain Behav. Immun. 2025, 123, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Barnabé, C.F.; Marchon, R.G.; Pinto, M.V.C.; Gregório, B.M.; Fortuna-Costa, A.; Sampaio, F.J.B.; De Souza, D.B. Effects of Chronic Stress and Comfort Food in Testicular Morphology in Adult Wistar Rats. Int. Braz. J. Urol. 2025, 51, e20240515. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jiang, L.; Wang, Q.; Hu, Q.; Zhong, T.; Wu, J.; Chen, X.; Liu, T. Chronic unpredictable stress during adolescence exerts sex-specific effects on depressive-like behavior and neural activation triggered by tail suspension test. Behav. Brain Res. 2025, 477, 115314. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vidal, I. El análisis de redes sociales en la investigación en salud pública: Una revisión sistemática [Social network analysis in public health research: A systematic review]. Rev. Salud Publica 2019, 21, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Carrier, K.; Abdel-Baki, A.; Thériault, L.; Karelis, A.D.; Lecomte, T.; Romain, A.J. Effects of Physical Activity on Disordered Eating Behaviours in Individuals With a Psychotic Disorder. Early Interv. Psychiatry 2025, 19, e13611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Y.; Wang, X. The effects of physical activity on social physique anxiety in college students-the mediating and moderating role of mental toughness and negative physical self. BMC Psychol. 2025, 13, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sambuco, N. Cognition, emotion, and the default mode network. Brain Cogn. 2024, 182, 106229. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, R.; Melguizo-Ibáñez, E.; Zurita-Ortega, F.; Ubago-Jiménez, J.L. Development and validation of a mental hyperactivity questionnaire for the evaluation of chronic stress in higher education. BMC Psychol. 2024, 12, 392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandino, L.; Binder, J.R. How does the “default mode” network contribute to semantic cognition? Brain Lang. 2024, 252, 105405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acuña, A.; Morales, S.; Uriarte-Gaspari, L.; Aguirre, N.; Brandani, A.; Huart, N.; Mattos, J.; Pérez, A.; Cuña, E.; Waiter, G.; et al. Increased default mode network activation in depression and social anxiety during upward social comparison. Soc. Cogn. Affect. Neurosci. 2025, 20, nsaf012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raichle, M.E. Two views of brain function. Trends Cogn. Sci. 2010, 14, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Akiki, T.J.; Averill, C.L.; Wrocklage, K.M.; Scott, J.C.; Averill, L.A.; Schweinsburg, B.; Alexander-Bloch, A.; Martini, B.; Southwick, S.M.; Krystal, J.H.; et al. Default mode network abnormalities in posttraumatic stress disorder: A novel network-restricted topology approach. Neuroimage 2018, 176, 489–498. [Google Scholar] [CrossRef]
- Zidda, F.; Andoh, J.; Pohlack, S.; Winkelmann, T.; Dinu-Biringer, R.; Cavalli, J.; Ruttorf, M.; Nees, F.; Flor, H. Default mode network connectivity of fear- and anxiety-related cue and context conditioning. Neuroimage 2018, 165, 190–199. [Google Scholar] [CrossRef]
- Park, S.M.; Jung, H.Y. Respiratory sinus arrhythmia biofeedback alters heart rate variability and default mode network connectivity in major depressive disorder: A preliminary study. Int. J. Psychophysiol. 2020, 158, 225–237. [Google Scholar] [CrossRef]
- Spunt, R.P.; Adolphs, R. Folk explanations of behavior: A specialized use of a domain-general mechanism. Psychol. Sci. 2015, 26, 724–736. [Google Scholar] [CrossRef]
- Satpute, A.B.; Lindquist, K.A. The default mode network’s role in discrete emotion. Trends Cogn. Sci. 2019, 23, 851–864. [Google Scholar] [CrossRef]
- Ko, Y.W.; Kim, S.M.; Kang, K.D.; Han, D.H. Changes in Functional Connectivity Between Default Mode Network and Attention Network in Response to Changes in Aerobic Exercise Intensity. Psychiatry Investig. 2023, 20, 27–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naderi, H.; Dehghan, H.; Dehrouyeh, S.; Tajik, E. Academic burnout among undergraduate nursing studentrs: Predicting the role of sleep quality and healthy lifestyle. Res. Dev. Med. Educ. 2021, 10, 16. [Google Scholar] [CrossRef]
- Marvaldi, M.; Mallet, J.; Dubertret, C.; Moro, M.R.; Guessoum, S.B. Anxiety, depression, trauma-related disorders and sleep among health workers during the COVID-19 pandemic: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 126, 252–264. [Google Scholar] [CrossRef]
- Santos, R.V.T.; Tufik, S.; De Mello, M.T. Exercise, sleep and cytokines: Is there a relation? Sleep Med. Rev. 2007, 11, 231–239. [Google Scholar] [CrossRef]
- Zagaar, M.; Dao, A.; Alhaider, I.; Alkadhi, K. Regular treadmill exercise prevents sleep deprivation-induced disruption of synaptic plasticity and associated signaling cascade in the dentate gyrus. Mol. Cell. Neurosci. 2013, 56, 375–383. [Google Scholar] [CrossRef]
- Rahmani, M.; Rahmani, F.; Rezaei, N. The brain-derived neurotrophic factor: Missing link between sleep deprivation, insomnia, and depression. Neurochem. Res. 2020, 45, 221–231. [Google Scholar] [CrossRef]
- Van Cauter, E.; Plat, L. Physiology of growth hormone secretion during sleep. J. Pediatr. 1996, 128, S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Mantilla-Toloza, S.C.; Gómez-Conesa, A. El cuestionario Internacional de Actividad Física. Un instrumento adecuado para el seguimiento de la actividad física poblacional. Rev. Iberoam. Fisioter. Kinesol. 2007, 10, 48–52. [Google Scholar]
- Dinger, M.K.; Behrens, T.K.; Han, J.L. Validity and reliability of the international physical activity questionnaire in college students. Am. J. Health Educ. 2006, 37, 337–343. [Google Scholar] [CrossRef]
- Sanz-Martín, D.; Zurita-Ortega, F.; Cachón-Zagalaz, J.; Melguizo-Ibánez, E. Relationship between Mediterranean diet, physical activity and emotional intelligence in Spanish undergraduates. Retos 2024, 55, 307–316. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Favela-Ramírez, C.A.; Castro-Robles, A.I.; Bojórquez-Díaz, C.I.; Chan Barocio, N.L. Propiedades psicométricas del índice de calidad de sueño de Pittsburgh en deportistas. Rev. Iberoam. Cienc. Act. Fís. Deporte 2022, 11, 29–46. [Google Scholar] [CrossRef]
- Kline, R.B. Beyond Significance Testing: Reforming Data Analysis Methods in Behavioral Research; American Psychological Association: Washington, DC, USA, 2004. [Google Scholar]
- Marôco, J. Análise de Equações Estruturais Fundamentos Teóricos, Software & Aplicações; ReportNumber, Lda: Lisbon, Portugal, 2021. [Google Scholar]
- Kyriazos, T.A. Applied psychometrics: Sample size and sample power considerations in factor analysis (EFA, CFA) and SEM in general. Psychology 2018, 9, 2207–2230. [Google Scholar] [CrossRef]
- Maydeu-Olivares, A. Maximum likelihood estimation of structural equation models for continuous data: Standard errors and goodness of fit. Struct. Equ. Model. 2017, 24, 383–394. [Google Scholar] [CrossRef]
- Kuckartz, U.; Rädiker, S.; Ebert, T.; Schehl, J. Statistik Eine Verständliche Einführung; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Militana, A.R.; Donahue, M.J.; Sills, A.K.; Solomon, G.S.; Gregory, A.J.; Strother, M.K.; Morgan, V.L. Alterations in default-mode network connectivity may be influenced by cerebrovascular changes within 1 week of sports related concussion in college varsity athletes: A pilot study. Brain Imaging Behav. 2016, 10, 559–568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, H.; Bruchmann, M.; Straube, T. Altered putamen activation for social comparison-related feedback in social anxiety disorder: A pilot study. Neuropsychobiology 2023, 82, 359–572. [Google Scholar] [CrossRef]
- Stalder, C.; Kirschbaum, B.M.; Kudielka, E.K.; Adam, J.C.; Pruessner, S.; Wüst, A. Clow Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 2016, 63, 414–432. [Google Scholar] [CrossRef]
- Hakamata, Y.; Komi, S.; Moriguchi, Y.; Izawa, S.; Motomura, Y.; Sato, E.; Mizukami, S.; Kim, Y.; Hanakawa, T.; Inoue, Y.; et al. Amygdala-centered functional connectivity affects daily cortisol levels: A putative link with anxiety. Sci. Fict. Represent. 2017, 7, 8313. [Google Scholar]
- Lee, D.Y.; Kim, E.; Choi, M.H. Aspectos técnicos y clínicos del cortisol como marcador bioquímico del estrés crónico. BMB Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef]
- Hehr, A.; Huntley, E.D.; Marusak, H.A. Getting a Good Night’s Sleep: Associations Between Sleep Duration and Parent-Reported Sleep Quality on Default Mode Network Connectivity in Youth. J. Adolesc. Health 2023, 72, 933–942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, H.; Zhou, Q.; Yang, J.; Lu, Q.; Qiu, H.; He, C.; Yan, H. Altered functional connectivity of the default mode and frontal control networks in patients with insomnia. CNS Neurosci. Ther. 2023, 29, 2318–2326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, P. Stress buffering effects of physical activity in adolescents: The moderating role of physical activity attitudes. BMC Public Health 2025, 25, 463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lastella, M.; Vincent, G.E.; Duffield, R.; Roach, G.D.; Halson, S.L.; Heales, L.J.; Sargent, C. Can sleep be used as an indicator of overtraining and overexertion in athletes? Front. Physiol. 2018, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, R. A Grandes Males Grandes Remedios: Borrón Y Mente Nueva; Uno: Madrid, Spain, 2021. [Google Scholar]
- Worthen, M.; Cash, E. Stress Management. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 14 August 2025. [Google Scholar] [PubMed]
| M | SD | SWEK | KUR | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|---|---|
| 1. MH | 1.21 | 0.69 | 0.390 | −0.507 | 0.449 ** | 0.319 ** | 0.376 ** | 0.494 ** |
| 2. PAS | 1.14 | 0.72 | 0.381 | 0.146 | 0.315 ** | 0.464 ** | 0.590 ** | |
| 3. UM | 0.30 | 0.78 | 0.652 | 0.810 | 0.256 ** | 0.517 ** | ||
| 4. SCT | 1.88 | 0.88 | 0.684 | 0.424 | 0.382 ** | |||
| 5. SD | 0.74 | 0.42 | 0.850 | 0.906 |
| Direction of Causal Relationships | Regression Weights | Standardized Regression Weights | ||||
|---|---|---|---|---|---|---|
| Estimate | Estimation Error | Critical Radio | p | β | ||
| MILD PA | MH → PAS | 0.735 | 0.66 | 11.143 | 0.049 | 0.671 |
| MODERATE PA | MH → PAS | 0.426 | 0.057 | 7.525 | 0.428 | |
| VIGOROUS PA | MH → PAS | 0.364 | 0.075 | 4.828 | 0.343 | |
| MILD PA | MH → SCT | 0.602 | 0.090 | 6.714 | 0.024 | 0.479 |
| MODERATE PA | MH → SCT | 0.391 | 0.078 | 5.036 | 0.302 | |
| VIGOROUS PA | MH → SCT | 0.531 | 0.088 | 6.006 | 0.413 | |
| MILD PA | MH → UM | 0.392 | 0.101 | 3.892 | 0.073 | −0.096 |
| MODERATE PA | MH → UM | 0.158 | 0.064 | 2.455 | 0.169 | |
| VIGOROUS PA | MH → UM | −0.123 | 0.136 | −0.903 | 0.309 | |
| MILD PA | PAS → UM | 0.168 | 0.087 | 1.929 | 0.054 | 0.449 |
| MODERATE PA | PAS → UM | 0.148 | 0.062 | 2.381 | 0.158 | |
| VIGOROUS PA | PAS → UM | 0.523 | 0.115 | 4.540 | 0.141 | |
| MILD PA | UM →SCT | 0.075 | 0.074 | 1.014 | 0.310 | 0.115 |
| MODERATE PA | UM →SCT | 0.063 | 0.045 | 1.397 | 0.088 | |
| VIGOROUS PA | UM →SCT | 0.117 | 0.085 | 1.377 | 0.077 | |
| MILD PA | MH → SD | 0.035 | 0.028 | 1.250 | 0.211 | 0.352 |
| MODERATE PA | MH → SD | 0.096 | 0.031 | 3.037 | 0.162 | |
| VIGOROUS PA | MH → SD | 0.225 | 0.052 | 4.355 | 0.193 | |
| MILD PA | TCS → SD | 0.035 | 0.028 | 1.25 | 0.076 | 0.075 |
| MODERATE PA | TCS → SD | 0.004 | 0.022 | 0.168 | 0.008 | |
| VIGOROUS PA | TCS → SD | 0.038 | 0.032 | 1.176 | 0.072 | |
| MILD PA | VPS → SD | 0.197 | 0.033 | 5.971 | 0.258 | 0.264 |
| MODERATE PA | PAS → SD | 0.266 | 0.030 | 8.747 | 0.448 | |
| VIGOROUS PA | VAS → SD | 0.154 | 0.047 | 3.315 | 0.338 | |
| MILD PA | UM → SD | 0.192 | 0.028 | 6.789 | 0.006 | 0.252 |
| MODERATE PA | UM → SD | 0.187 | 0.030 | 6.175 | 0.298 | |
| VIGOROUS PA | UM → SD | 0.126 | 0.031 | 4.098 | 0.393 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-García, R.; González-Forte, C.; Granero-Molina, J.; Melguizo-Ibáñez, E. Modulation Effect of Physical Activity on Sleep Quality and Mental Hyperactivity in Higher-Education Students. Healthcare 2025, 13, 1040. https://doi.org/10.3390/healthcare13091040
Fernández-García R, González-Forte C, Granero-Molina J, Melguizo-Ibáñez E. Modulation Effect of Physical Activity on Sleep Quality and Mental Hyperactivity in Higher-Education Students. Healthcare. 2025; 13(9):1040. https://doi.org/10.3390/healthcare13091040
Chicago/Turabian StyleFernández-García, Rubén, Cristina González-Forte, José Granero-Molina, and Eduardo Melguizo-Ibáñez. 2025. "Modulation Effect of Physical Activity on Sleep Quality and Mental Hyperactivity in Higher-Education Students" Healthcare 13, no. 9: 1040. https://doi.org/10.3390/healthcare13091040
APA StyleFernández-García, R., González-Forte, C., Granero-Molina, J., & Melguizo-Ibáñez, E. (2025). Modulation Effect of Physical Activity on Sleep Quality and Mental Hyperactivity in Higher-Education Students. Healthcare, 13(9), 1040. https://doi.org/10.3390/healthcare13091040








