Could Immersive Virtual Reality Facilitate the Recovery of Survivors of Critical Illness? A Systematic Review
Abstract
1. Introduction
2. Methods and Materials
2.1. Search and Study Identification
2.2. Methodological Quality
2.3. Data Collection
3. Results
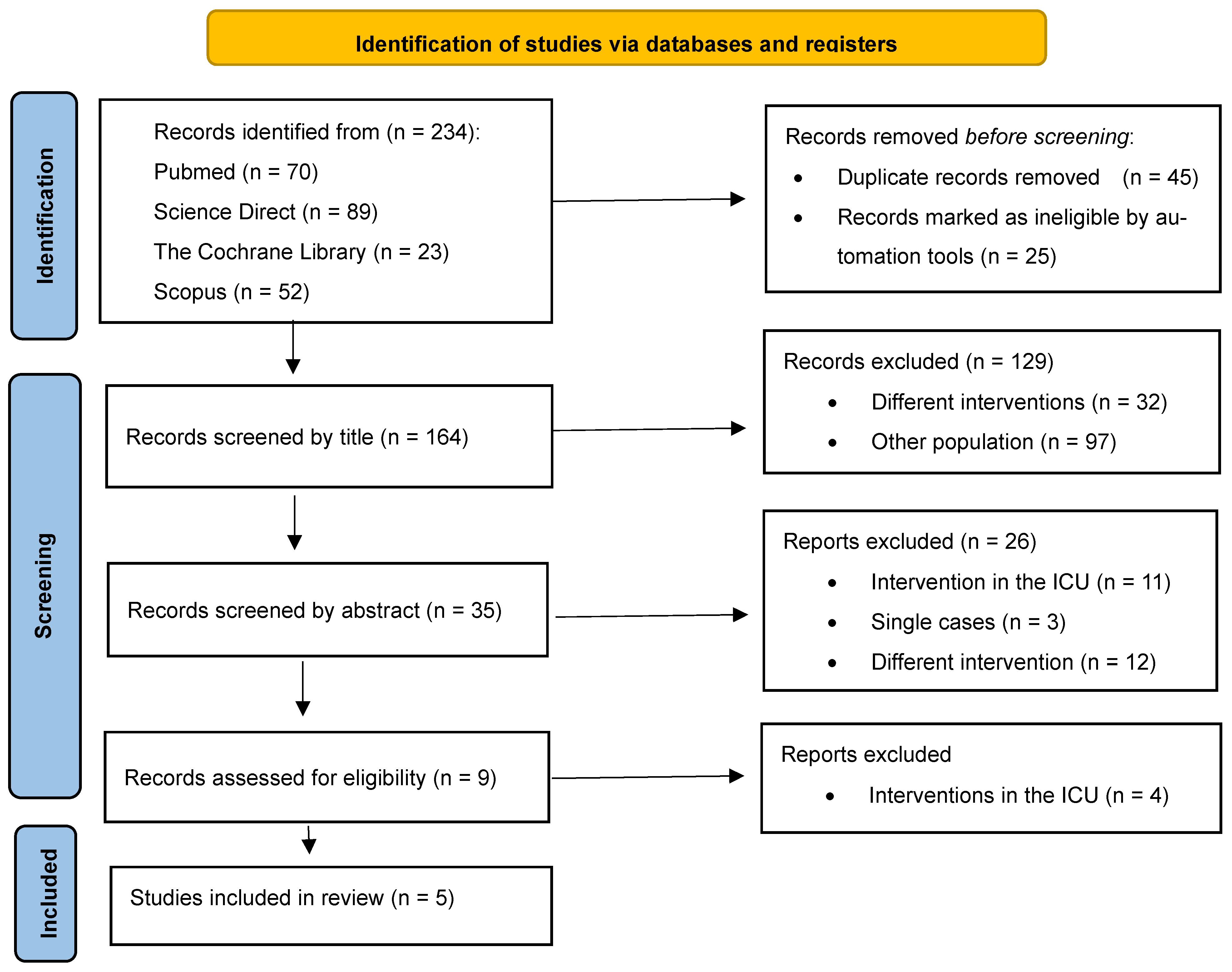
3.1. Identification of Studies
3.2. Methodological Quality
3.3. Description of Studies
3.3.1. Population
3.3.2. Interventions
3.3.3. Feasibility and Safety
3.3.4. Effects of Intervention
4. Discussion
Clinical Considerations
5. Limitations
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Ayenew, T.; Gete, M.; Gedfew, M.; Getie, A.; Afenigus, A.D.; Edmealem, A.; Amha, H.; Alem, G.; Tiruneh, B.G.; Messelu, M.A. Prevalence of Post-intensive care syndrome among intensive care unit-survivors and its association with intensive care unit length of stay: Systematic review and meta-analysis. PLoS ONE 2025, 2, e0323311. [Google Scholar] [CrossRef] [PubMed]
- Schembari, G.; Santonocito, C.; Messina, S.; Caruso, A.; Cardia, L.; Rubulotta, F.; Noto, A.; Bignami, E.G.; Sanfilippo, F. Post-Intensive Care Syndrome as a Burden for Patients and Their Caregivers: A Narrative Review. J. Clin. Med. 2024, 13, 5881. [Google Scholar] [CrossRef] [PubMed]
- Denehy, L.; Elliott, D. Strategies for post ICU rehabilitation. Curr. Opin. Crit. Care 2012, 18, 503–508. [Google Scholar] [CrossRef]
- Renner, C.; Jeitziner, M.M.; Albert, M.; Brinkmann, S.; Diserens, K.; Dzialowski, I.; Heidler, M.D.; Lück, M.; Nusser-Müller-Busch, R.; Sandor, P.S.; et al. Guideline on multimodal rehabilitation for patients with post-intensive care syndrome. Crit. Care 2023, 27, 301. [Google Scholar] [CrossRef]
- Rai, S.; Anthony, L.; Needham, D.M.; Georgousopoulou, E.N.; Sudheer, B.; Brown, R.; Mitchell, I.; van Haren, F. Barriers to rehabilitation after critical illness: A survey of multidisciplinary healthcare professionals caring for ICU survivors in an acute care hospital. Aust. Crit. Care 2020, 33, 264–271. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Z.; Xue, D.D.; Zhang, R.; Cheng, Y. Barriers and facilitators to offering post-intensive care follow-up services from the perspective of critical care professionals: A qualitative study. Nurs. Crit. Care 2024, 29, 682–694. [Google Scholar] [CrossRef]
- Cox, N.S.; Dal Corso, S.; Hansen, H.; McDonald, C.F.; Hill, C.J.; Zanaboni, P.; Alison, J.A.; O’Halloran, P.; Macdonald, H.; Holland, A.E. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst. Rev. 2021, 1, CD013040. [Google Scholar] [CrossRef]
- Savoric, T.; Aziz, S.; Ling, R.R.; Antlej, K.; Arnab, S.; Subramaniam, A. Systematic review: The impact of virtual reality interventions on stress and anxiety in intensive care units. J. Crit. Care 2025, 90, 155164. [Google Scholar] [CrossRef]
- Li, M.; Race, M.; Huang, F.; Escalon, M.X. Role of Virtual Reality to Promote Mobilization in the Critical Care Setting: A Narrative Review. Am. J. Phys. Med. Rehabil. 2025, 104, 487–494. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Foley, N.C.; Bhogal, S.K.; Teasell, R.W.; Bureau, Y.; Speechley, M.R. Estimates of quality and reliability with the physiotherapy evidence-based database scale to assess the methodology of randomized controlled trials of pharmacological and nonpharmacological interventions. Phys. Ther. 2006, 86, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- de Vries, M.; Beumeler, L.F.E.; van der Meulen, J.; Bethlehem, C.; den Otter, R.; Boerma, E.C. The feasibility of virtual reality therapy for upper extremity mobilization during and after intensive care unit admission. PeerJ 2025, 13, e18461. [Google Scholar] [CrossRef]
- Vlake, J.H.; van Bommel, J.; Wils, E.J.; Korevaar, T.I.; Taccone, F.; Schut, A.F.; Elderman, J.H.; Labout, J.A.; Raben, A.M.; Dijkstra, A.; et al. Effect of intensive care unit-specific virtual reality (ICU-VR) to improve psychological well-being in ICU survivors: Study protocol for an international, multicentre, randomised controlled trial-the HORIZON-IC study. BMJ Open 2022, 12, e061876. [Google Scholar] [CrossRef]
- Vlake, J.H.; Wils, E.J.; van Bommel, J.; Korevaar, T.I.M.; Gommers, D.; van Genderen, M.E. Virtual Reality Tailored to the Needs of Post-ICU Patients: A Safety and Immersiveness Study in Healthy Volunteers. Crit. Care Explor. 2021, 3, e0388. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, Y.; Tang, Q.; Yang, M.; Lan, A.; Xiao, H.; Wei, J.; Cao, X.; Xian, Y.; Yang, Q.; et al. Effects of early cognitive rehabilitation training on cognitive function and quality of life in critically ill patients with cognitive impairment: A randomised controlled trial. Aust. Crit. Care 2023, 36, 708–715. [Google Scholar] [CrossRef]
- Despoti, A.; Patsaki, I.; Alexandropoulou, A.; Magkouti, E.; Tzoumi, D.; Leventakis, N.; Roussou, G.; Papathanasiou, A.; Dimitriadi, N.; Presvelou, P.; et al. Comparing virtual reality with traditional methods in cognitive rehabilitation in PICS syndrome. Appl. Neuropsychol. Adult. 2025, 21, 1–12. [Google Scholar] [CrossRef]
- Roussou, G.; Despoti, A.; Patsaki, I.; Tzoumi, D.; Leventakis, N.; Dimitriadi, N.; Papathanasiou, A.; Alexandropoulou, A.; Nanas, S.; Karatzanos, E. Suitability, usability and safety of fully immersive virtual reality applications for motor and cognitive rehabilitation in stroke patients preliminary data. Health Res. J. 2024, 10, 193–205. [Google Scholar] [CrossRef]
- Jawed, Y.T.; Golovyan, D.; Lopez, D.; Khan, S.H.; Wang, S.; Freund, C.; Imran, S.; Hameed, U.B.; Smith, J.P.; Kok, L.; et al. Feasibility of a virtual reality intervention in the intensive care unit. Heart Lung 2021, 50, 748–753. [Google Scholar] [CrossRef]
- Haghedooren, E.; Haghedooren, R.; Langer, D.; Gosselink, R. Feasibility and safety of interactive virtual reality upper limb rehabilitation in patients with prolonged critical illness. Aust. Crit. Care 2024, 37, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Herbst, N.P.; Danesh, V.; Lewis, A.; Sevin, C.M. Multidisciplinary Team Approaches to Assessing and Addressing Post Intensive Care Syndrome. Crit. Care Clin. 2025, 41, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Chen, X.; Ding, B.; Guo, X.; Gai, Y. Efficacy of virtual reality in alleviating post-ICU syndrome symptoms: A systematic review and meta-analysis. Nurs. Crit. Care 2025, 30, e70004. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Spence, C. Enhancing Presence, Immersion, and Interaction in Multisensory Experiences Through Touch and Haptic Feedback. Virtual Worlds 2025, 4, 3. [Google Scholar] [CrossRef]
- Mehrinejad Khotbehsara, M.; Soar, J.; Lokuge, S.; Mehrinejad Khotbehsara, E.; Ip, W.K. The Potential of Virtual Reality-Based Multisensory Interventions in Enhancing Cognitive Function in Mild Cognitive Impairment: A Systematic Review. J. Clin. Med. 2025, 14, 5475. [Google Scholar] [CrossRef]
- Drigas, A.; Sideraki, A. Brain Neuroplasticity Leveraging Virtual Reality and Brain–Computer Interface Technologies. Sensors 2024, 24, 5725. [Google Scholar] [CrossRef]
- Wankhede, N.L.; Koppula, S.; Ballal, S.; Doshi, H.; Kumawat, R.; Raju, S.; Arora, I.; Sammeta, S.S.; Khalid, M.; Zafar, A.; et al. Virtual reality modulating dynamics of neuroplasticity: Innovations in neuro-motor rehabilitation. Neuroscience 2025, 566, 97–111. [Google Scholar] [CrossRef]
- Marotta, N.; Calafiore, D.; Curci, C.; Lippi, L.; Ammendolia, V.; Ferraro, F.; Invernizzi, M.; de Sire, A. Integrating virtual reality and exergaming in cognitive rehabilitation of patients with Parkinson disease: A systematic review of randomized controlled trials. Eur. J. Phys. Rehabil. Med. 2022, 58, 818–826. [Google Scholar] [CrossRef]
- Kim, B.R.; Chun, M.H.; Kim, L.S.; Park, J.Y. Effect of virtual reality on cognition in stroke patients. Ann. Rehabil. Med. 2011, 35, 450–459. [Google Scholar] [CrossRef]
- Jones, C.; Bäckman, C.; Capuzzo, M.; Egerod, I.; Flaatten, H.; Granja, C.; Rylander, C.; Griffiths, R.D.; RACHEL Group. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: A randomised, controlled trial. Crit. Care 2010, 14, R168. [Google Scholar] [CrossRef]
- Huang, W.; Gao, Y.; Zhou, L.; Xiao, X.; Xu, H.; Lu, L.; Deng, J.; Wu, J. Effects of ICU diaries on psychological disorders and sleep quality in critically ill patients and their family members: A systematic review and meta-analysis. Sleep Med. 2024, 122, 84–91. [Google Scholar] [CrossRef]
- Rashidi, E.; Razban, F.; Asadi, N. The effect of nurse-initiated diary intervention on posttraumatic stress disorder and recall of memories in ICU survivors: A randomized controlled trial. BMC Psychiatry 2024, 24, 158. [Google Scholar] [CrossRef] [PubMed]
- Chillura, A.; Bramanti, A.; Tartamella, F.; Pisano, M.F.; Clemente, E.; Lo Scrudato, M.; Cacciato, G.; Portaro, S.; Calabrò, R.S.; Naro, A. Advances in the rehabilitation of intensive care unit acquired weakness: A case report on the promising use of robotics and virtual reality coupled to physiotherapy. Medicine 2020, 99, e20939. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.; Huang, W.C. The Impact of Virtual Reality on Early Rehabilitation in Cardiac Intensive Care Units. Circulation 2023, 148, S1.14908. [Google Scholar] [CrossRef]
- Keller, J.; Štětkářová, I.; Macri, V.; Kühn, S.; Pětioký, J.; Gualeni, S.; Simmons, C.D.; Arthanat, S.; Zilber, P. Virtual reality-based treatment for regaining upper extremity function induces cortex grey matter changes in persons with acquired brain injury. J. Neuroeng. Rehabil. 2020, 17, 127. [Google Scholar] [CrossRef]
- Georgiev, D.D.; Georgieva, I.; Gong, Z.; Nanjappan, V.; Georgiev, G.V. Virtual Reality for Neurorehabilitation and Cognitive Enhancement. Brain Sci. 2021, 11, 221. [Google Scholar] [CrossRef]
- Luu, T.P.; He, Y.; Brown, S.; Nakagame, S.; Contreras-Vidal, J.L. Gait adaptation to visual kinematic perturbations using a real-time closed-loop brain-computer interface to a virtual reality avatar. J. Neural Eng. 2016, 13, 036006. [Google Scholar] [CrossRef]
- Ong, T.L.; Ruppert, M.M.; Akbar, M.; Rashidi, P.; Ozrazgat-Baslanti, T.; Bihorac, A.; Suvajdzic, M. Improving the Intensive Care Patient Experience With Virtual Reality—A Feasibility Study. Crit. Care Explor. 2020, 2, e0122. [Google Scholar] [CrossRef]
- He, Y.; Yang, Q.; Dai, X.; Chen, T.; Wu, H.; Li, K.; Zhu, S.; Liu, Y.; Lei, H. Effects of virtual reality technology on early mobility in critically ill adult patients: A systematic review and meta-analysis. Front. Neurol. 2025, 15, 1469079. [Google Scholar] [CrossRef]
- Kho, M.E.; Damluji, A.; Zanni, J.M.; Needham, D.M. Feasibility and observed safety of interactive video games for physical rehabilitation in the intensive care unit: A case series. J. Crit. Care 2012, 27, 219.e1–219.e6. [Google Scholar] [CrossRef]
- Bargeri, S.; Scalea, S.; Agosta, F.; Banfi, G.; Corbetta, D.; Filippi, M.; Sarasso, E.; Turolla, A.; Castellini, G.; Gianola, S. Effectiveness and safety of virtual reality rehabilitation after stroke: An overview of systematic reviews. EclinicalMedicine 2023, 64, 102220. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.K.; Soar, J.; Fong, K.; Wang, S.Y.; James, C. An Exploratory Study on Virtual Reality Technology for Fall Prevention in Older Adults with Mild Cognitive Impairment. Sensors 2025, 25, 3123. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.M.; Jeitziner, M.M.; Sänger, S.D.; Knobel, S.E.J.; Marchal-Crespo, L.; Müri, R.M.; Schefold, J.C.; Jakob, S.M.; Nef, T. Comparing the Relaxing Effects of Different Virtual Reality Environments in the Intensive Care Unit: Observational Study. JMIR Perioper. Med. 2019, 2, e15579. [Google Scholar] [CrossRef] [PubMed]
- Garrett, B.; Taverner, T.; Gromala, D.; Tao, G.; Cordingley, E.; Sun, C. Virtual Reality Clinical Research: Promises and Challenges. JMIR Serious Games 2018, 6, e10839. [Google Scholar] [CrossRef]
- Cong, X.; Li, T. Design and Development of Virtual Medical System Interface Based on VR-AR Hybrid Technology. Comput. Math. Methods Med. 2020, 2020, 7108147. [Google Scholar] [CrossRef]
- Abbas, Q.; Jeong, W.; Lee, S.W. Explainable AI in Clinical Decision Support Systems: A Meta-Analysis of Methods, Applications, and Usability Challenges. Healthcare 2025, 13, 2154. [Google Scholar] [CrossRef]
- Abbas, S.R.; Seol, H.; Abbas, Z.; Lee, S.W. Exploring the Role of Artificial Intelligence in Smart Healthcare: A Capability and Function-Oriented Review. Healthcare 2025, 13, 1642. [Google Scholar] [CrossRef]
- de Queiroz, R.S.; Saquetto, M.B.; Martinez, B.P.; Andrade, E.A.; da Silva, P.A.M.P.; Gomes-Neto, M. Evaluation of the description of active mobilisation protocols for mechanically ventilated patients in the intensive care unit: A systematic review of randomized controlled trials. Heart Lung 2018, 47, 253–260. [Google Scholar] [CrossRef]
- Queiroz, R.S.; Saquetto, M.B.; Martinez, B.P.; Cazeta, B.B.R.; Hodgson, C.; Gomes-Neto, M. Progressive active mobilization with dose control and training load in critically ill patients (PROMOB): Protocol for a randomized controlled trial. PLoS ONE 2020, 15, e0238352. [Google Scholar] [CrossRef]
| Search strategy |
|
| Search string | # 1 OR # 2 AND # 3 AND # 5 # 1 OR # 2 AND # 4 AND # 5 |
| 1 * | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vlake et al., 2021 [18] | Y | Y | Y | Y | Ν | Ν | Ν | Y | Ν | Y | Y | 6/10 |
| Vlake et al., 2022 [17] | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | 6/10 |
| Dong et al., 2022 [19] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5/10 |
| Study(Design)—Country | Population | Intervention | Control | Outcomes | Results (Between-Group Comparison When Applicable) |
|---|---|---|---|---|---|
| De Vries et al., 2025 [16] (pre-post study)—Netherlands | 10 patients from ICU to hospital discharge | 4 VR therapy sessions (puzzles) (3 times/week, 20 min) | - | Morton Mobility index Hand Grip | MMI: p < 0.05 HG: p = ns |
| Vlake et al., 2022 [17] (RCT)—Netherlands | COVID-19 3 months post hospital discharge (IG: 45/CG: 44) | 14 min-long informational video | Scenery VR | IES-r HADS SF-36 EQ-5D | Psychological distress: p < 0.05 at 3 months but not at 4 and 6 months HADS and IES-r: p = ns at all points. |
| Vlake et al., 2021 [18] (RCT)—Netherlands | (IG: 25/CG: 25) After ICU discharge | Informational video | Scenery | Immersive Tendencies Questionnaire, Simulator Sickness Questionnaire, Presence Questionnaire, IES-r, BDI-II, MCS-12, PCS-12, EQ-5D Adverse events | Only IES-R, BDI and MCS-12 (p < 0.05) at 1 month No simulation sickness, no change in vital signs, no adverse events |
| Dong et al., 2023 [19] (RCT)—China | (IG: 68/CG: 68) After ICU discharge with cognitive impairment | Cognitive rehabilitation + Music Therapy + Aerobic Training | Music + Aerobic training | MOCA SF-36 | p < 0.05 3 and 6 months |
| Despoti et al., 2025 [20] (nonRCT)—Greece | (IG: 15/CG: 15) Post Hospital discharge– PICS populations | 2 VR games 12 sessions (3 times a week, for 4 weeks). Each session lasted 30 min | Pencil–paper cognitive training | ACE-R (general cognitive function), MMSE (general cognitive function), FAB (executive function), GDS (depression) | Only Visuospatial abilities and fluency p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patsaki, I.; Tzoumi, D.; Kalyviotis, M.; Despoti, A.; Karatzanos, E.; Nanas, S.; Magira, E. Could Immersive Virtual Reality Facilitate the Recovery of Survivors of Critical Illness? A Systematic Review. Healthcare 2025, 13, 2942. https://doi.org/10.3390/healthcare13222942
Patsaki I, Tzoumi D, Kalyviotis M, Despoti A, Karatzanos E, Nanas S, Magira E. Could Immersive Virtual Reality Facilitate the Recovery of Survivors of Critical Illness? A Systematic Review. Healthcare. 2025; 13(22):2942. https://doi.org/10.3390/healthcare13222942
Chicago/Turabian StylePatsaki, Irini, Dimitra Tzoumi, Marios Kalyviotis, Akylina Despoti, Eleftherios Karatzanos, Serafim Nanas, and Eleni Magira. 2025. "Could Immersive Virtual Reality Facilitate the Recovery of Survivors of Critical Illness? A Systematic Review" Healthcare 13, no. 22: 2942. https://doi.org/10.3390/healthcare13222942
APA StylePatsaki, I., Tzoumi, D., Kalyviotis, M., Despoti, A., Karatzanos, E., Nanas, S., & Magira, E. (2025). Could Immersive Virtual Reality Facilitate the Recovery of Survivors of Critical Illness? A Systematic Review. Healthcare, 13(22), 2942. https://doi.org/10.3390/healthcare13222942







