A Randomized Trial in Older Adults of a Flavor-Enhanced Coconut Oil-Based Mouthwash: Clinical Safety, Antimicrobial Efficacy, and User Satisfaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Volunteers and Ethical Issues
2.2. Sample Size Determination
2.3. FCoMW Formulation
2.4. Evaluation of Oral Health Parameters, Safety, and Satisfaction
2.5. Clinical Anticandidal and Antibacterial Efficacy
2.6. Statistical Analysis
3. Results
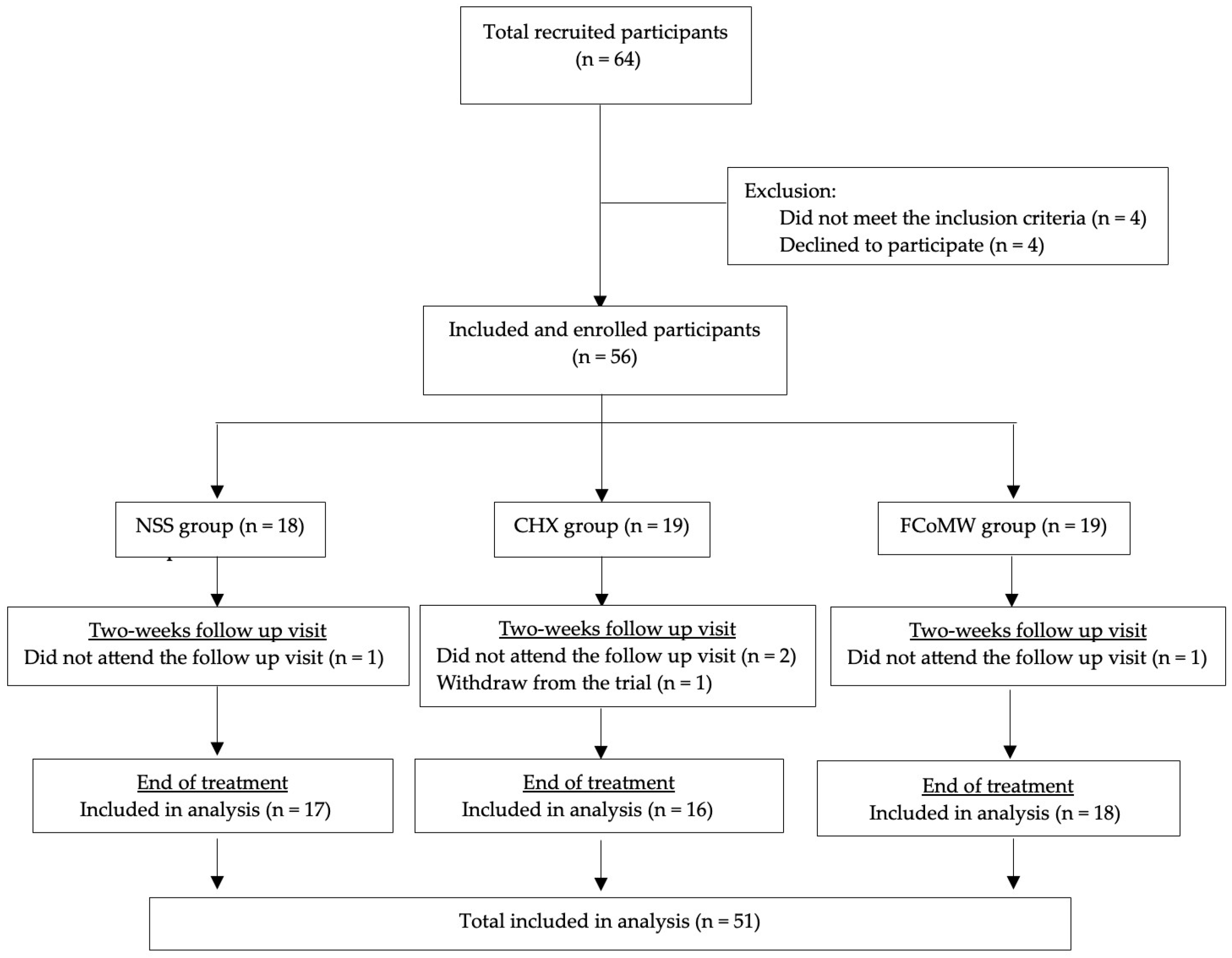
3.1. Demographic Characteristics of Participants
3.2. Evaluation of Oral Health Parameters, Safety, and Satisfaction
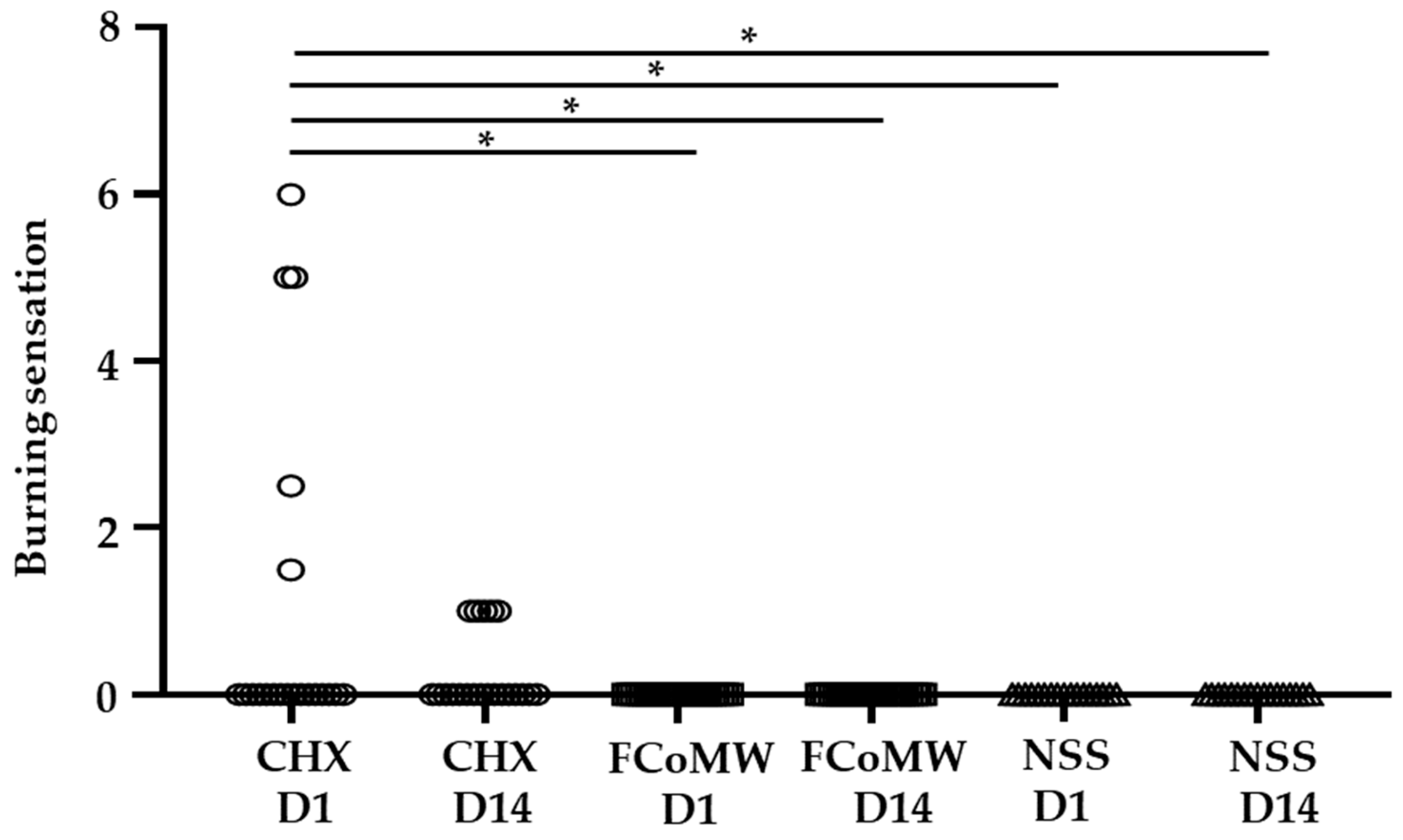
- Burning Sensation
- Oral Mucosal Alterations
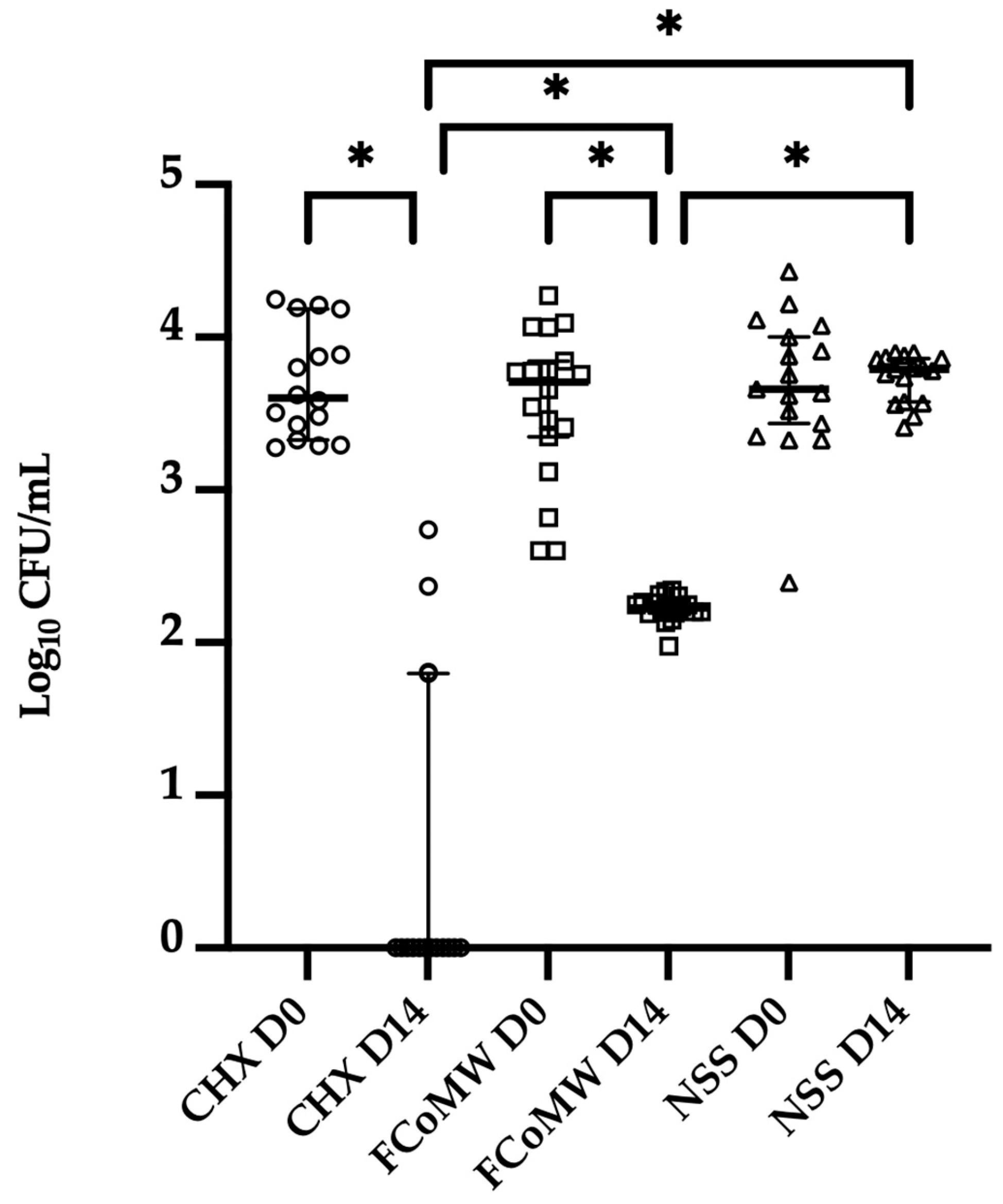
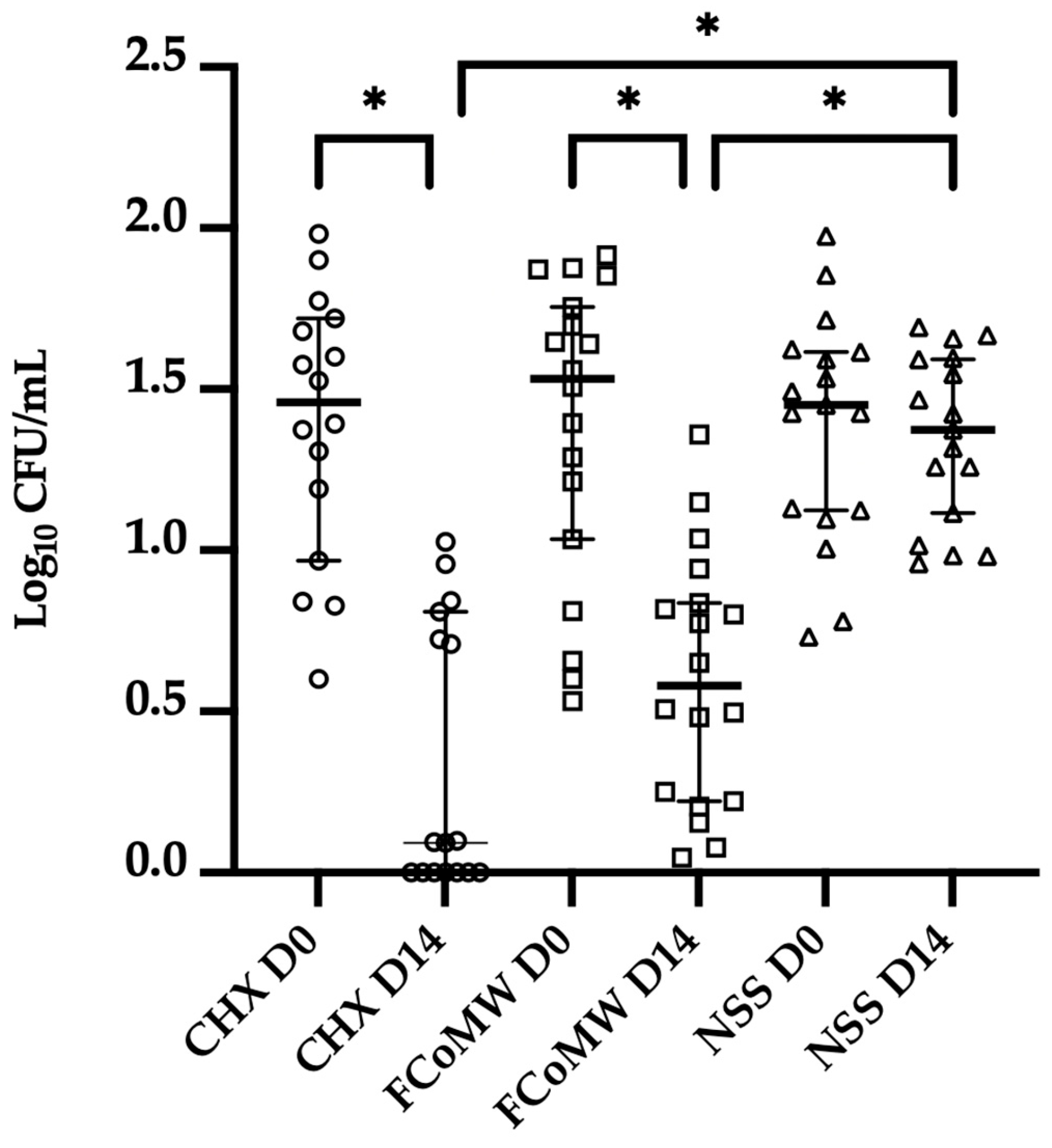
3.3. Clinical Anticandidal and Antibacterial Efficacy
4. Discussion
4.1. Antibacterial and Antifungal Efficacies
4.2. Safety and Biocompatibility
4.3. Effects on Oral Moisture and Plaque Control
4.4. User Satisfaction and Acceptability
4.5. Clinical Implications
4.6. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| s | Second (s) |
| min | Minute (s) |
| h | Hour (s) |
| Pa.sec | Pascal × second |
| CFU | Colony forming unit |
| mL | Millilitre (s) |
References
- Brookes, Z.L.S.; Belfield, L.A.; Ashworth, A.; Casas-Agustench, P.; Raja, M.; Pollard, A.J.; Bescos, R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021, 113, 103768. [Google Scholar] [CrossRef]
- Kim, J.; Sudbery, P. Candida albicans, a major human fungal pathogen. J. Microbiol. 2011, 49, 171–177. [Google Scholar] [CrossRef]
- Kong, E.F.; Kucharíková, S.; Van Dijck, P.; Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Clinical implications of oral candidiasis: Host tissue damage and disseminated bacterial disease. Infect. Immun. 2015, 83, 604–613. [Google Scholar] [CrossRef]
- Perić, M.; Miličić, B.; Kuzmanović Pfićer, J.; Živković, R.; Arsić Arsenijević, V. A Systematic Review of Denture Stomatitis: Predisposing Factors, Clinical Features, Etiology, and Global Candida spp. Distribution. J. Fungi 2024, 10, 328. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance toward chlorhexidine in oral bacteria—Is there cause for concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Gürgan, C.A.; Zaim, E.; Bakirsoy, I.; Soykan, E. Short-term side effects of 0.2% alcohol-free chlorhexidine mouthrinse used as an adjunct to non-surgical periodontal treatment: A double-blind clinical study. J. Periodontol. 2006, 77, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Amith, H.V.; Ankola, A.V.; Nagesh, L. Effect of oil pulling on plaque and gingivitis. J. Oral Health Community Dent. 2007, 1, 12–18. [Google Scholar]
- Asokan, S.; Emmadi, P.; Chamundeswari, R. Effect of oil pulling on plaque induced gingivitis: A randomized, controlled, triple-blind study. Indian J. Dent. Res. 2009, 20, 47–51. [Google Scholar] [CrossRef]
- Woolley, J.; Gibbons, T.; Patel, K.; Sacco, R. The effect of oil pulling with coconut oil to improve dental hygiene and oral health: A systematic review. Heliyon 2020, 6, e04789. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Khiyani, M.F.; Nauman, H.; Zafar, M.S.; Shah, A.H.; Khalil, H.S. Oil pulling and importance of traditional medicine in oral health maintenance. Int. J. Health Sci. 2017, 11, 65–70. [Google Scholar]
- Quimby, A.E.; Hogan, D.; Khalil, D.; Hearn, M.; Nault, C.; Johnson-Obaseki, S. Coconut oil as a novel approach to managing radiation-induced xerostomia: A primary feasibility study. Int. J. Otolaryngol. 2020, 2020, 8537643. [Google Scholar] [CrossRef] [PubMed]
- Permata, D.; Saputra, D.A.; Sutedjo, B.S.; Meilawaty, Z. Evaluating the effectiveness of coconut oil (Cocus nucifera) anti-bacterial benefits in caries prevention: A literature review. Int. J. Sci. Res. Arch. 2024, 11, 212–215. [Google Scholar] [CrossRef]
- Peedikayil, F.C.; Sreenivasan, P.; Narayanan, A. Effect of coconut oil in plaque related gingivitis—A preliminary report. Niger. Med. J. 2015, 56, 143–147. [Google Scholar] [CrossRef]
- Owittayakul, D.; Palee, K.; Khongkhunthian, S.; Langkapin, W.; Wanachantararak, P.; Bhatia, P. Efficacy of coconut oil and 0.12% chlorhexidine mouthrinses in reduction of plaque and gingivitis: A two-week randomized clinical trial. J. Dent. Assoc. Thail. 2018, 68, 360–369. [Google Scholar] [CrossRef]
- Siripaiboonpong, N.; Matangkasombut, O.; Pengcharoen, H.; Boonchaiyapluk, B.; Rujiraprasert, P.; Petcharat, P.; Chantarangsu, S.; Srithanyarat, S.S. Clinical effect of virgin coconut oil pulling in comparison with palm oil pulling on gingival health: A crossover randomized clinical controlled trial. Nat. Life Sci. Commun. 2023, 22, e2023053. [Google Scholar] [CrossRef]
- Abllah, Z.; Shahnon, S.K.; Nuawi, N.N.; Ardini, Y.D. The effect of virgin coconut oil (VCO) on physiological halitosis. a randomized controlled pilot trial. IIUM Med. J. Malays. 2020, 18, 221. [Google Scholar] [CrossRef]
- de Oliveira, S.F.; Lôbo, I.P.; da Cruz, R.S.; Andrioli, J.L.; da Mata, C.; Soares, G.A.; Santos, E.D.C.; Aguiar-Oliveira, E.; Franco, M.; da Conceição, A.O. Antimicrobial activity of coconut oil-in-water emulsion on Staphylococcus epidermidis and Escherichia coli EPEC associated to Candida kefyr. Heliyon 2018, 4, e00924. [Google Scholar] [CrossRef]
- Widianingrum, D.C.; Noviandi, C.T.; Salasia, S.I.O. Antibacterial and immunomodulator activities of virgin coconut oil (VCO) against Staphylococcus aureus. Heliyon 2019, 5, e02612. [Google Scholar] [CrossRef]
- Shilling, M.; Matt, L.; Rubin, E.; Visitacion, M.P.; Haller, N.A.; Grey, S.F.; Woolverton, C.J. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J. Med. Food 2013, 16, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Rofiee, M.S.; Somchit, M.N.; Zuraini, A.; Sulaiman, M.R.; Teh, L.K.; Salleh, M.Z.; Long, K. Hepatoprotective activity of dried- and fermented-processed virgin coconut oil. Evid. Based Complement. Altern. Med. 2011, 2011, 142739. [Google Scholar] [CrossRef] [PubMed]
- Ogbolu, D.O.; Oni, A.A.; Daini, O.A.; Oloko, A.P. In vitro antimicrobial properties of coconut oil on Candida species in Ibadan, Nigeria. J. Med. Food 2007, 10, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Intarakaewsri, T.; Owittayakul, D.; Wanachantararak, P. Development of virgin coconut oil mouthwash against Candida albicans biofilms. CM Dent. J. 2020, 41, 55–64. [Google Scholar]
- Marasri, P.; Sookkhee, S.; Wanachantararak, P.; Owittayakul, D. Anti-inflammatory activity and wound healing ability of coconut oil mouthwash on gingival fibroblast cell in vitro. Nat. Life Sci. Commun. 2024, 23, e2024059. [Google Scholar] [CrossRef]
- Chanpa, P.; Owittayakul, D.; Wanachantararak, P.; Chaiyana, W.; Sookkhee, S. Formulation of coconut oil mouthwash with mixed emulsifier and its growth inhibition of Candida albicans biofilms. Nat. Life Sci. Commun. 2023, 22, 1–16. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsion Science and Technology: A General Introduction; Wiley: Hoboken, NJ, USA, 2009; pp. 1–56. [Google Scholar]
- Epstein, J.B.; Pearsall, N.N.; Truelove, E.L. Quantitative relationships between Candida albicans in saliva and the clinical status of human subjects. J. Clin. Microbiol. 1980, 12, 475–476. [Google Scholar] [CrossRef]
- Uludamar, A.; Özyeşil, A.G.; Ozkan, Y.K. Clinical and microbiological efficacy of three different treatment methods in the management of denture stomatitis. Gerodontology 2011, 28, 104–110. [Google Scholar] [CrossRef]
- Ngamjarus, C. n4Studies: Sample size calculation for an epidemiological study on a smart device. Siriraj Med. J. 2016, 68, 160–170. [Google Scholar]
- Silness, J.; Loe, H. Periodontal disease in pregnancy. II. correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Loretz, L.J.; Bailey, J.E. CTFA Safety Evaluation Guidelines; Cosmetic, Toiletry, and Fragrance Association: Washington, DC, USA, 2007. [Google Scholar]
- Chen, H.; Li, Q.; Li, M.; Liu, S.; Yao, C.; Wang, Z.; Zhao, Z.; Liu, P.; Yang, F.; Li, X.; et al. Microbial characteristics across different tongue coating types in a healthy population. J. Oral Microbiol. 2021, 13, 1946316. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Figueiredo, L.C.; Faveri, M.; Stewart, B.; de Vizio, W. The effectiveness of a preprocedural mouthrinse containing cetylpyridinium chloride in reducing bacteria in the dental office. J. Am. Dent. Assoc. 2010, 141, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Hemaid, A.S.S.; Abdelghany, M.M.E.; Abdelghany, T.M. Isolation and identification of Candida spp. from immunocompromised patients. Bull. Natl. Res. Cent. 2021, 45, 163. [Google Scholar] [CrossRef]
- Olver, W.J.; Stafford, J.; Cheetham, P.; Boswell, T.C. Comparison of Candida ID medium with sabouraud-chloramphenicol agar for the isolation of yeasts from clinical haematology surveillance specimens. J. Med. Microbiol. 2002, 51, 221–224. [Google Scholar] [CrossRef]
- Alomeir, N.; Zeng, Y.; Fadaak, A.; Wu, T.T.; Malmstrom, H.; Xiao, J. Effect of nystatin on Candida albicans-Streptococcus mutans duo-species biofilms. Arch. Oral Biol. 2023, 145, 105582. [Google Scholar] [CrossRef]
- Peng, T.R.; Cheng, H.Y.; Wu, T.W.; Ng, B.K. Effectiveness of Oil Pulling for Improving Oral Health: A Meta-Analysis. Healthcare 2022, 10, 1991. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Tjitda, P.J.P.; Nitti, F.; Jumina, J.; Detha, A.I.R. Antimicrobial properties of lauric acid and monolaurin in virgin coconut oil: A review. ChemBioEng Rev. 2022, 9, 442–461. [Google Scholar] [CrossRef]
- Griessl, T.; Zechel-Gran, S.; Olejniczak, S.; Weigel, M.; Hain, T.; Domann, E. High-resolution taxonomic examination of the oral microbiome after oil pulling with standardized sunflower seed oil and healthy participants: A pilot study. Clin. Oral Investig. 2021, 25, 2689–2703. [Google Scholar] [CrossRef]
- Peckys, D.B.; De Jong, N.; Hannig, M. Oil droplet formation on pellicle covered tooth surfaces studied with environmental scanning electron microscopy. J. Microsc. 2019, 274, 158–167. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, CD008676. [Google Scholar] [CrossRef]
- Kuroyama, M.; Kagawa, H.; Kitada, S.; Maekura, R.; Mori, M.; Hirano, H. Exogenous lipoid pneumonia caused by repeated sesame oil pulling: A report of two cases. BMC Pulm. Med. 2015, 15, 135. [Google Scholar] [CrossRef]
- Ripari, F.; Filippone, F.; Zumbo, G.; Covello, F.; Zara, F.; Vozza, I. The Role of Coconut Oil in Treating Patients Affected by Plaque-Induced Gingivitis: A Pilot Study. Eur. J. Dent. 2020, 14, 558–565. [Google Scholar] [CrossRef]
- Gupta, D.; Bhaskar, D.J.; Gupta, R.K.; Karim, B.; Jain, A.; Singh, R.; Karim, W. A randomized controlled clinical trial of Ocimum sanctum and chlorhexidine mouthwash on dental plaque and gingival inflammation. J. Ayurveda Integr. Med. 2014, 5, 109–116. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Kuśka-Kielbratowska, A.; Fiegler-Rudol, J.; Niemczyk, W.; Mertas, A.; Skaba, D.; Wiench, R. The Prevalence and Drug Susceptibility of Candida Species and an Analysis of Risk Factors for Oral Candidiasis-A Retrospective Study. Antibiotics 2025, 14, 876. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Variables | NSS % (n = 17) | 0.12% w/v CHX % (n = 16) | FCoMW % (n = 18) | Total % (n = 51) | p-Value | |
|---|---|---|---|---|---|---|
| 1. Gender | Female | 29.40 (5) | 25.00 (4) | 38.88 (7) | 31.37 (16) | 0.669 |
| Male | 70.60 (12) | 75.00 (12) | 61.11 (11) | 68.63 (35) | ||
| 2. Age | 60–69 | 58.80 (10) | 68.75 (11) | 50.00 (9) | 58.82 (30) | 0.431 |
| 70–79 | 35.30 (6) | 31.25 (5) | 50.00 (9) | 39.22 (20) | ||
| >80 | 5.90 (1) | 0.00 (0) | 0.00 (0) | 1.96 (1) | ||
| 3. Underlying disease | Had | 64.70 (11) | 31.25 (5) | 72.22 (13) | 56.86 (29) | 0.006 * |
| Hypertension | 47.10 (8) | 31.25 (5) | 44.44 (8) | 41.18 (21) | ||
| Diabetes | 5.90 (1) | 25.00 (4) | 27.78 (5) | 29.41 (15) | ||
| Hyperlipidemia | 11.80 (2) | 18.75 (3) | 38.89 (7) | 23.53 (12) | ||
| Others | 29.40 (5) | 12.50 (2) | 11.11 (2) | 17.65 (9) | ||
| None | 35.30 (6) | 68.75 (11) | 27.78 (5) | 43.14 (22) | ||
| 4. History of head and neck radiotherapy | No | 100.00 (17) | 100.00 (16) | 94.44 (17) | 98.04 (50) | 0.393 |
| Yes | 0.00 (0) | 0.00 (0) | 5.56 (1) | 1.96 (1) | ||
| 5. History of mouthwash allergy | No | 100.00 (17) | 100.00 (16) | 100.00 (18) | 100.00 (51) | NA |
| Yes | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | ||
| 6. Xerostomia | No | 100.00 (17) | 100.00 (16) | 100.00 (18) | 100.00 (51) | NA |
| Yes | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | ||
| 7. Frequency of brushing teeth | <2 times/day | 0.00 (0) | 18.75 (3) | 5.56 (1) | 7.84 (4) | 0.691 |
| 2 times/days | 82.40 (14) | 62.50 (10) | 72.22 (13) | 72.55 (37) | ||
| >2 times/days | 17.60 (3) | 18.75 (3) | 22.22 (4) | 19.61 (10) | ||
| 8. Using mouthwash | No | 58.80 (10) | 56.25 (9) | 66.67 (12) | 60.78 (31) | 0.804 |
| Yes | 41.20 (7) | 43.75 (7) | 33.33 (6) | 39.22 (20) | ||
| 8.1. Frequency | Everyday | 17.64 (3) | 25.00 (4) | 11.11 (2) | 23.53 (12) | |
| Sometimes | 23.53 (4) | 18.75 (3) | 22.22 (4) | 15.69 (8) | ||
| 8.2. Type of mouthwash | Commercial mouthwash | 35.29 (6) | 43.75 (7) | 27.78 (5) | 35.29 (18) | |
| Therapeutic mouthwash | 5.88 (1) | 0.00 (0) | 5.56 (1) | 3.92 (2) | ||
| 9. Presence of removable denture | No | 41.18 (7) | 25.00 (4) | 33.33 (6) | 33.33 (17) | 0.230 |
| Yes | 58.82 (10) | 75.00 (12) | 66.67 (12) | 66.67 (34) | ||
| 9.1 Denture’s age (n = 34) | <5 years | 23.53 (4) | 50.00 (8) | 33.33 (6) | 35.29 (18) | |
| 5 years | 35.29 (6) | 25.00 (4) | 33.33 (6) | 31.37 (16) | ||
| 9.2 Frequency of denture cleaning (n = 34) | No | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | |
| Once/day | 0.00 (0) | 12.50 (2) | 5.56 (1) | 5.88 (3) | ||
| Twice/day | 35.29 (6) | 25.00 (4) | 16.67 (3) | 25.49 (13) | ||
| After every meal | 23.53 (4) | 37.50 (6) | 44.44 (8) | 51.43 (18) | ||
| 9.3 Method of cleaning (n = 34) | Toothbrush + toothpaste | 35.29 (6) | 18.75 (3) | 22.22 (4) | 25.49 (13) | |
| Toothbrush + soap | 5.88 (1) | 18.75 (3) | 22.22 (4) | 15.69 (8) | ||
| Water | 17.65 (3) | 37.50 (6) | 22.22 (4) | 25.49 (13) | ||
| 9.4 Overnight removal (n = 34) | No | 0.00 (0) | 0.00 (0) | 5.56 (1) | 1.96 (1) | |
| Yes | 58.82 (10) | 75.00 (12) | 61.11 (11) | 64.71 (33) | ||
| 9.5 Denture hygiene (n = 34) | Good | 41.18 (7) | 68.75 (11) | 44.44 (8) | 50.98 (26) | |
| Poor | 17.65 (3) | 6.25 (1) | 22.22 (4) | 15.69 (8) |
| Parameter | NSS (n = 17) | 0.12% w/v CHX (n = 16) | FCoMW (n = 18) | p-Value (Between Group) |
|---|---|---|---|---|
| Plaque index | ||||
| Baseline | 1.22 ± 0.22 | 1.38 ± 0.21 | 1.22 ± 0.31 | 0.098 |
| Day 14 | 1.20 ± 0.24 | 1.23 ± 0.26 | 1.13 ± 0.31 | 0.578 |
| Mean changes (Δ) (Baseline-Day 14) | −0.02 ± 0.15 | −0.16 ± 0.13 | −0.09 ± 0.25 | 0.092 |
| p-value (within group) | 0.677 | <0.001 ** | 0.269 | |
| Oral moisture level | ||||
| Baseline | 30.09 ± 1.99 | 30.12 ± 1.38 | 28.40 ± 2.93 | 0.042 * |
| Day 14 | 30.72 ± 0.79 | 30.81 ± 1.65 | 29.51 ± 2.11 | 0.040 * |
| Mean changes (Δ) (Baseline-Day 14) | 0.63 ± 2.09 | 0.69 ± 2.27 | 1.12 ± 3.21 | 0.833 |
| p-value (within group) | 0.217 | 0.246 | 0.169 |
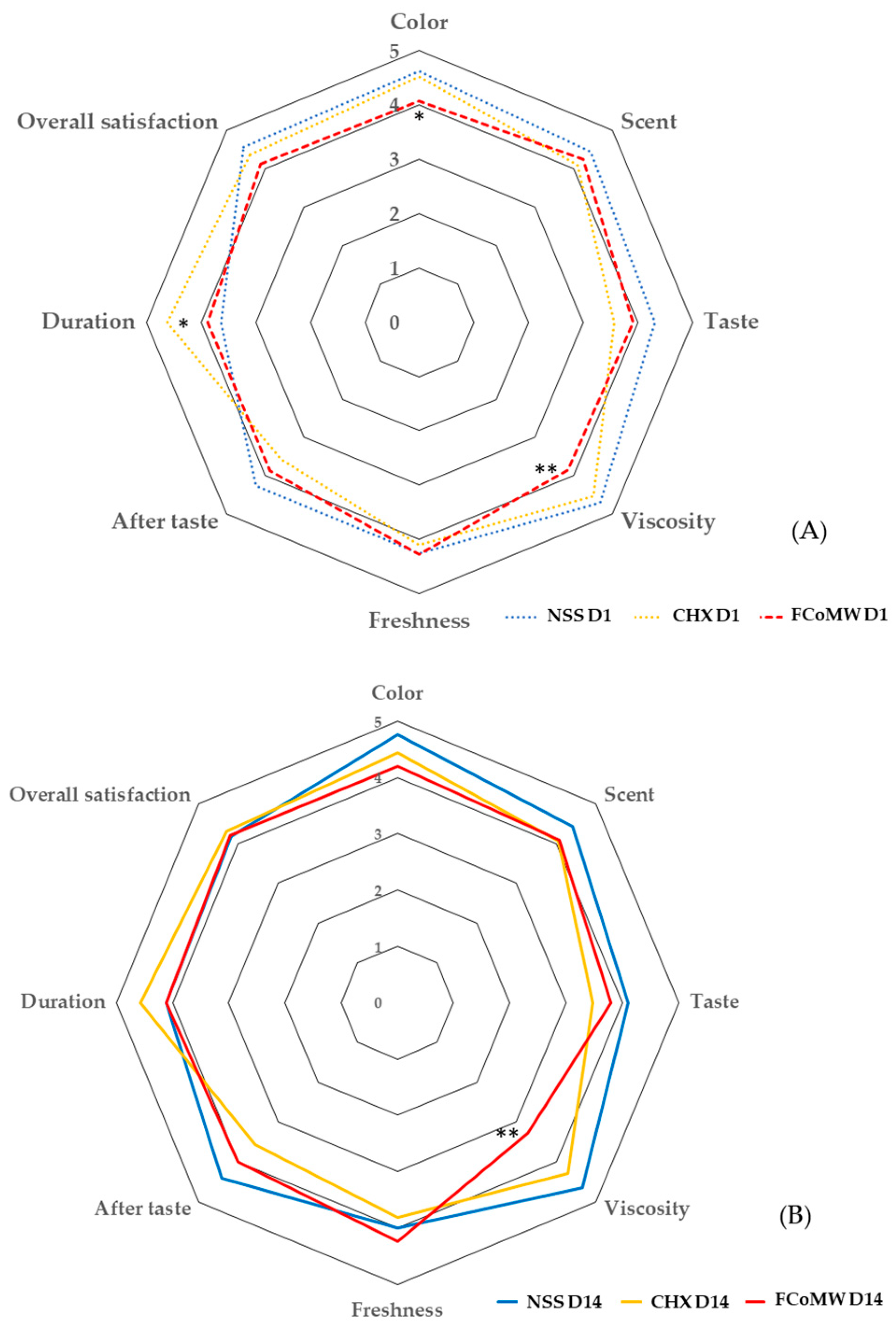
| Parameters | Day | Levels of Satisfaction | NSS (n = 17), %(n) | 0.12% w/v CHX (n = 16), %(n) | FCoMW (n = 18), %(n) | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± S.D. | Median (IQR) | Mean ± S.D. | Median (IQR) | Mean ± S.D. | Median (IQR) | |||
| Color | 1 | 4.65 ± 0.61 a | 5 (4–5) | 4.69 ± 0.48 b | 5 (4–5) | 4.17 ± 0.79 a,b | 4 (3.75–5) | |
| 4–5 | 94.12% (16) | 100 (16) | 77.78 (14) | |||||
| 3 | 6.25% (1) | 0 (0) | 22.22 (4) | |||||
| 1–2 | 0 (0) | 0 (0) | 0 (0) | |||||
| 14 | 4.70 ± 0.59 | 5 (4–5) | 4.50 ± 0.73 | 5 (4–5) | 4.28 ± 0.84 | 5 (3–5) | ||
| 4–5 | 94.12 (16) | 77.78 (14) | 72.22 (13) | |||||
| 3 | 5.88 (1) | 12.5 (2) | 27.78 (5) | |||||
| 1–2 | 0 (0) | 0 (0) | 0 (0) | |||||
| Scent | 1 | 4.35 ± 0.70 | 4 (4–5) | 4.25 ± 1.00 | 5 (3.25–5) | 4.22 ± 0.64 | 4 (4–5) | |
| 4–5 | 88.24 (15) | 75.00 (12) | 88.89 (16) | |||||
| 3 | 11.76 (2) | 18.75 (3) | 11.11 (2) | |||||
| 1–2 | 0 (0) | 6.25 (1) | 0 (0) | |||||
| 14 | 4.35 ± 0.79 | 5 (4–5) | 4.50 ± 0.73 | 5 (4–5) | 4.11 ± 0.90 | 4 (3–5) | ||
| 4–5 | 82.35 (14) | 88.24 (15) | 66.67 (12) | |||||
| 3 | 17.65 (3) | 12.50 (2) | 33.33 (6) | |||||
| 1–2 | 0 (0) | 0 (0) | 0 (0) | |||||
| Viscosity | 1 | 4.71 ± 0.69 c | 5 (5–5) | 4.63 ± 0.73 d | 5 (4–5) | 3.56 ± 0.98 c,d | 3 (3–4.25) | |
| 4–5 | 88.24 (15) | 93.75 (15) | 44.45 (8) | |||||
| 3 | 11.76 (2) | 6.25 (1) | 44.44 (8) | |||||
| 1–2 | 0 (0) | 0 (0) | 11.11 (2) | |||||
| 14 | 4.35 ± 0.93 e | 5 (5–5) | 4.44 ± 0.73 f | 5 (4–5) | 3.22 ± 0.81 e,f | 3 (3–4) | ||
| 4–5 | 82.35 (14) | 87.5 (14) | 33.33 (6) | |||||
| 3 | 11.76 (2) | 12.50 (2) | 50.00 (9) | |||||
| 1–2 | 5.88 (1) | 0 (0) | 16.67 (3) | |||||
| Freshness | 1 | 4.18 ± 0.81 | 4 (4–5) | 4.19 ± 0.83 | 4 (3.25–5) | 4.11 ± 0.83 | 4 (3–5) | |
| 4–5 | 88.24 (15) | 75.00 (12) | 72.22 (13) | |||||
| 3 | 5.88 (1) | 25.00 (4) | 27.78 (5) | |||||
| 1–2 | 5.88 (1) | 0 (0) | 0 (0) | |||||
| 14 | 4.00 ± 0.87 | 4 (4–5) | 4.00 ± 0.97 | 4 (3–5) | 4.28 ± 0.75 | 4 (4–5) | ||
| 4–5 | 76.47 (13) | 68.75 (11) | 83.33 (15) | |||||
| 3 | 17.65 (3) | 25.00 (4) | 16.67 (3) | |||||
| 1–2 | 5.88 (1) | 6.25 (1) | 0 (0) | |||||
| Taste | 1 | 4.47 ± 0.62 | 5 (4–5) | 3.69 ± 1.35 | 4 (3–5) | 3.94 ± 0.80 | 4 (3–5) | |
| 4–5 | 94.12 (16) | 68.75 (11) | 66.67 (12) | |||||
| 3 | 5.88 (1) | 11.11 (2) | 33.33 (6) | |||||
| 1–2 | 0 (0) | 18.75 (3) | 0 (0) | |||||
| Taste | 14 | 4.12 ± 0.78 | 5 (4–5) | 3.63 ± 1.50 | 4 (2.25–5) | 3.78 ± 0.80 | 4 (3–4.25) | |
| 4–5 | 88.24 (15) | 56.25 (9) | 55.56 (10) | |||||
| 3 | 5.88 (1) | 18.75 (3) | 44.44 (8) | |||||
| 1–2 | 5.88 (1) | 25.00 (4) | 0 (0) | |||||
| Aftertaste | 1 | 4.24 ± 0.75 | 4 (4–5) | 3.75 ± 1.39 | 4 (2.25–5) | 3.89 ± 0.83 | 4 (3–4.25) | |
| 4–5 | 82.35 (14) | 62.5 (10) | 72.22 (13) | |||||
| 3 | 17.65 (3) | 12.50 (2) | 22.22 (4) | |||||
| 1–2 | 0 (0) | 25.00 (4) | 5.56 (1) | |||||
| 14 | 4.24 ± 0.75 | 4 (3–5) | 3.63 ± 1.45 | 4 (2.25–5) | 4.00 ± 0.84 | 4 (3–4.25) | ||
| 4–5 | 82.35 (14) | 62.5 (10) | 66.67 (12) | |||||
| 3 | 17.65 (3) | 12.50 (2) | 33.33 (6) | |||||
| 1–2 | 0 (0) | 25.00 (4) | 0 (0) | |||||
| Duration | 1 | 3.71 ± 1.36 g | 4 (3–5) | 4.75 ± 0.57 g,h | 5 (5–5) | 3.72 ± 1.02 h | 4 (3–5) | |
| 4–5 | 64.71 (11) | 93.75 (15) | 55.56 (10) | |||||
| 3 | 17.65 (3) | 6.25 (1) | 33.33 (6) | |||||
| 1–2 | 17.65 (3) | 0 (0) | 11.11 (2) | |||||
| 14 | 4.12 ± 1.11 | 4 (3.5–5) | 4.63 ± 0.72 | 5 (4.25–5) | 4.11 ± 0.90 | 4 (3–5) | ||
| 4–5 | 76.47 (13) | 87.5 (14) | 66.67 (12) | |||||
| 3 | 17.65 (3) | 12.50 (2) | 33.33 (6) | |||||
| 1–2 | 5.88 (1) | 0 (0) | 0 (0) | |||||
| Overall satisfaction | 1 | 4.53 ± 0.62 | 5 (4–5) | 4.31 ± 0.95 | 5 (4–5) | 4.06 ± 0.80 | 4 (3–5) | |
| 4–5 | 94.12 (16) | 81.25 (13) | 72.22 (13) | |||||
| 3 | 5.88 (1) | 12.50 (2) | 27.78 (5) | |||||
| 1–2 | 0 (0) | 6.25 (1) | 0 (0) | |||||
| 14 | 4.18 ± 0.73 | 4 (4–5) | 4.38 ± 0.72 | 4.5 (4–5) | 4.33 ± 0.69 | 4 (4–5) | ||
| 4–5 | 82.35 (14) | 87.5 (14) | 88.89 (16) | |||||
| 3 | 17.65 (3) | 12.50 (2) | 11.11 (2) | |||||
| 1–2 | 0 (0) | 0 (0) | 0 (0) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soodsakorn, A.; Chaiyana, W.; Ittichaicharoen, J.; Wanachantararak, P.; Wongtapin, M.; Sookkhee, S.; Owittayakul, D. A Randomized Trial in Older Adults of a Flavor-Enhanced Coconut Oil-Based Mouthwash: Clinical Safety, Antimicrobial Efficacy, and User Satisfaction. Healthcare 2025, 13, 2941. https://doi.org/10.3390/healthcare13222941
Soodsakorn A, Chaiyana W, Ittichaicharoen J, Wanachantararak P, Wongtapin M, Sookkhee S, Owittayakul D. A Randomized Trial in Older Adults of a Flavor-Enhanced Coconut Oil-Based Mouthwash: Clinical Safety, Antimicrobial Efficacy, and User Satisfaction. Healthcare. 2025; 13(22):2941. https://doi.org/10.3390/healthcare13222941
Chicago/Turabian StyleSoodsakorn, Arpasiri, Wantida Chaiyana, Jitjiroj Ittichaicharoen, Phenphichar Wanachantararak, Marut Wongtapin, Siriwoot Sookkhee, and Darunee Owittayakul. 2025. "A Randomized Trial in Older Adults of a Flavor-Enhanced Coconut Oil-Based Mouthwash: Clinical Safety, Antimicrobial Efficacy, and User Satisfaction" Healthcare 13, no. 22: 2941. https://doi.org/10.3390/healthcare13222941
APA StyleSoodsakorn, A., Chaiyana, W., Ittichaicharoen, J., Wanachantararak, P., Wongtapin, M., Sookkhee, S., & Owittayakul, D. (2025). A Randomized Trial in Older Adults of a Flavor-Enhanced Coconut Oil-Based Mouthwash: Clinical Safety, Antimicrobial Efficacy, and User Satisfaction. Healthcare, 13(22), 2941. https://doi.org/10.3390/healthcare13222941









