Comparison of Acute Irisin and Cognitive Responses to Different Exercise Modalities Among Late Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
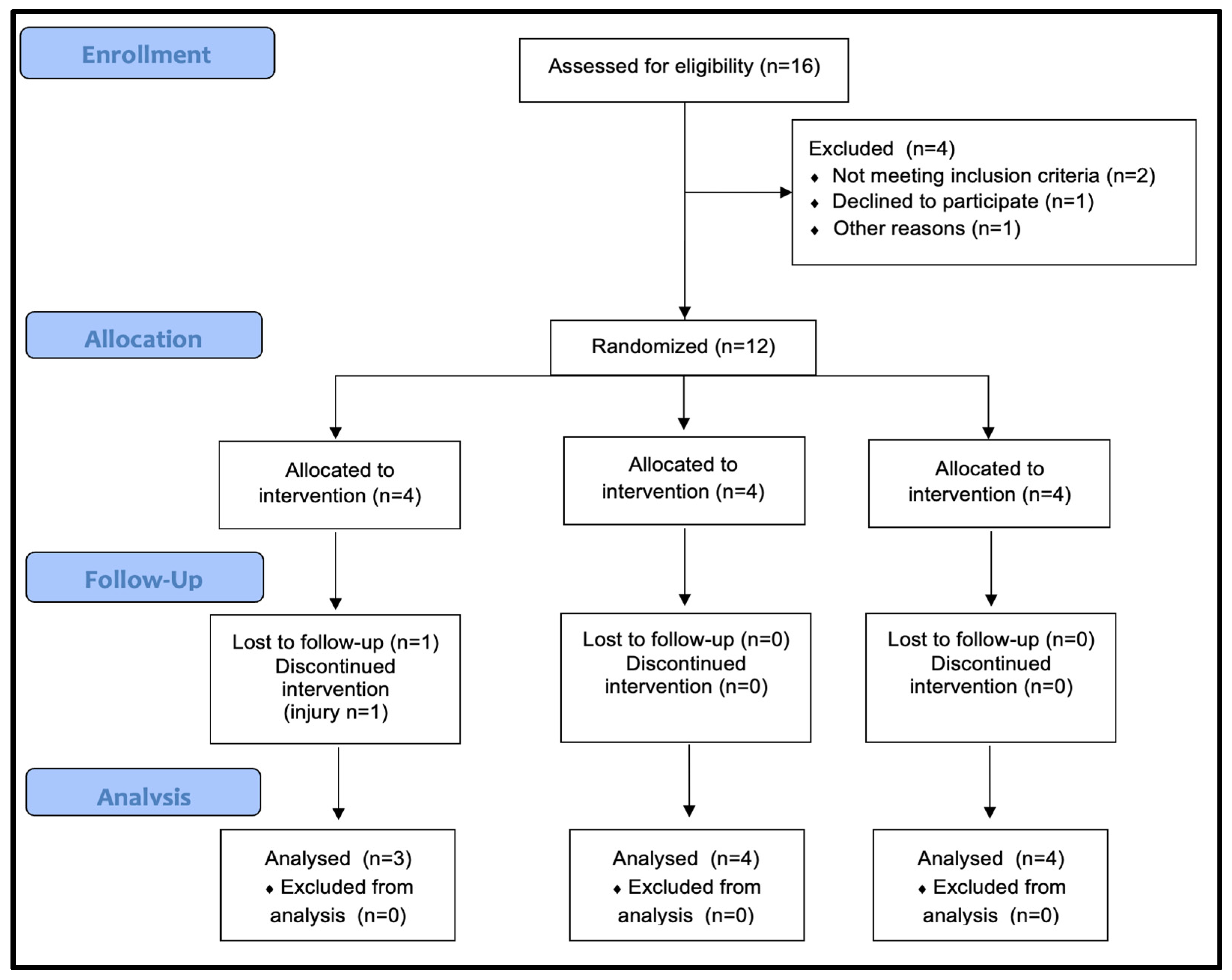
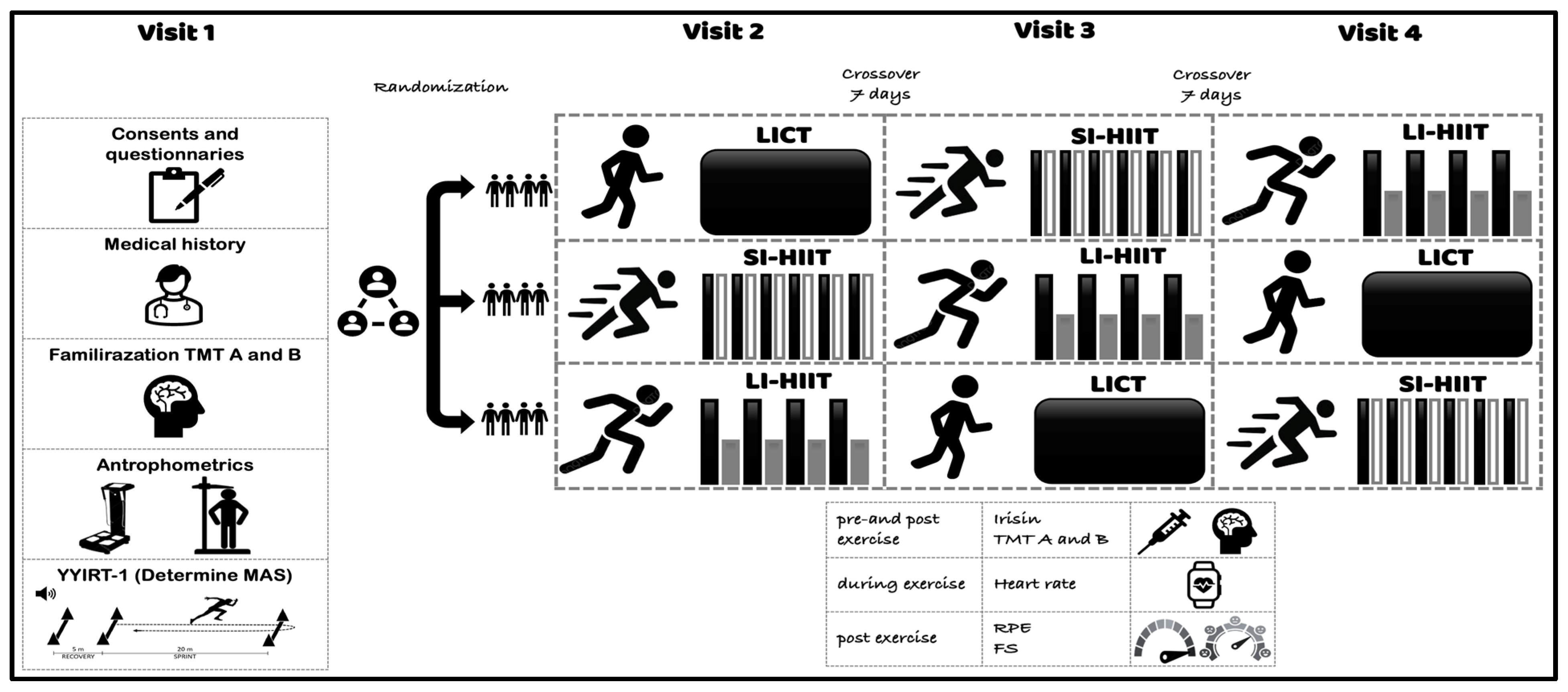
2.2. Procedures
Exercise Interventions
2.3. Measurements
2.3.1. Anthropometric Assessments
2.3.2. Exercise Intensity
2.3.3. Cognitive Function Performance
2.3.4. The Feeling Scale (FS)
2.3.5. Biochemical Analysis
2.4. Statistical Analyses
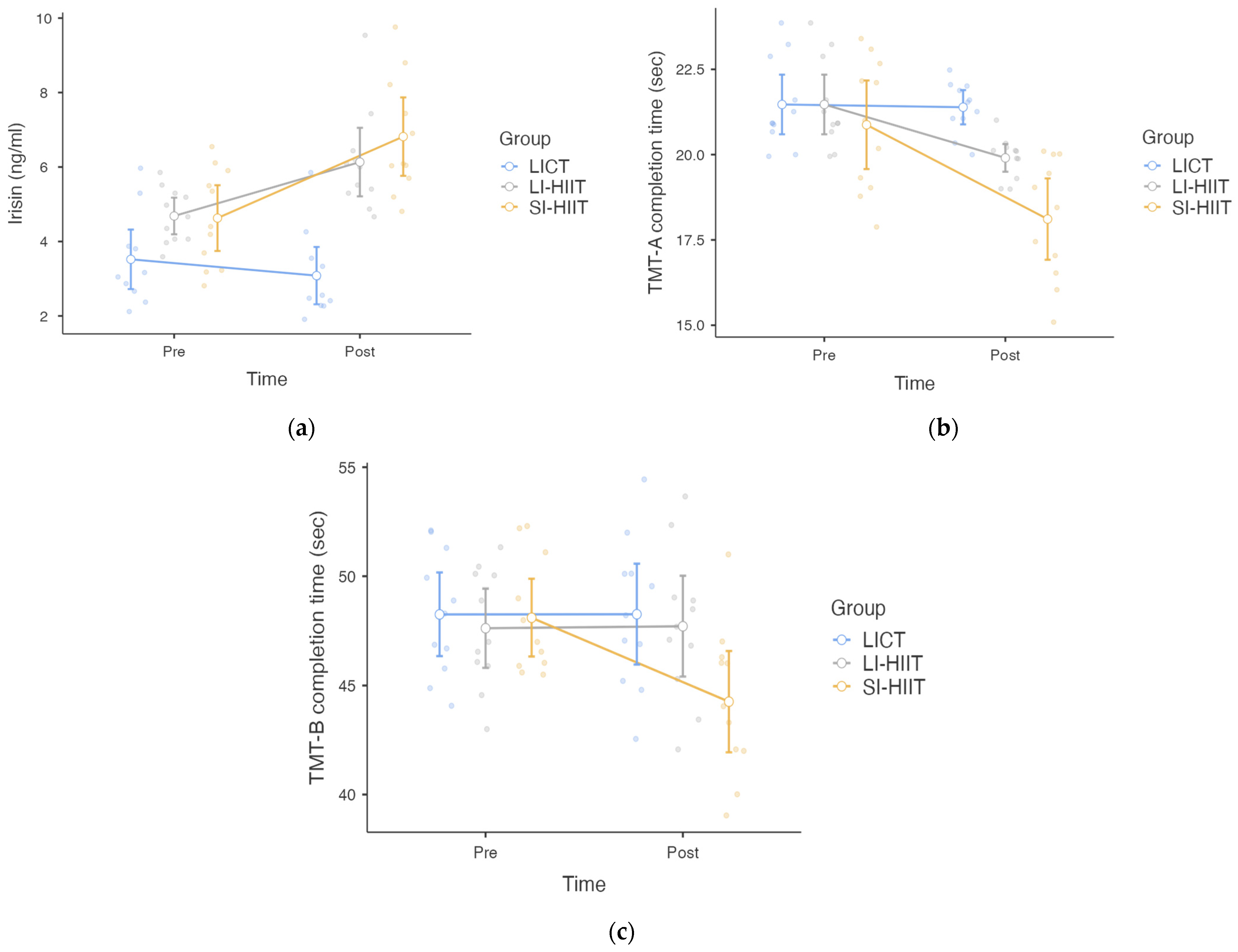
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| FNDC5 | Fibronectin type III domain-containing protein 5 |
| FS | Feeling Scale |
| LICT | Low-intensity continuous training |
| LI-HIIT | Long-interval high-intensity interval training |
| MAS | Maximal aerobic speed |
| PGC-1α | Peroxisome proliferator-activated receptor γ coactivator-1α |
| SI-HIIT | Short-interval high-intensity interval training |
| TMT | Trail Making Test |
| VO2max | Maximal oxygen uptake |
| YYIRT-1 | Yo-Yo Intermittent Recovery Test Level-1 |
References
- Gaesser, G.A.; Hall, S.E.; Angadi, S.S.; Poole, D.C.; Racette, S.B. Increasing the health span: Unique role for exercise. J. Appl. Physiol. 2025, 138, 1285–1308. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 3, 1–72. [Google Scholar] [CrossRef]
- Oliveira, R.G.; Guedes, D.P. Physical Activity, Sedentary Behavior, Cardiorespiratory Fitness and Metabolic Syndrome in Adolescents: Systematic Review and Meta-Analysis of Observational Evidence. PLoS ONE 2016, 11, e0168503. [Google Scholar] [CrossRef]
- Cao, M.; Quan, M.; Zhuang, J. Effect of High-Intensity Interval Training versus Moderate-Intensity Continuous Training on Cardiorespiratory Fitness in Children and Adolescents: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 1533. [Google Scholar] [CrossRef] [PubMed]
- Racil, G.; Ben Ounis, O.; Hammouda, O.; Kallel, A.; Zouhal, H.; Chamari, K.; Amri, M. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur. J. Appl. Physiol. 2013, 113, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Gejl, A.K.; Bugge, A.; Ernst, M.T.; Mortensen, E.L.; Gejl, K.D.; Andersen, L.B. Effects of 9 weeks of high-or moderate-intensity training on cardiorespiratory fitness, inhibitory control, and plasma brain-derived neurotrophic factor in Danish adolescents—A randomized controlled trial. Scand. J. Med. Sci. Sports 2024, 34, e14703. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle. Part II: Anaerobic energy, neuromuscular load and practical applications. Sports Med. 2013, 43, 927–954. [Google Scholar] [CrossRef]
- Calverley, T.A.; Ogoh, S.; Marley, C.J.; Steggall, M.; Marchi, N.; Brassard, P.; Lucas, S.J.E.; Cotter, J.D.; Roig, M.; Ainslie, P.N.; et al. HIITing the brain with exercise: Mechanisms, consequences and practical recommendations. J. Physiol. 2020, 598, 2513–2530. [Google Scholar] [CrossRef]
- Afzalpour, M.E.; Chadorneshin, H.T.; Foadoddini, M.; Eivari, H.A. Comparing interval and continuous exercise training regimens on neurotrophic factors in rat brain. Physiol. Behav. 2015, 147, 78–83. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Qi, J.Y.; Yang, L.K.; Wang, X.S.; Wang, M.; Li, X.B.; Feng, B.; Wu, Y.M.; Zhang, K.; Liu, S.B. Irisin: A promising treatment for neurodegenerative diseases. Neuroscience 2022, 498, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Dzięgiel, P.; Nowińska, K. The Role of FNDC5/Irisin in Cardiovascular Disease. Cells 2024, 13, 277. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Wrann, C.D. FNDC5/irisin—Their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 2015, 1, 55–61. [Google Scholar] [CrossRef]
- Pesce, M.; La Fratta, I.; Paolucci, T.; Grilli, A.; Patruno, A.; Agostini, F.; Bernetti, A.; Mangone, M.; Paoloni, M.; Invernizzi, M.; et al. From Exercise to Cognitive Performance: Role of Irisin. Appl. Sci. 2021, 11, 7120. [Google Scholar] [CrossRef]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 2021, 3, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, J.M.; Alviña, K. Multiple Roles in Neuroprotection for the Exercise Derived Myokine Irisin. Front. Aging Neurosci. 2021, 13, 649929. [Google Scholar] [CrossRef]
- Cosio, P.L.; Pelaez, M.; Cadefau, J.A.; Farran-Codina, A. Systematic Review and Meta-Analysis of Circulating Irisin Levels Following Endurance Training: Results of Continuous and Interval Training. Biol. Res. Nurs. 2023, 25, 367–381. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ando, D.; Goto, K.; Kiuchi, M.; Yamakita, M.; Koyama, K. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J. Exp. Med. 2014, 233, 135–140. [Google Scholar] [CrossRef]
- Colpitts, B.H.; Rioux, B.V.; Eadie, A.L.; Brunt, K.R.; Sénéchal, M. Irisin response to acute moderate intensity exercise and high intensity interval training in youth of different obesity statuses: A randomized crossover trial. Physiol. Rep. 2022, 10, e15198. [Google Scholar] [CrossRef]
- Archundia-Herrera, C.; Macias-Cervantes, M.; Ruiz-Muñoz, B.; Vargas-Ortiz, K.; Kornhauser, C.; Perez-Vazquez, V. Muscle irisin response to aerobic vs HIIT in overweight female adolescents. Diabetol. Metab. Syndr. 2017, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Löffler, D.; Müller, U.; Scheuermann, K.; Friebe, D.; Gesing, J.; Bielitz, J.; Erbs, S.; Landgraf, K.; Wagner, I.V.; Kiess, W.; et al. Serum irisin levels are regulated by acute strenuous exercise. J. Clin. Endocrinol. Metab. 2015, 100, 1289–1299. [Google Scholar] [CrossRef]
- Lagzdina, R.; Rumaka, M.; Gersone, G.; Tretjakovs, P. Circulating Irisin in Healthy Adults: Changes after Acute Exercise, Correlation with Body Composition, and Energy Expenditure Parameters in Cross-Sectional Study. Medicina 2020, 56, 274. [Google Scholar] [CrossRef]
- Parada-Sánchez, S.G.; Macias-Cervantes, M.H.; Pérez-Vázquez, V.; Vargas-Ortiz, K. The Effects of Different Types of Exercise on Circulating Irisin Levels in Healthy Individuals and in People with Overweight, Metabolic Syndrome and Type 2 Diabetes. Physiol. Res. 2022, 71, 457–475. [Google Scholar] [CrossRef]
- Fox, J.; Rioux, B.V.; Goulet, E.D.B.; Johanssen, N.M.; Swift, D.L.; Bouchard, D.R.; Loewen, H.; Sénéchal, M. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: A meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 16–28. [Google Scholar] [CrossRef]
- Tine Kartinah, N.; Rosalyn Sianipar, I.; Nafi’ah, R. The Effects of Exercise Regimens on Irisin Levels in Obese Rats Model: Comparing High-Intensity Intermittent with Continuous Moderate-Intensity Training. Biomed. Res. Int. 2018, 2018, 4708287. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.; Haque, M.; Johal, L.; Mathur, P.; Nel, W.; Rais, A.; Sandhu, R.; Sharma, S. Maturation of the adolescent brain. Neuropsychiatry Dis. Treat. 2013, 9, 449–461. [Google Scholar]
- Poletti, M. Adolescent brain development and executive functions: A prefrontal framework for developmental psychopathologies. Clin. Neuropsychiatry J. Treat. Eval. 2009, 6, 155–165. [Google Scholar]
- Herting, M.M.; Keenan, M.F.; Nagel, B.J. Aerobic Fitness Linked to Cortical Brain Development in Adolescent Males: Preliminary Findings Suggest a Possible Role of BDNF Genotype. Front. Hum. Neurosci. 2016, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, K.P.M.D.; de Oliveira, V.H.; Medeiros, G.C.B.S.D.; Mata, Á.N.D.S.; García, D.Á.; Martínez, D.G.; Leitão, J.C.; Knackfuss, M.I.; Piuvezam, G. The effects of exercise on BDNF levels in adolescents: A systematic review with meta-analysis. Int. J. Environ. Res. Public Health 2020, 17, 6056. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Fernandes, M.S.; Ordônio, T.F.; Santos, G.C.J.; Santos, L.E.R.; Calazans, C.T.; Gomes, D.A.; Santos, T.M. Effects of physical exercise on neuroplasticity and brain function: A systematic review in human and animal studies. Neural Plast. 2020, 2020, 8856621. [Google Scholar] [CrossRef]
- Chaddock-Heyman, L.; Erickson, K.I.; Holtrop, J.L.; Voss, M.W.; Pontifex, M.B.; Raine, L.B.; Hillman, C.H.; Kramer, A.F. Aerobic fitness is associated with greater white matter integrity in children. Front. Hum. Neurosci. 2014, 8, 584. [Google Scholar] [CrossRef]
- Talbot, J.S.; Perkins, D.R.; Tallon, C.M.; Dawkins, T.G.; Douglas, A.J.M.; Beckerleg, R.; Crofts, A.; Wright, M.E.; Davies, S.; Steventon, J.J.; et al. Cerebral blood flow and cerebrovascular reactivity are modified by maturational stage and exercise training status during youth. Exp. Physiol. 2023, 108, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Pouzesh Jadidi, G. Comparison of Whole-Body Electrical Stimulation with Aerobic Exercise on Body Composition and Serum Irisin Level in Overweight Adolescents. Phys. Act. Child. 2024, 1, 80–85. [Google Scholar] [CrossRef]
- Pandis, N.; Chung, B.; Scherer, R.W.; Elbourne, D.; Altman, D.G. CONSORT 2010 statement: Extension checklist for reporting within person randomised trials. BMJ 2017, 357, j2835. [Google Scholar] [CrossRef]
- Boutron, I.; Altman, D.G.; Moher, D.; Schulz, K.F.; Ravaud, P.; CONSORT NPT Group. CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann. Intern. Med. 2017, 167, 40–47. [Google Scholar] [CrossRef]
- Oberste, M.; Javelle, F.; Sharma, S.; Joisten, N.; Walzik, D.; Bloch, W.; Zimmer, P. Effects and Moderators of Acute Aerobic Exercise on Subsequent Interference Control: A Systematic Review and Meta-Analysis. Front. Psychol. 2019, 10, 2616. [Google Scholar] [CrossRef]
- Arney, B.E.; Glover, R.; Fusco, A.; Cortis, C.; de Koning, J.J.; van Erp, T.; Jaime, S.; Mikat, R.P.; Porcari, J.P.; Foster, C. Comparison of RPE (Rating of Perceived Exertion) Scales for Session RPE. Int. J. Sports Physiol. Perform. 2019, 14, 994–996. [Google Scholar] [CrossRef]
- Bangsbo, J.; Iaia, F.M.; Krustrup, P. The Yo-Yo intermittent recovery test: A useful tool for evaluation of physical performance in intermittent sports. Sports Med. 2008, 38, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Türkeş, N.; Can, H.; Kurt, M.; Dikeç, B.E. A Study to Determine the Norms for The Trail Making Test for the Age Range of 20–49 in Turkey. Turk. Psikiyatri. Derg. 2015, 26, 189–196. [Google Scholar]
- Hardy, C.J.; Rejeski, W.J. Not what, but how one feels: The measurement of affect during exercise. J. Sport Exerc. Psychol. 1989, 11, 304–317. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 2013; 567p. [Google Scholar]
- Contrepois, K.; Wu, S.; Moneghetti, K.J.; Hornburg, D.; Ahadi, S.; Tsai, M.S.; Metwally, A.A.; Wei, E.; Lee-McMullen, B.; Quijada, J.V.; et al. Molecular choreography of acute exercise. Cell 2020, 181, 1112–1130. [Google Scholar] [CrossRef]
- Delezie, J.; Handschin, C. Endocrine Crosstalk Between Skeletal Muscle and the Brain. Front. Neurol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Hecksteden, A.; Wegmann, M.; Steffen, A.; Kraushaar, J.; Morsch, A.; Ruppenthal, S.; Kaestner, L.; Meyer, T. Irisin and exercise training in humans—Results from a randomized controlled training trial. BMC Med. 2013, 11, 235. [Google Scholar] [CrossRef]
- Li, P.; Tong, L.; Bi, X. High-intensity training and irisin response: A possible molecular cross-talk for irisin response. Curr. Res. Physiol. 2025, 8, 100163. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef] [PubMed]
- Léger, C.; Quirié, A.; Méloux, A.; Fontanier, E.; Chaney, R.; Basset, C.; Lemaire, S.; Garnier, P.; Prigent-Tessier, A. Impact of Exercise Intensity on Cerebral BDNF Levels: Role of FNDC5/Irisin. Int. J. Mol. Sci. 2024, 25, 1213. [Google Scholar] [CrossRef]
- Kabasakalis, A.; Nikolaidis, S.; Tsalis, G.; Christoulas, K.; Mougios, V. Effects of sprint interval exercise dose and sex on circulating irisin and redox status markers in adolescent swimmers. J. Sports Sci. 2019, 37, 827–832. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Bishop, D.; Tarnopolsky, M.A.; Gibala, M.J. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1303–R1310. [Google Scholar] [CrossRef]
- Choi, J.W.; Balakrishnan, R. Aerobic exercise-induced myokine irisin release: A novel strategy to promote neuroprotection and improve cognitive function. Neural Regen. Res. 2026, 21, 306–307. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.; Wang, Y.; Cao, R.; Zhang, Z.; Fu, L. What Is the Relationship Between Body Mass Index, Sex Hormones, Leptin, and Irisin in Children and Adolescents? A Path Analysis. Front. Pediatr. 2022, 10, 823424. [Google Scholar] [CrossRef]
- Karampatsou, S.I.; Genitsaridi, S.M.; Michos, A.; Kourkouni, E.; Kourlaba, G.; Kassari, P.; Manios, Y.; Charmandari, E. The Effect of a Life-Style Intervention Program of Diet and Exercise on Irisin and FGF-21 Concentrations in Children and Adolescents with Overweight and Obesity. Nutrients 2021, 13, 1274. [Google Scholar] [CrossRef] [PubMed]
- Martland, R.; Mondelli, V.; Gaughran, F.; Stubbs, B. Can high-intensity interval training improve physical and mental health outcomes? A meta-review of 33 systematic reviews across the lifespan. J. Sports Sci. 2020, 38, 430–469. [Google Scholar] [CrossRef] [PubMed]
- Sudo, M.; Costello, J.T.; McMorris, T.; Ando, S. The effects of acute high-intensity aerobic exercise on cognitive performance: A structured narrative review. Front. Behav. Neurosci. 2022, 16, 957677. [Google Scholar] [CrossRef] [PubMed]
- McIlvain, G.; Magoon, E.M.; Clements, R.G.; Merritt, A.; Hiscox, L.V.; Schwarb, H.; Johnson, C.L. Acute effects of high-intensity exercise on brain mechanical properties and cognitive function. Brain Imaging Behav. 2024, 18, 863–874. [Google Scholar] [CrossRef]
- Mekari, S.; Earle, M.; Martins, R.; Drisdelle, S.; Killen, M.; Bouffard-Levasseur, V.; Dupuy, O. Effect of high intensity interval training compared to continuous training on cognitive performance in young healthy adults: A pilot study. Brain Sci. 2020, 10, 81. [Google Scholar] [CrossRef]
- Kao, S.C.; Westfall, D.R.; Soneson, J.; Gurd, B.; Hillman, C.H. Comparison of the acute effects of high-intensity interval training and continuous aerobic walking on inhibitory control. Psychophysiology 2017, 54, 1335–1345. [Google Scholar] [CrossRef]
- Yue, T.; Su, H.; Cheng, M.Y.; Wang, Y.; Bao, K.; Qi, F. High-Intensity Interval Training Improves Inhibitory Control and Working Memory in Healthy Young Adults. J. Hum. Kinet. 2025, 98, 41–56. [Google Scholar] [CrossRef]
- Mou, H.; Tian, S.; Fang, Q.; Qiu, F. The Immediate and Sustained Effects of Moderate-Intensity Continuous Exercise and High-Intensity Interval Exercise on Working Memory. Front. Psychol. 2022, 13, 766679. [Google Scholar] [CrossRef]
- Chang, Y.K.; Ren, F.F.; Li, R.H.; Ai, J.Y.; Kao, S.C.; Etnier, J.L. Effects of acute exercise on cognitive function: A meta-review of 30 systematic reviews with meta-analyses. Psychol. Bull. 2025, 151, 240–259. [Google Scholar] [CrossRef]
- Birinci, Y.Z.; Pancar, S.; Soylu, Y.; Topçu, H.; Koçyiğit, A.; Sarandöl, E.; Şimşek, H.; Şahin, Ş. Acute Neurochemical, Psychophysiological, and Cognitive Responses to Small-Sided Games vs. Running-Based HIIT in Young, Male Soccer Players. Healthcare 2025, 13, 1738. [Google Scholar] [CrossRef]
- Liu, L.; Xin, X.; Zhang, Y. The effects of physical exercise on cognitive function in adolescents: A systematic review and meta-analysis. Front. Psychol. 2025, 16, 1556721. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Kirk, I.J.; Waldie, K.E. High-intensity training enhances executive function in children in a randomized, placebo-controlled trial. eLife 2017, 6, e25062. [Google Scholar] [CrossRef]
- Hatch, L.M.; Dring, K.J.; Williams, R.A.; Sunderland, C.; Nevill, M.E.; Cooper, S.B. Effect of Differing Durations of High-Intensity Intermittent Activity on Cognitive Function in Adolescents. Int. J. Environ. Res. Public Health 2021, 18, 11594. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Amigo, T.; Salinas-Gallardo, G.; Mendoza, E.; Ovalle-Fernández, C.; Ibarra-Mora, J.; Gómez-Álvarez, N.; Carrasco-Beltrán, H.; Páez-Herrera, J.; Hurtado-Almonácid, J.; Yañez-Sepúlveda, R.; et al. Effectiveness of school-based active breaks on classroom behavior, executive functions and physical fitness in children and adolescent: A systematic review. Front. Public Health 2025, 13, 1469998. [Google Scholar] [CrossRef]
- Leahy, A.A.; Mavilidi, M.F.; Smith, J.J.; Hillman, C.H.; Eather, N.; Barker, D.; Lubans, D.R. Review of High-Intensity Interval Training for Cognitive and Mental Health in Youth. Med. Sci. Sports Exerc. 2020, 52, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Costigan, S.A.; Eather, N.; Plotnikoff, R.C.; Hillman, C.H.; Lubans, D.R. High-Intensity Interval Training for Cognitive and Mental Health in Adolescents. Med. Sci. Sports Exerc. 2016, 48, 1985–1993. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Hill, T.; Polk, J.D. BDNF, endurance activity, and mechanisms underlying the evolution of hominin brains. Am. J. Phys. Anthropol. 2019, 168, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Minuti, A.; Raffaele, I.; Scuruchi, M.; Lui, M.; Muscarà, C.; Calabrò, M. Role and Functions of Irisin: A Perspective on Recent Developments and Neurodegenerative Diseases. Antioxidants 2025, 14, 554. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Santos, C.; Castrillón, C.I.; Miranda, R.A.; Monteiro, P.A.; Inoue, D.S.; Campos, E.Z.; Hofmann, P.; Lira, F.S. Inflammatory Cytokines and BDNF Response to High-Intensity Intermittent Exercise: Effect the Exercise Volume. Front. Physiol. 2016, 7, 509. [Google Scholar] [CrossRef] [PubMed]
- Slusher, A.L.; Patterson, V.T.; Schwartz, C.S.; Acevedo, E.O. Impact of high intensity interval exercise on executive function and brain derived neurotrophic factor in healthy college aged males. Physiol. Behav. 2018, 191, 116–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Lin, S. Irisin: A bridge between exercise and neurological diseases. Heliyon 2022, 8, e12352. [Google Scholar] [CrossRef]
- Winn, N.C.; Grunewald, Z.I.; Liu, Y.; Heden, T.D.; Nyhoff, L.M.; Kanaley, J.A. Plasma Irisin Modestly Increases during Moderate and High-Intensity Afternoon Exercise in Obese Females. PLoS ONE 2017, 12, e0170690. [Google Scholar] [CrossRef]
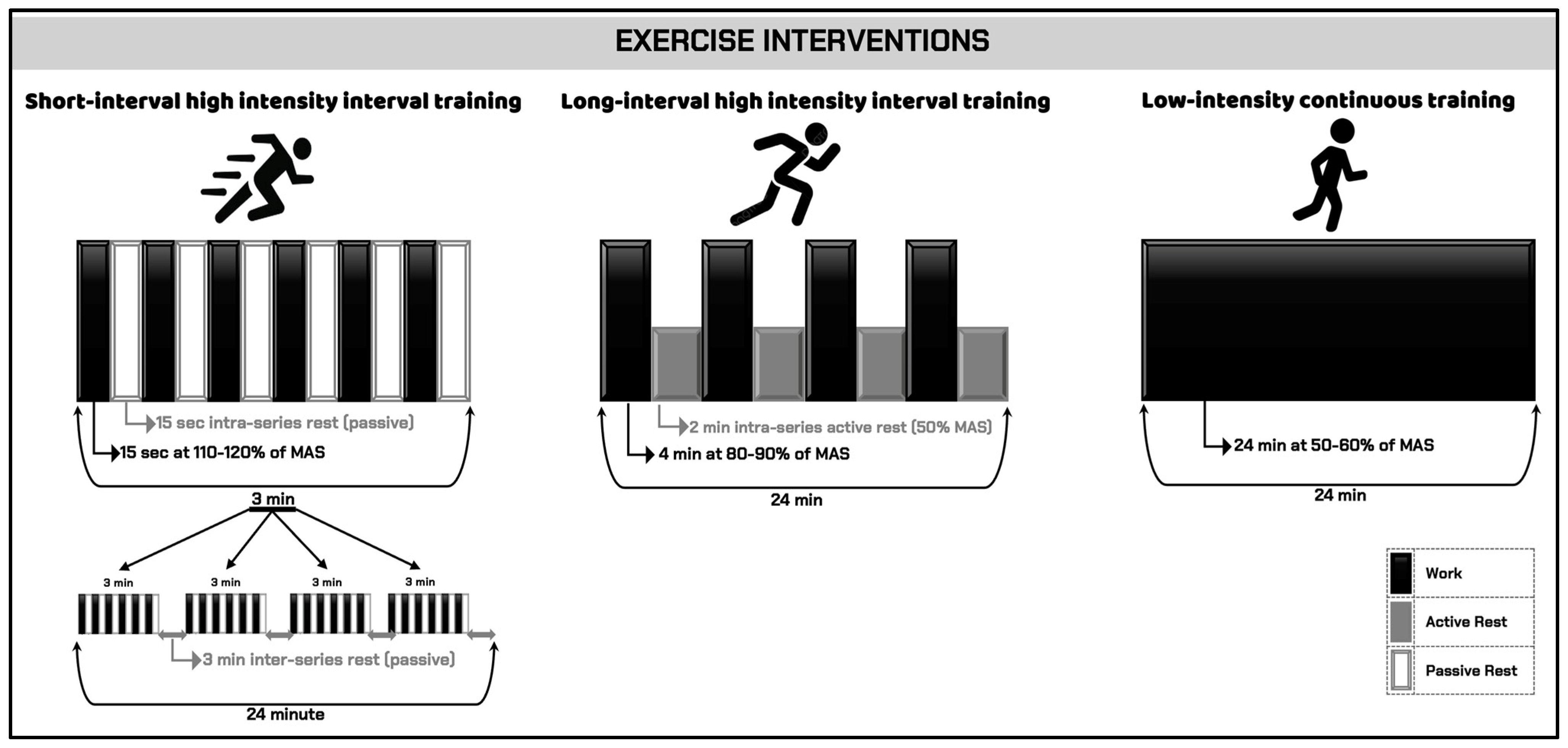
| Session | No. × Duration of Bout | Work Velocity | No. × Duration of Series | Intraseries Recovery | Interseries Recovery | Total Duration |
|---|---|---|---|---|---|---|
| SI-HIIT | 6 × 15 s | 110–120% MAS | 4 × 3 min | 15 s passive | 3 min passive | 24 min |
| LI-HIIT | 1 × 4 min | 80–90% MAS | 4 × 4 min | – | 2 min active (50% MAS) | 24 min |
| LICT | 1 × 24 min | 50–60% MAS | 1 × 24 min | – | – | 24 min |
| LICT | LI-HIIT | SI-HIIT | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Irisin (ng/mL) | 3.52 ± 1.19 | 3.08 ± 1.15 | 4.69 ± 0.73 | 6.13 ± 1.37 | 4.63 ± 1.31 | 6.82 ± 1.57 |
| TMT-A completion time (s) | 21.47 ± 1.30 | 20.84 ± 0.76 | 20.85 ± 1.36 | 19.90 ± 0.60 | 20.87 ± 1.93 | 18.11 ± 1.77 |
| TMT-B completion time (s) | 48.26 ± 2.84 | 48.27 ± 3.43 | 47.63 ± 2.70 | 47.72 ± 3.43 | 48.11 ± 2.65 | 44.26 ± 3.46 |
| LICT | LI-HIIT | SI-HIIT | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
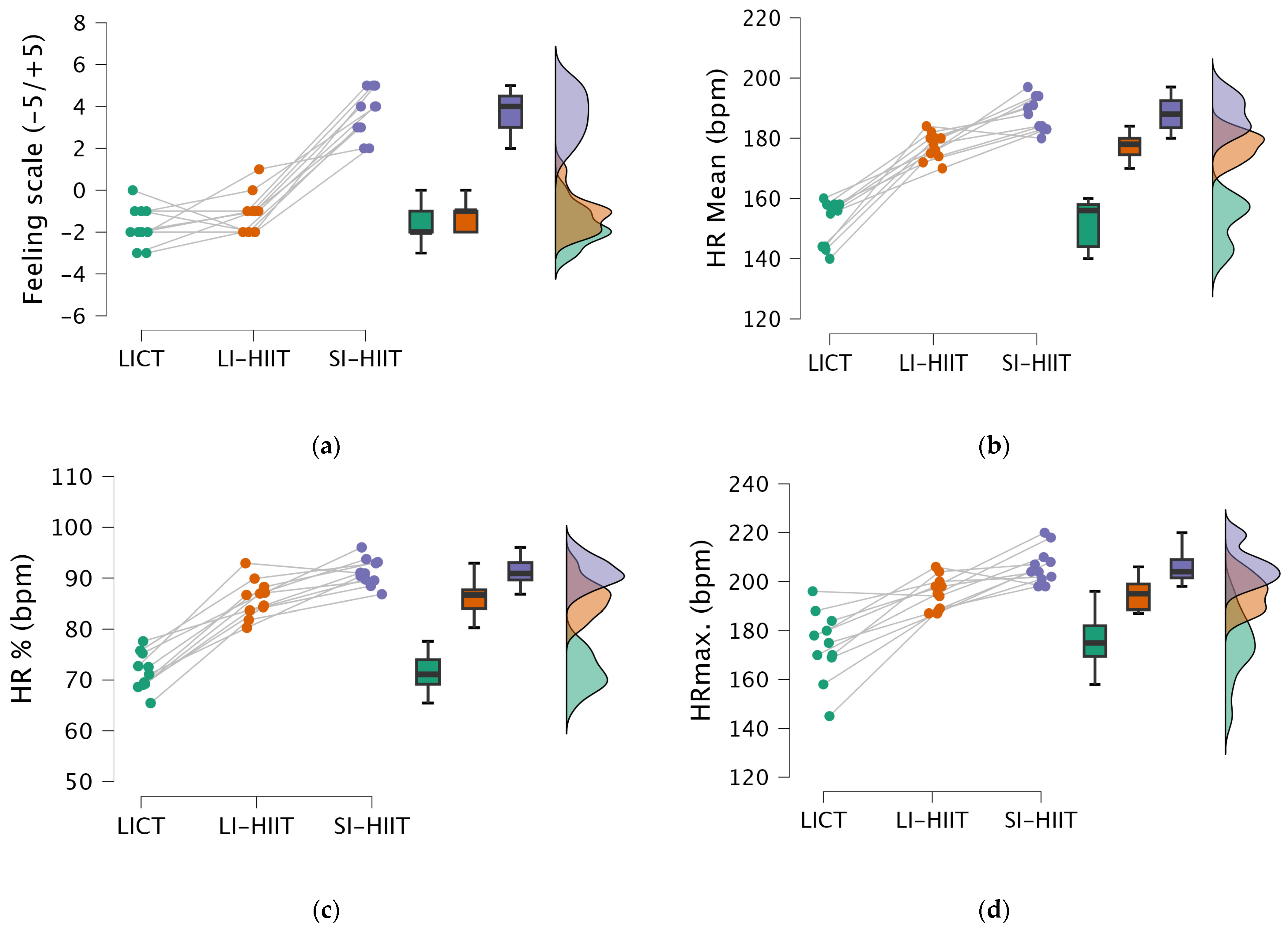
| Feeling Scale (score) | −1.73 ± 0.90 | −1.10 ± 0.94 | 3.63 ± 1.12 |
| HRmean (bpm) | 147.46 ± 5.20 | 177.36 ± 4.34 | 188 ± 5.59 |
| HR% | 71.54 ± 3.63 | 86.04 ± 3.62 | 91.15 ± 2.63 |
| HRmax (bpm) | 173.91 ± 14.10 | 195.10 ± 6.77 | 206.36 ± 7.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birinci, Y.Z.; Pancar, S. Comparison of Acute Irisin and Cognitive Responses to Different Exercise Modalities Among Late Adolescents. Healthcare 2025, 13, 3242. https://doi.org/10.3390/healthcare13243242
Birinci YZ, Pancar S. Comparison of Acute Irisin and Cognitive Responses to Different Exercise Modalities Among Late Adolescents. Healthcare. 2025; 13(24):3242. https://doi.org/10.3390/healthcare13243242
Chicago/Turabian StyleBirinci, Yakup Zühtü, and Serkan Pancar. 2025. "Comparison of Acute Irisin and Cognitive Responses to Different Exercise Modalities Among Late Adolescents" Healthcare 13, no. 24: 3242. https://doi.org/10.3390/healthcare13243242
APA StyleBirinci, Y. Z., & Pancar, S. (2025). Comparison of Acute Irisin and Cognitive Responses to Different Exercise Modalities Among Late Adolescents. Healthcare, 13(24), 3242. https://doi.org/10.3390/healthcare13243242







