Effects of Physical Exercise on Cardiometabolic Health in Individuals with Autism Spectrum Disorder: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Database and Search Strategy
2.2. Study Criteria and Eligibility
2.3. Quality Assessment
2.4. Data Extraction
2.5. Primary Outcomes
2.6. Secondary Outcomes
3. Results
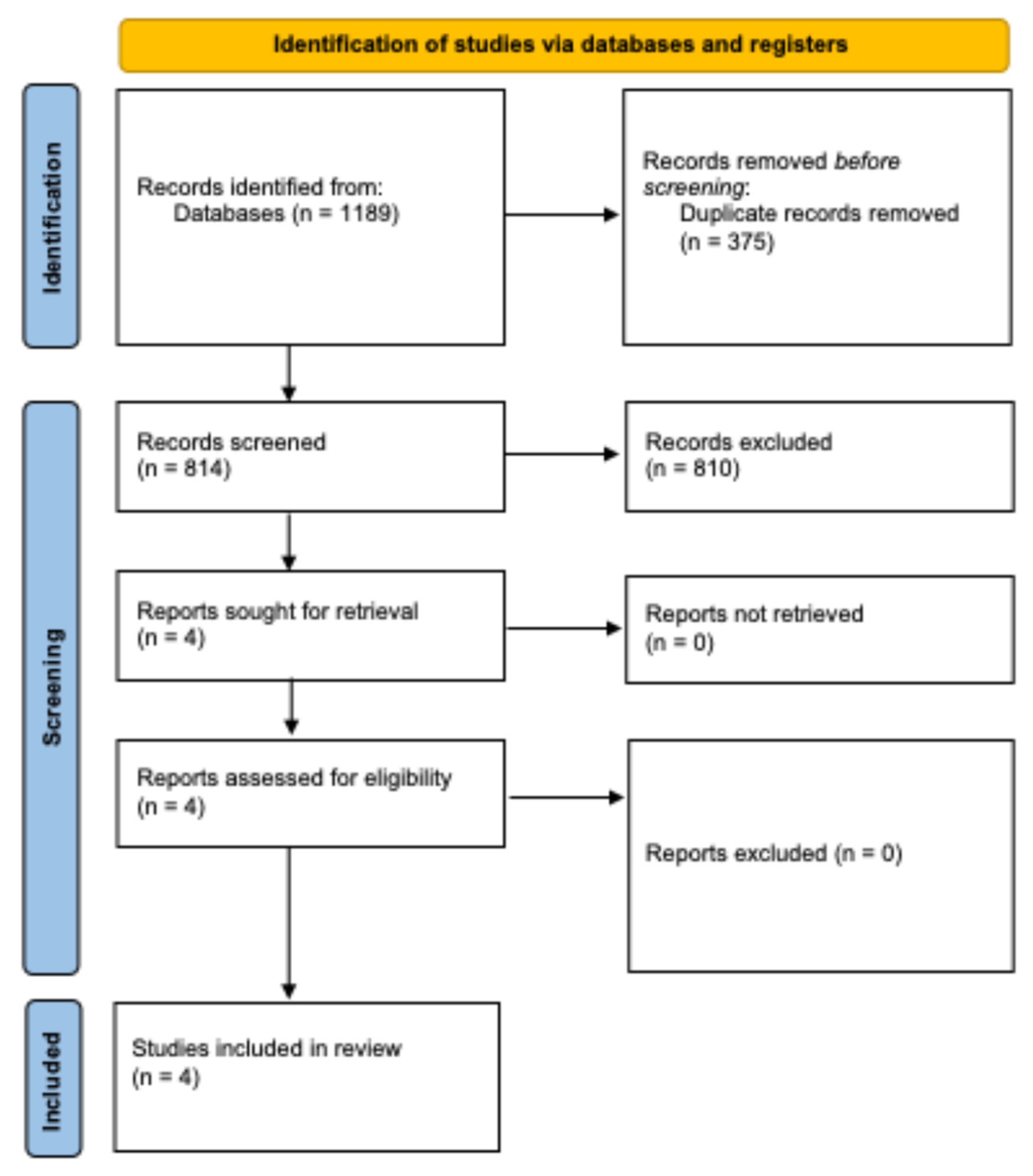
3.1. Study Selection
3.2. Characteristics of Participants and Groups
3.3. Characteristics of Interventions
3.4. Results of Primary Outcomes
3.5. Methodological Quality Assessment
3.6. Results of Secondary Outcomes
4. Discussion
4.1. Primary Outcomes
4.2. Secondary Outcomes
4.3. Practical Application
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Takumi, T.; Tamada, K.; Hatanaka, F.; Nakai, N.; Bolton, P.F. Behavioral neuroscience of autism. Neurosci. Biobehav. Rev. 2020, 110, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, K.K.; Orsso, C.E.; Richard, C.; Haqq, A.M.; Zwaigenbaum, L. Risk Factors for Unhealthy Weight Gain and Obesity among Children with Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 3285. [Google Scholar] [CrossRef]
- Heffernan, K.S.; Columna, L.; Russo, N.; Myers, B.A.; Ashby, C.E.; Norris, M.L.; Barreira, T.V. Brief Report: Physical Activity, Body Mass Index and Arterial Stiffness in Children with Autism Spectrum Disorder: Preliminary Findings. J. Autism Dev. Disord. 2018, 48, 625–631. [Google Scholar] [CrossRef]
- Dhanasekara, C.S.; Ancona, D.; Cortes, L.; Hu, A.; Rimu, A.H.; Robohm-Leanvitt, C.; Payne, D.; Wakefield, S.M.; Mastergeorge, A.M.; Kahathuduwa, C.N. Association Between Autism Spectrum Disorders and Cardiometabolic Diseases: A Systematic Review and Meta-analysis. JAMA Pediatr. 2023, 177, 248–257. [Google Scholar] [CrossRef]
- Davis, J. Individuals with autism are at a higher associated risk of developing cardiometabolic diseases. Evid. Based Nurs. 2023, 27, 91. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Huang, Y.C.; Huang, W.L. Heart rate variability in individuals with autism spectrum disorders: A meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Bricout, V.A.; Pace, M.; Dumortier, L.; Baillieul, F.; Favre-Juvin, A.; Guinot, M. Reduced Cardiorespiratory Capacity in Children with Autism Spectrum Disorders. J. Clin. Med. 2018, 7, 361. [Google Scholar] [CrossRef]
- Parisi, L.; Salerno, M.; Maltese, A.; Tripi, G.; Romano, P.; Di Folco, A.; Di Filippo, T. Autonomic Regulation In Autism Spectrum Disorders. Acta Medica Mediterr. 2017, 33, 491–494. [Google Scholar] [CrossRef]
- Gehricke, J.G.; Chan, J.; Farmer, J.G.; Fenning, R.M.; Steinberg-Epstein, R.; Misra, M.; Parker, R.A.; Neumeyer, A.M. Physical activity rates in children and adolescents with autism spectrum disorder compared to the general population. Res. Autism Spectr. Disord. 2020, 70, 101490. [Google Scholar] [CrossRef]
- Boucher, T.Q.; McIntyre, C.L.; Iarocci, G. Facilitators and Barriers to Physical Activity Involvement as Described by Autistic Youth with Mild Intellectual Disability. Adv. Neurodev. Disord. 2022, 7, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Du, C.; Liu, J.; Tan, G. Meta-Analysis on Intervention Effects of Physical Activities on Children and Adolescents with Autism. Int. J. Environ. Res. Public Health 2020, 17, 1950. [Google Scholar] [CrossRef] [PubMed]
- Healy, S.; Nacario, A.; Braithwaite, R.E.; Hopper, C. The effect of physical activity interventions on youth with autism spectrum disorder: A meta-analysis. Autism Res. 2018, 11, 818–833. [Google Scholar] [CrossRef] [PubMed]
- AHA. American Heart Association Recommendations for Physical Activity in Kids Infographic|American Heart Association. Available online: https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-in-kids-infographic (accessed on 14 January 2025).
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1978, 52, 377–384. [Google Scholar] [CrossRef]
- O’Connor, S.R.; Tully, M.A.; Ryan, B.; Bradley, J.M.; Baxter, G.D.; McDonough, S.M. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: A comparison study. BMC Res. Notes 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Hooper, P.; Jutai, J.W.; Strong, G.; Russell-Minda, E. Age-related macular degeneration and low-vision rehabilitation: A systematic review. Can. J. Ophthalmol. 2008, 43, 180–187. [Google Scholar] [CrossRef]
- Kirk, E.P.; Klein, S. Pathogenesis and pathophysiology of the cardiometabolic syndrome. J. Clin. Hypertens. 2009, 11, 761–765. [Google Scholar] [CrossRef]
- Khambhati, J.; Allard-Ratick, M.; Dhindsa, D.; Lee, S.; Chen, J.; Sandesara, P.B.; O’Neal, W.; Quyyumi, A.A.; Wong, N.D.; Blumenthal, R.S.; et al. The art of cardiovascular risk assessment. Clin. Cardiol. 2018, 41, 677–684. [Google Scholar] [CrossRef]
- Nevill, A.M.; Stewart, A.D.; Olds, T.; Duncan, M.J. A new waist-to-height ratio predicts abdominal adiposity in adults. Res. Sports Med. 2020, 28, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Raghuveer, G.; Hartz, J.; Lubans, D.R.; Takken, T.; Wiltz, J.L.; Mietus-Snyder, M.; Perak, A.M.; Baker-Smith, C.; Pietris, N.; Edwards, N.M. Cardiorespiratory Fitness in Youth: An Important Marker of Health: A Scientific Statement From the American Heart Association. Circulation 2020, 142, E101–E118. [Google Scholar] [CrossRef] [PubMed]
- Ades, P.A.; Savage, P.D.; Toth, M.J.; Harvey-Berino, J.; Schneider, D.J.; Bunn, J.Y.; Audein, M.C.; Ludlow, M. High-Caloric Expenditure Exercise: A New Approach to Cardiac Rehabilitation for Overweight Coronary Patients Ades: High-Caloric Exercise Overweight Coronary Patients. Circulation 2009, 119, 2671. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Ellie Wilson, C.; Robertson, D.M.; Ecker, C.; Daly, E.M.; Hammond, N.; Galanopoulos, A.; Dud, L.; Murphy, D.G.; McAlonan, G.M. Autism spectrum disorder in adults: Diagnosis, management, and health services development. Neuropsychiatr. Dis. Treat. 2016, 12, 1669–1686. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Yu, J.; Jee, Y.S. Educational exercise program affects to physical fitness and gross motor function differently in the severity of autism spectrum disorder. J. Exerc. Rehabil. 2020, 16, 410–417. [Google Scholar] [CrossRef]
- Pitetti, K.H.; Rendoff, A.D.; Grover, T.; Beets, M.W. The efficacy of a 9-month treadmill walking program on the exercise capacity and weight reduction for adolescents with severe autism. J. Autism Dev. Disord. 2007, 37, 997–1006. [Google Scholar] [CrossRef]
- Toscano, C.V.A.; Carvalho, H.M.; Ferreira, J.P. Exercise Effects for Children With Autism Spectrum Disorder: Metabolic Health, Autistic Traits, and Quality of Life. Percept. Mot. Skills 2018, 125, 126–146. [Google Scholar] [CrossRef]
- Pierantozzi, E.; Morales, J.; Fukuda, D.H.; Garcia, V.; Gomez, A.M.; Guerra-Balic, M.; Carballeira, E. Effects of a Long-Term Adapted Judo Program on the Health-Related Physical Fitness of Children with ASD. Int. J. Environ. Res. Public Health 2022, 19, 16731. [Google Scholar] [CrossRef]
- Westerterp, K.R. Exercise, energy balance and body composition. Eur. J. Clin. Nutr. 2018, 72, 1246–1250. [Google Scholar] [CrossRef]
- Soares, R.; Brasil, I.; Monteiro, W.; Farinatti, P. Effects of physical activity on body mass and composition of school-age children and adolescents with overweight or obesity: Systematic review focusing on intervention characteristics. J. Bodyw. Mov. Ther. 2023, 33, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Karpe, F.; Lafontan, M.; Frayn, K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol. Rev. 2012, 92, 157–191. [Google Scholar] [CrossRef] [PubMed]
- Belanger, M.J.; Rao, P.; Robbins, J.M. Exercise, Physical Activity, and Cardiometabolic Health: Pathophysiologic Insights. Cardiol. Rev. 2022, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.L.; Himali, J.J.; Torjesen, A.; Tsao, C.W.; Beiser, A.; Hamburg, N.M.; DeCarli, C.; Vasan, R.S.; Seshadri, S.; Pase, M.P.; et al. Inter-Relations of Orthostatic Blood Pressure Change, Aortic Stiffness, and Brain Structure and Function in Young Adults. J. Am. Heart Assoc. 2017, 6, e006206. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.C.; Lira, F.S.; Gonçalves, D.C. Reverse Cholesterol Transport: Molecular Mechanisms and the Non-medical Approach to Enhance HDL Cholesterol. Front. Physiol. 2018, 9, 526. [Google Scholar] [CrossRef]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef]
- Evans, P.L.; McMillin, S.L.; Weyrauch, L.A.; Witczak, C.A. Regulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise Training. Nutrients 2019, 11, 2432. [Google Scholar] [CrossRef]
- Figueira, F.R.; Umpierre, D.; Bock, P.M.; Waclawovsky, G.; Guerra, A.P.; Donelli, A.; Andrades, M.; Casali, K.R.; Schaan, B.D. Effect of exercise on glucose variability in healthy subjects: Randomised crossover trial. Biol. Sport 2019, 36, 141–148. [Google Scholar] [CrossRef]
- Peter Adams, O. The impact of brief high-intensity exercise on blood glucose levels. Diabetes Metab. Syndr. Obes. 2013, 6, 113–122. [Google Scholar] [CrossRef]
- Srinivasan, S.M.; Pescatello, L.S.; Bhat, A.N. Current perspectives on physical activity and exercise recommendations for children and adolescents with autism spectrum disorders. Phys. Ther. 2014, 94, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; Murillo, A.G. Is There a Correlation between Dietary and Blood Cholesterol? Evidence from Epidemiological Data and Clinical Interventions. Nutrients 2022, 14, 2168. [Google Scholar] [CrossRef]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Fraguas, D.; Díaz-Caneja, C.M.; Pina-Camacho, L.; Moreno, C.; Durán-Cutilla, M.; Ayora, M.; González-Vioque, E.; De Matteis, M.; Hendren, R.L.; Arango, C.; et al. Dietary Interventions for Autism Spectrum Disorder: A Meta-analysis. Pediatrics 2019, 144, e20183218. [Google Scholar] [CrossRef]
- Pagidipati, N.J.; Taub, P.R.; Ostfeld, R.J.; Kirkpatrick, C.F. Dietary patterns to promote cardiometabolic health. Nat. Rev. Cardiol. 2025, 22, 38–46. [Google Scholar] [CrossRef]
- Chen, H.; Xu, J.; Xie, H.; Huang, Y.; Shen, X.; Xu, F. Effects of physical activity on heart rate variability in children and adolescents: A systematic review and meta-analysis. Cienc. Saude Coletiva 2022, 27, 1827–1842. [Google Scholar] [CrossRef]
- Garavaglia, L.; Gulich, D.; Defeo, M.M.; Mailland, J.T.; Irurzun, I.M. The effect of age on the heart rate variability of healthy subjects. PLoS ONE 2021, 16, e0255894. [Google Scholar] [CrossRef]
- Bernardi, J.; Aromolaran, K.A.; Aromolaran, A.S. Neurological Disorders and Risk of Arrhythmia. Int. J. Mol. Sci. 2020, 22, 188. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef]
- Wakabayashi, I. Age-dependent influence of gender on the association between obesity and a cluster of cardiometabolic risk factors. Gend. Med. 2012, 9, 267–277. [Google Scholar] [CrossRef]
- Zhang, X.E.; Cheng, B.; Wang, Q.; Wan, J.J. Association of gender-specific risk factors in metabolic and cardiovascular diseases: An NHANES-based cross-sectional study. J. Investig. Med. 2018, 66, 22–31. [Google Scholar] [CrossRef]
- Stevens, J.; Katz, E.G.; Huxley, R.R. Associations between gender, age and waist circumference. Eur. J. Clin. Nutr. 2010, 64, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Szatmari, P. Sex and gender impacts on the behavioural presentation and recognition of autism. Curr. Opin. Psychiatry 2020, 33, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, M. Improving the quality of life of children and adolescents with autism spectrum disorders through athletic-based therapy programs. Educ. Dev. Psychol. 2019, 36, 68–74. [Google Scholar] [CrossRef]
- Magnuson, K.M.; Constantino, J.N. Characterisation of depression in children with autism spectrum disorders. J. Dev. Behav. Pediatr. 2011, 32, 332–340. [Google Scholar] [CrossRef]
- Awick, E.A.; Ehlers, D.K.; Aguiñaga, S.; Daugherty, A.M.; Kramer, A.F.; McAuley, E. Effects of a Randomised Exercise Trial on Physical Activity, Psychological Distress and Quality of Life in Older Adults. Gen. Hosp. Psychiatry 2017, 49, 44. [Google Scholar] [CrossRef]
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef]
- Levante, A.; Martis, C.; Antonioli, G.; Dima, M.; Duma, L.; Perrone, M.; Russo, L.; Lecciso, F. The Effect of Sports Activities on Motor and Social Skills in Autistic Children and Adolescents: A Systematic Narrative Review. Curr. Dev. Disord. Rep. 2023, 10, 155–174. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Diet and Health. Calories: Total Macronutrient Intake, Energy Expenditure, and Net Energy Stores. 1989. Available online: https://www.ncbi.nlm.nih.gov/books/NBK218769/ (accessed on 15 January 2025).
- Batrakoulis, A.; Jamurtas, A.Z.; Metsios, G.S.; Perivoliotis, K.; Liguori, G.; Feito, Y.; Riebe, D.; Thompson, W.R.; Angelopoulos, T.J.; Krustrup, P.; et al. Comparative Efficacy of 5 Exercise Types on Cardiometabolic Health in Overweight and Obese Adults: A Systematic Review and Network Meta-Analysis of 81 Randomized Controlled Trials. Circ. Cardiovasc. Qual. Outcomes 2022, 15, E008243. [Google Scholar] [CrossRef]
- Sowa, M.; Meulenbroek, R. Effects of physical exercise on Autism Spectrum Disorders: A meta-analysis. Res. Autism Spectr. Disord. 2012, 6, 46–57. [Google Scholar] [CrossRef]
- Kirkovski, M.; Enticott, P.G.; Fitzgerald, P.B. A review of the role of female gender in autism spectrum disorders. J. Autism Dev. Disord. 2013, 43, 2584–2603. [Google Scholar] [CrossRef] [PubMed]
| Study | Groups and Gender (M/F) | Age (Years) | Weight (kg) | Height (cm) | BMI (kg/m2) | Waist Circumference (cm) | Cholesterol (Total, HDL-C, and LDL-C) (mg/dL) | Glucose (mg/dL) | Triglycerides (mg/dL) | HR (ppm) | VO2max (mL/kg/min) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [28] | EG: n = 5 CG: n = 5 M:6 F:4 | 14–19 EG: 16.6 ± 1.9 CG: 17.4 ± 1.1 | EG: 98.0 ± 18.3 CG: 93.0 ± 32.3 | EG: 173.0 ± 7.8 CG: 172.6 ± 8.4 | TWG: 33 ± 7.8 CG: 30.9 ± 8.49 | NR | NR | NR | NR | NR | NR |
| [29] | EG: n = 46 CG: n = 18 M: 56 F: 8 | 6–12 | EG:35.9 (13.6) CG: 51.2 (29.1) | EG: 128.2 (14.7) CG: 148.7 (27.2) | EG: 21.4 (6.2) CG: 21.1 (5.4) | EG: 67.9 (16.3) CG: 48.6 (11.1) | Total cholesterol: EG: 167.4 (31.3) CG: 201.0 (49.1) HDL-C: EG:50.3 (14.4) CG: 45.6 (15.5) LDL-C: EG: 100.9 (29.16) CG:119.9 (34.8) | EG: 71.6 (10.2) CG: 89.2 (11.7) | EG: 91.7 (47.0) CG: 139.2 (57.2) | NR | NR |
| [30] | EG: n = 21 CG: n = 19 M/F: NR | Mean: 11.07 (± 1.73) EG: 11.1 ± 1.9 CG: 11.0 ± 1.5 | Mean: 47.71 (± 16.71) EG: 47.7 ± 12.5 CG: 47.6 ± 10.2 | Mean: 145.9 (± 15.81) EG: 147.0 ± 15.7 CG: 144.5 ± 15.9 | NR | EG: 72.5 ± 7.5 CG: 73.5 ± 4.9 Allometric index (WHT5): EG: 0.60 ± 0.05 CG: 0.61 ± 0.04 | NR | NR | NR | EG:73.60 ± 7.0 CG: 72.3 ± 7.8 | |
| [27] | Mild group: n = 17, Severe group n = 18 M: 35 | 20–29 Mild ASD: 22.82 ± 2.70 Severe ASD: 22.83 ± 2.81 | Mild ASD: 73.02 ± 12.63 Severe ASD: 72.09 ± 10.32 | Mild ASD: 174.01 ± 7.16 Severe ASD: 173.39 ± 8.56 | Mild ASD: 24.18 ± 4.32 Severe ASD: 24.14 ± 4.18 | NR | NR | NR | NR | NR |
| Study | Type of Exercise and Duration of Intervention | Frequency | Protocol | Training Sessions (reps or min) | Control Group Intervention |
|---|---|---|---|---|---|
| [28] | Treadmill walking 9 months (39 weeks) | The initial frequency was 2 times/wk Progression of 1 day every 2 weeks Peak frequency of 5 times/wk | Initial speed: 2.4–3.5 mph (according to individual capacity) Progression: 0.1–0.3 mph every 2–3 weeks Peak: 2.7–4.1 mph | Initial: 8 min Progression: 1–2 min every 2–3 weeks Peak: 20 min | ‘Leisure activity’ 30 min, 3 times/wk |
| [29] | Coordination and strength exercise 48 weeks (11 months) | 2 times/wk | Climbing and support on the bar: 5.0 s Release to the basket: different weights (0.5, 1.0, and 2.0 kg) Work with elastics: NR Walking on steps and inclined plane: three steps (12 and 15 cm) and inclined plane of 0.78 cm in length and 30 cm Step box with target: three sets of sequenced steps of dimension 60 × 28 × 14 cm Sequenced march: running on a sequence of five arcs | 40 min 5 min of preparation 30 min of warm-up and exercise 5 min of return to calm state (relaxation) | Usual levels of daily activity + standardized care |
| [30] | Judo program supervised by judo instructors, 6 months (26 weeks) | 1/wk | Different types of movements and falling techniques: From ‘walking in all directions’ to ‘change in direction’ activities, from stable movements to unstable movements. Judo analytical techniques and judo games: Progressively increasing body contact with games, simplifying movements to focus on essential judo movements. Ground control techniques and throws: Incremental change to already known movements, progression from repetitive movements to those more relevant to the understanding and purpose of judo Repetitions of basic movements in different directions and planes (pulling, pushing, holding, and lifting). | 90 min sessions 15–20 min (movement and falling techniques) 25–30 min (judo games) 25–30 min (ground control techniques and throws) 20–30 min (basic movement) | No extracurricular sports activities |
| [27] | Supervised educational exercise program, 12 weeks (2.7 months) | 2 times/wk | From week 1 to 4: Game Catching a tail, 15 min Playing tug-of-war, 15 min Floorball Play for passing and receiving a ball, 15 min Passing a ball (slapper and air pass), 15 min. From week 5 to 8: Floorball Mini-short game, 15 min Basketball Dribble (a hand dribble and then both-hands dribble), 15 min Pass (chest, overhand, one hand, and bound), 15 min Shot (middle, layup, and pass and shoot), 15 min Inline skating Skating adaptation (wearing, falling, and standing), 10 min Walking with an expert, 5 min. From week 9 to 12: Inline skating Pushing (kneeling and left or right foot push), 15 min Turn (return to target and turn left/right/front/back), 15 min Jumping play Sprint, jump over obstacles, running, and jump, 20 min High jump and triple jump, 20 min. | 5 min of warm-up 40 to 45 min of workout 10 min of stretching | NR (no control group) |
| Study | Weight (kg) | BMI (kg/m2) | Waist Circumference (cm) | Cholesterol (Total, HDL-C and LDL-C (mg/dL) | Glucose (mg/dL) | Triglycerides (mg/dL) | HRbaseline (ppm) | VO2max (ML/kg/min) | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| [28] | EG: non-significant reduction in weight, 92.9 ± 15.5 p = 0.065. CG: non-significant reduction in weight 91.2 ± 30.9, p = 0.215. | EG: a significant decrease, 30.2 ± 6.33, p = 0.016. CG: no significant changes, 30.0 ± 7.35, p = 0.315. | NR | NR | NR | NR | NR | NR | Calorie expenditure Average at month 9: 914.62 ± 233.4 mean ± S.D. increase in calorie expenditure for EG participants. |
| [29] | No significant changes. EG: −1.9 [0.9; 4.7]. CG: −0.2 [2.1; 2.6]. | No significant changes. EG: −1.9 [0.9; 4.7]. CG: −0.2 [2.1; 2.6]. | EG: no significant changes, +0.4 [3.6; 4.5]. CG: increased by +1.8 [1.6; 5.2]. | EG: increased HDL-C, + 5.2 [2.2; 8.1]. CG: increased HDL-C + 0.9 [−1.6; 3.4]. EG: decreased LDL-C, −7.7 [−14.5; −0.9]. CG: increased LDL-C, + 2 [−3.8; 7.8]. EG: decreased total cholesterol, −10.1 [−19.0; 1.3]. CG: increased total cholesterol + 0.2 [−7.2; 7.7]. | EG: no significant changes, −1.5 [4.7; 1.6]. CG: increase of +2.8 [0.2; 5.5]. | EG: no significant change, + 33.1 [−8.6; 74.9]. CG: decreased by −38.1 [−73.5; −2.72]. | NR | NR | Autistic traits scale: EG: decreased by −8.1 [−12.2; −4]. CG: decreased by −1.4 [−4.9; 2.1]. Motor profile scale: EG: decreased by −2.4 [−3.3; −1.5]. CG: negligible change of 0.1 [−0.7; 0.9]. Physical health: EG: increased by 13.3 [7.7; 18.9]. CG: increased by 3.1 [−1.8; 8.1]. Psychosocial health: EG: increased by 15.2 [9.8; 20.7]. CG: decreased by −2.4 [−7.1; 2.4]. |
| [30] | NR | NR | EG: decreased WC by −1.86 [−2.63; −1.09]. CG: negligible change of +0.32 [−0.49; 1.23]. Allometric index (WHT5): Decreased in both EG and CG. EG has a very large decrease, −0.024 [−0.030; −0.019]. CG: medium decrease, −0.008 [−0.015; −0.002]. | NR | NR | NR | Decreased in EG and CG. EG: medium decrease of 68.3 ± 4.4, p < 0.001, Apost–pre = 28.0%. CG: negligible decrease of 70.6 ± 5.5, p = 0.018, Apost–pre = 45.6%. | EG: medium increase of 55.2 ± 7.5, p < 0.001, Apost–pre = 66.6%; CG: no significant changes, 54.2 ± 6.2; p = 0.609, Apost–pre = 51.4%. | NR |
| [27] | Mild ASD: decreased ~2.34%. Severe ASD: increased ~1.70%. | Mild ASD: decreased by ~2.34%. Severe ASD: increased ~1.70%. | NR | NR | NR | NR | NR | NR | Shuttle run test (s): Mild ASD increased by ~8.49%. Severe ASD: decreased by ~2.88%. Fat mass (kg): Mild ASD: decreased by ~0.28%. Severe ASD: increased by ~5.60%. Strength (kg): Mild ASD: increased by ~16.92%. Severe ASD: increased by ~17.20%. Muscle endurance (rep): Mild ASD: increased by ~11.34%. Severe ASD: decreased by ~12.48%. Basal metabolic rate: Mild ASD: increased by ~0.75%. severe ASD: decreased by ~1.39%. |
| First Author, Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | Total | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 16/28 | Fair |
| [29] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 21/28 | Good |
| [30] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 14/28 | Poor |
| [27] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 15/28 | Fair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charlier, L.; Cordeiro, L.; Cavalcante Neto, J.L.; Signini, É.D.F.; Barbosa-Silva, J.; Corbellini, C.; Lipka, A.; de Abreu, R.M. Effects of Physical Exercise on Cardiometabolic Health in Individuals with Autism Spectrum Disorder: A Systematic Review. Healthcare 2025, 13, 439. https://doi.org/10.3390/healthcare13040439
Charlier L, Cordeiro L, Cavalcante Neto JL, Signini ÉDF, Barbosa-Silva J, Corbellini C, Lipka A, de Abreu RM. Effects of Physical Exercise on Cardiometabolic Health in Individuals with Autism Spectrum Disorder: A Systematic Review. Healthcare. 2025; 13(4):439. https://doi.org/10.3390/healthcare13040439
Chicago/Turabian StyleCharlier, Léa, Léa Cordeiro, Jorge Lopes Cavalcante Neto, Étore De Favari Signini, Jordana Barbosa-Silva, Camilo Corbellini, Antoine Lipka, and Raphael Martins de Abreu. 2025. "Effects of Physical Exercise on Cardiometabolic Health in Individuals with Autism Spectrum Disorder: A Systematic Review" Healthcare 13, no. 4: 439. https://doi.org/10.3390/healthcare13040439
APA StyleCharlier, L., Cordeiro, L., Cavalcante Neto, J. L., Signini, É. D. F., Barbosa-Silva, J., Corbellini, C., Lipka, A., & de Abreu, R. M. (2025). Effects of Physical Exercise on Cardiometabolic Health in Individuals with Autism Spectrum Disorder: A Systematic Review. Healthcare, 13(4), 439. https://doi.org/10.3390/healthcare13040439







