Abstract
Background/Objective: Cancer significantly impairs physical function and quality of life. Exercise has gained recognition as a therapeutic strategy; however, its long-term efficacy in terms of multidimensional outcomes in patients with cancer remains underexplored. This review aimed to evaluate and summarize the available evidence regarding the effects of multicomponent exercise programs on pain and psychosocial outcomes in individuals with cancer. Methods: A systematic review was conducted in accordance with PRISMA guidelines and registered in PROSPERO (CRD42022321183). Comprehensive searches were performed in MEDLINE, OVID, LILACS, Scopus, PEDro, OTseeker, The Cochrane Library, EBSCO, and Google Scholar, without date restrictions. Search terms included “exercise” and “cancer.” Only randomized controlled trials (RCTs) were included. Methodological quality and risk of bias were assessed using the PEDro scale. Results: Sixteen randomized controlled trials met the inclusion criteria. Multicomponent exercise programs combining aerobic, resistance, and flexibility training significantly improved muscle strength, balance, and quality of life. Several studies reported meaningful reductions in pain intensity and interference, whereas others found no significant changes. Overall, exercise interventions demonstrated superior effects compared with usual care or educational controls across physical and psychosocial outcomes. Conclusions: Multicomponent exercise is a safe and feasible intervention for adults with cancer, including those with advanced disease or complex clinical profiles. Evidence supports consistent benefits in physical function and quality of life, with partially consistent yet favorable effects on pain. Programs integrating multiple exercise modalities appear most effective and should be considered as part of comprehensive oncological care to enhance therapeutic outcomes and long-term well-being.
1. Introduction
Cancer is the leading cause of death worldwide and the second most frequent cause of morbidity and mortality in the Americas, following cardiovascular diseases. In 2020, nearly 10 million deaths globally were attributed to cancer. In the Americas, approximately 4 million new cases were diagnosed, and 1.4 million deaths were reported due to this disease [1,2]. According to GLOBOCAN [3], the most common cancers in terms of new cases across both sexes and all age groups were breast (11.7%), lung (11.4%), colorectal (10%), and prostate cancer (7.3%). The leading causes of cancer-related mortality were lung (18%), colorectal (9.4%), liver (8.3%), and gastric cancer (7.7%) [1,3]. Approximately 57% of new cancer cases and 47% of cancer-related deaths occurred in individuals aged 69 years or younger [3].
The primary causes of cancer stem from interactions between individual genetic predispositions and three categories of external agents: physical, chemical, and biological carcinogens [3]. About one-third of cancer-related deaths are attributable to modifiable risk factors, including tobacco use, high body mass index, alcohol consumption, low intake of fruits and vegetables, and physical inactivity [1,2]. Cancer mortality can be reduced through early diagnosis and timely treatment, as early detection increases treatment success rates, improves survival, and decreases morbidity [2].
Cancer-related symptoms include fever, severe fatigue, weight loss or feeding difficulties, pain, swelling or palpable masses, and abnormal bleeding. Tumors may also secrete substances that alter energy metabolism or elicit immune responses, contributing to these manifestations. Many of these symptoms are nonspecific and may be associated with other conditions or with adjuvant therapies such as chemotherapy or radiotherapy [4]. Physical complications, including fatigue, pain, and functional impairments, substantially affect daily activities [5]. Pain is a major determinant of reduced quality of life, frequently associated with sleep disturbances, social withdrawal, anorexia, and decreased physical activity [6]. Alterations in body image and sexual function further contribute to emotional distress [7,8].
Clinically significant stress, depression, anxiety, and deterioration in quality of life are also common after cancer treatment [9,10]. Proposed mechanisms include psychosocial distress related to the disease, such as heightened fear of recurrence [11], concerns about family and financial burden [12], and difficulties reintegrating into social life [13]. Pain perception is modulated by complex interactions among biological variables—including gonadal hormones, genetic factors, differences in nociceptive processing pathways, and structural or functional variations in the central nervous system [14]—as well as psychosocial variables such as depression, anxiety, catastrophizing, cultural background, social learning, perceived relevance of pain, sex, age, and perceived social support [15].
Reducing pain and improving psychosocial well-being to optimize health-related quality of life (HRQoL) is a key objective across all phases of cancer management and survivorship [16]. Physical exercise has been proposed as an effective adjunct to cancer treatment, demonstrating multiple benefits for intrinsic tumor responses and cancer-related symptoms [17]. Additionally, exercise may reduce the risk of cancer recurrence [18,19]. Recent evidence suggests that multicomponent exercise programs defined as planned, structured, and repetitive interventions that combine more than one type of exercise, such as aerobic, resistance, balance, and flexibility training, are safe and well tolerated by patients with cancer when appropriately designed and supervised. The effectiveness of such interventions is strongly influenced by the planned intensity, frequency, and duration of the combined exercises. Beyond safety, physical exercise has demonstrated substantial benefits in this population, including reductions in cancer-related fatigue, improvements in cardiorespiratory fitness, muscle strength, and functional capacity, as well as enhancements in psychological well-being and overall quality of life. Exercise has also been associated with better treatment tolerance, fewer adverse effects, and potential improvements in survival outcomes. Nevertheless, current evidence remains limited, and further comprehensive studies are required to determine the most effective strategies for structuring multicomponent programs in this population [20,21,22]. Against this background, the aim of this systematic review was to evaluate and summarize the available evidence regarding the effects of multicomponent exercise programs on pain and psychosocial outcomes in individuals with cancer.
2. Materials and Methods
This study followed the Cochrane Collaboration guidelines for systematic reviews [23] and the PRISMA checklist criteria [24]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under ID CRD42022321183 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022321183, accessed on 6 November 2025).
2.1. Eligibility Criteria
Studies were selected based on the PICO framework, which is detailed as follows:
P (Population): Adult patients diagnosed with cancer or cancer survivors.
I (Intervention): Multicomponent exercise refers to programs that incorporate more than one type of exercise, most commonly strength, balance, and aerobic or endurance training. Additional components may include flexibility, coordination, power, or joint mobility, depending on the intended therapeutic goals and the scope of the intervention. For this review, inclusion criteria required the implementation of at least three combined exercise modalities [25,26,27].
C (Comparison): Conventional management or other non-pharmacological interventions.
O (Outcomes): Pain, catastrophizing, depression, disability index, life expectancy, quality of life, physical function, and pharmacological behavior.
Study type: Randomized controlled trials (RCTs) were included.
Exclusion criteria: Studies with interventions involving fewer than three exercise components or those incorporating additional non-exercise modalities were excluded.
2.2. Information Sources and Search Strategy
Searches were conducted in MEDLINE, OVID, LILACS, Scopus, PEDro, OTseeker, The Cochrane Library (Cochrane Central Register of Controlled Trials), EBSCO, and Google Scholar. The primary search terms were “exercise” and “cancer.” The complete search strategy is detailed in Appendix A: “(((exercise[Title/Abstract]) OR (exercise therapy[MeSH Terms])) AND (multicomponent[Title/Abstract])) AND (cancer[Title/Abstract]).” No language or publication date restrictions were applied. Searches were concluded on 10 March 2025.
2.3. Study Selection
A calibration process was implemented for study selection. Two independent reviewers screened the records in a blinded manner. Titles and abstracts were assessed using an online reference management system. Duplicate records were identified and removed with EndNote. Rayyan was used to support blinded screening of titles and abstracts and to flag potentially ineligible studies based on predefined filters. All outputs from these tools were verified by the reviewers to ensure accuracy. Articles were included if both reviewers reached agreement; disagreements were resolved by a third blinded reviewer. Eligibility criteria were applied during full-text screening for final selection. Any discrepancies regarding eligibility, quality assessment, or data extraction were resolved by consensus.
2.4. Data Collection Process
Data extraction was conducted independently using a standardized Excel form. Extracted variables included first author and year, study design, country, sample size, age, cancer type and stage, time since diagnosis, study objective, use of pharmacological treatment, description of multicomponent exercise modalities, comparator or additional interventions, measurement scales, and outcomes related to pain, catastrophizing, depression, disability index, life expectancy, quality of life, physical function, adherence, and adverse events. Mean and standard deviation values were extracted for all reported outcomes. Data accuracy was confirmed by all authors.
2.5. Quality Assessment and Risk of Bias
The methodological quality of the included studies was evaluated using the PEDro scale [28]. Studies with a score ≥ 6 were eligible for inclusion. The scale assesses random allocation, allocation concealment, baseline comparability, blinding of participants, therapists, and assessors, completeness of outcome data for at least 85% of participants in one key outcome, intention-to-treat analysis, and between-group statistical comparisons with corresponding results.
3. Results
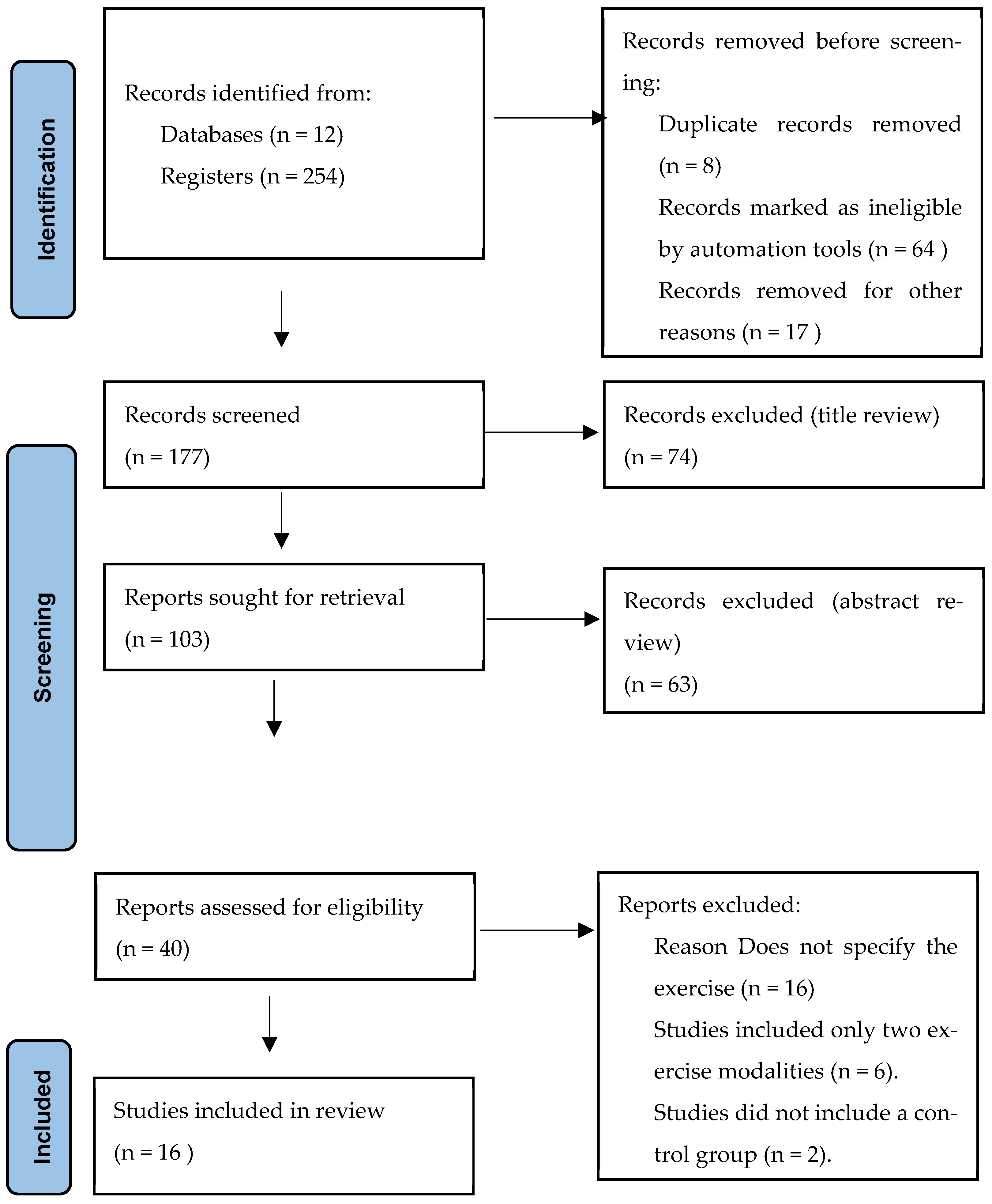
The systematic search across 12 databases retrieved 254 records. After removing 8 duplicates and excluding 64 records marked as ineligible by automation tools, 177 records remained for title and abstract screening. Of these, 74 were excluded at the title review stage and 63 at the abstract review stage. Consequently, 40 articles were assessed in full text, of which 16 met the inclusion criteria and were included in the final analysis (Figure 1 and Table 1).
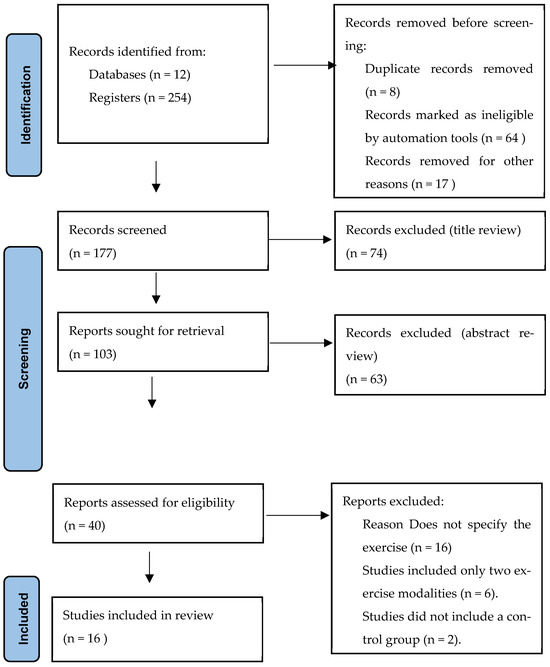
Figure 1.
Flow diagram of study selection process. Source: Page MJ et al. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71 [24].

Table 1.
Characteristics of studies evaluating multicomponent exercise in cancer patients.
3.1. Description of the Intervention
A total of 10 studies compared the intervention with conventional care, while 6 used alternative exercise modalities as comparators. Conventional care generally consisted of pharmacological management combined with routine clinical recommendations. Alternative comparators included stretching or relaxation programs, standard hospital care, home-based exercise, and impact-based exercise.
The interventions were delivered as multicomponent exercise programs incorporating aerobic, resistance, balance, flexibility, and strength training. Aerobic training involved walking, cycling, or treadmill exercise; resistance training was performed with elastic bands, free weights, or machines; and balance and flexibility components included pelvic floor exercises, yoga, and stretching. Intervention periods ranged from 8 to 12 weeks, with sessions of 60 to 90 min conducted two to three times per week.
Aerobic training was included in 100% of interventions, most frequently as walking [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Aerobic intensity was monitored via heart rate monitors and ratings of perceived exertion (RPE). All studies incorporated strength training [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44], employing bodyweight, elastic bands, or external weights. Flexibility training was also universal, typically performed through stretching at the end of sessions [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Additionally, two studies included pelvic floor training [31,36], one study reported respiratory training components [35], and four studies included balance training elements [33,40,41,42].
3.2. Description of Cancer Patient Characteristics
The most frequently reported cancer type was breast cancer [30,32,37,38,39,42,44], followed by prostate cancer [31,36,43], lung cancer [29,35], colorectal cancer [29,33], and mixed cancer populations [30,34,41]. Regarding disease stage, several studies included patients with advanced disease (stage IV) [29,33,43], while others enrolled patients across stages I–IV [38,39,40,41]. Populations comprised cancer survivors [30,31,32,34] and patients undergoing radiotherapy and/or chemotherapy [34]. Most studies included participants at least six months post-treatment.
3.3. Description of the Main Results of the Study
3.3.1. Pain Outcomes
Studies evaluating multicomponent exercise interventions in patients with cancer have reported heterogeneous effects on pain outcomes. Cheville et al. (2013) found no significant changes in pain intensity measured by the Numeric Rating Scale (NRS), with similar reductions in both groups (IG: −0.62 ± 2.59 vs. CG: −0.50 ± 2.01; p = 0.87) [29]. Conversely, Reis et al. (2018) observed significant improvements in multiple pain domains using the Brief Pain Inventory (BPI), including total pain (p = 0.0047), general intensity (p = 0.0082), worst (p = 0.0284) and least pain (p = 0.0365), as well as pain interference in daily life (p = 0.0201) [30]. Similarly, Mardani et al. (2021) reported a significant decrease in pain assessed by the EORTC QLQ-C30 (IG: 40.47 ± 16.31 vs. CG: 28.24 ± 15.84; p = 0.002) [31], whereas Zopf et al. (2015) found no significant between-group differences in the same instrument (IG: −2.37 points; CG: +3.33 points; both p > 0.05) [36]. In another trial, Do et al. (2015) demonstrated a substantial reduction in EORTC QLQ-C30 pain scores following an exercise program (IG pre = 40.9 ± 28.1; post = 19.4 ± 13.6; p < 0.001), with no significant change in controls [37]. Among cancer survivors, Fernández-Rodríguez et al. (2023) reported a trend toward pain reduction on the VAS and EQ-5D pain domains after a home-based multimodal program, though these differences were not statistically significant (p = 0.334) [41]. In contrast, Haines et al. (2010) identified a significant improvement in EQ-5D VAS scores favoring the intervention group (mean difference = 10.08; 95% CI 2.84–17.32; p = 0.006) [42]. Finally, Galvão et al. (2018) reported no between-group differences in bone pain assessed with the FACT-BP (p = 0.507) [43]. Collectively, these findings indicate that while several trials demonstrate clinically meaningful improvements in pain perception and interference, the overall evidence remains mixed, likely due to differences in cancer type, intervention components, and assessment instruments (see Table 2).

Table 2.
Pain, disability, quality of life and physical function outcomes in intervention and control groups.
3.3.2. Disability Outcomes
Regarding disability, a study using the World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) in patients with active cancer found no significant differences between groups [35]. The intervention included home-based aerobic exercise (30 min, ≥3 days/week, moderate-to-high intensity based on Borg 13–16 and target heart rate), resistance training for major muscle groups (2 sessions/week, 3 sets of 10–12 repetitions), stretching with elastic bands, and respiratory training (10 min, twice daily). Despite this structured multicomponent approach, changes in disability were not significant (WHODAS 2.0 mean difference −1.0, 95% CI −2.4 to 0.4; p = 0.152) (see Table 2).
3.3.3. Depression Outcomes
In cancer survivors, exercise interventions consistently demonstrated significant improvements in depressive symptoms compared with educational or usual care controls. In one supervised program [34], participants engaged in two 60-min sessions per week combining aerobic (20 min, 65–90% HRmax) and resistance training (progressing from 2 × 12 to 4 × 6 repetitions across upper and lower body exercises), complemented by encouragement to achieve 150 min/week of additional aerobic activity. Compared with the home-based group receiving only educational materials and telephone follow-up, the supervised group showed a marked reduction in HADS-D scores at week 12 (baseline 6.89 ± 4.20 to 4.00 ± 2.40), while the control remained unchanged (baseline 7.22 ± 2.49 to 7.67 ± 3.61). Similarly, another study [35] comparing exercise with educational interventions reported significant reductions in depressive symptoms favoring the exercise group. Furthermore, a multimodal supervised exercise program during radiotherapy and chemotherapy [44] produced greater improvements than routine nursing care, with significant between-group differences at mid- and post-intervention (HADS-D: t = −3.054, p = 0.003; t = −2.437, p = 0.019). Collectively, these findings highlight the robust effect of exercise, particularly supervised and multimodal programs, in reducing depression among cancer survivors.
3.3.4. Quality of Life Outcomes
Quality of life outcomes were assessed in fourteen trials using validated instruments such as the EORTC QLQ-C30 [31,36,37,40,42,44], FACT-G and related subscales [29,33,38,41], SF-36 [34], and EQ-5D [41,42]. Overall, multicomponent exercise interventions produced consistent improvements in quality of life compared to usual care or educational controls, although the magnitude of change varied across studies. Cheville et al. (2013) observed significant gains in mobility (p = 0.002), but not in global FACT-G scores (p = 0.54) [29]. In contrast, Mardani et al. (2021) reported marked improvements in global health status measured by the EORTC QLQ-C30 (IG pre 60.19 ± 13.95, post 72.57 ± 11.63; CG pre 61.12 ± 14.10, post 63.40 ± 12.80; p < 0.001) [31]. Similarly, Wang et al. (2021) found significant gains in the physical well-being subscale of FACT-ES (p = 0.023) [32], and Zimmer et al. (2018) reported an increase in FACT-G total scores favoring the intervention group (p = 0.028) [33].
Improvements were also observed in mental health and emotional function domains. Levin et al. (2018) reported significant enhancements in the SF-36 Mental Health Composite after 12 weeks of exercise compared to the control group (p = 0.005) [34]. Do et al. (2015) demonstrated substantial improvements in global health status using the EORTC QLQ-C30 (IG pre 58.0 ± 18.6; post 87.3 ± 13.7; CG pre 59.5 ± 17.9; post 61.0 ± 17.5; p < 0.001) [37], while Shinde et al. (2024) observed increases in FACT-B scores (p = 0.001) [38]. Fernández-Rodríguez et al. (2023) also reported significant improvements in total FACT scores following a home-based multimodal program (p = 0.036) [41].
In patients with active cancer, Haines et al. (2010) documented significant gains in global health on the EORTC QLQ-C30 favoring the exercise group (p = 0.04) [42], while Zhang et al. (2023) found significant benefits in functional, emotional, and social functioning, as well as reductions in fatigue and insomnia (all p < 0.05) [44]. Conversely, some studies, such as Zopf et al. (2015) and Ferrara et al. (2025), reported non-significant changes in overall quality of life [36,40]. Collectively, these findings indicate that multicomponent exercise programs typically integrating aerobic, resistance, and flexibility training are associated with meaningful improvements in both general and cancer-specific quality of life, particularly in physical, emotional, and global health domains (see Table 2).
3.3.5. Physical Function Outcomes
Across the included studies, multicomponent exercise interventions produced significant improvements in several indicators of physical function. Reis et al. (2018) reported higher aerobic capacity and handgrip strength in the intervention group compared with controls (VO2max: 20.68 ± 2.50 vs. 14.80 ± 2.46; p = 0.0001; handgrip: 24.79 ± 6.77 vs. 21.71 ± 7.44; p = 0.0001) [30]. Similarly, Wang et al. (2021) observed a significant increase in step counts during the 2-min step test (p = 0.036) [32], and Zimmer et al. (2018) demonstrated significant gains in leg press strength (p = 0.011) but no between-group differences in 6-min walk distance (p = 0.432) [33].
In patients with active cancer, Ferrara et al. (2025) reported improvements in lower limb strength (leg press 1RM: p = 0.153; knee extension 1RM: p = 0.018) and global performance measured by the SPPB (IG: 2.30 vs. CG: 0.38; p = 0.002) [40]. Fernández-Rodríguez et al. (2023) also found significant between-group differences favoring the intervention in total SPPB scores (6.21 ± 2.99 vs. 4.42 ± 3.03; p = 0.045) and gait performance (p = 0.035) [41]. Galvão et al. (2018) reported a significant increase in leg extension strength (mean difference 6.6 kg; 95% CI 0.6–12.7; p = 0.033) [43].
In breast cancer patients (stages I–III), Shinde et al. (2024) [39] observed significant improvements in lower limb function and aerobic performance following a multicomponent exercise intervention. The intervention group showed a notable increase in the sit-to-stand test (from 12.96 ± 4.24 to 17.12 ± 7.39; p = 0.0002) and in the 12-min walk test (from 1242.73 ± 205.68 m to 1309.37 ± 167.35 m; p = 0.008), while no significant changes were detected in the control group (p > 0.90). These findings further support the effectiveness of combined exercise modalities in enhancing functional capacity among individuals with cancer.
Other studies reported variable effects on aerobic capacity and general performance. Levin et al. (2018) found no between-group differences in 400-m walk performance (p = 0.466) [34], and Haines et al. (2010) observed non-significant differences in 6MWT distance (p = 0.34) and grip strength (p = 0.48) [42]. Conversely, significant within- and between-group improvements in physical function subscales were observed in studies using quality-of-life instruments, including EORTC QLQ-C30 (p < 0.001) [36,37]. Overall, exercise interventions combining aerobic, resistance, and flexibility training demonstrated consistent benefits in muscle strength, balance, and physical performance, while changes in aerobic capacity were heterogeneous across studies (see Table 2).
3.3.6. Additional Findings
Most studies did not evaluate overall survival, focusing instead on functional outcomes. Some reported indirect indicators; for instance, Zimmer et al. (2018) [33] observed maintenance of neuropathic symptoms and muscle strength in patients with metastatic colorectal cancer receiving the intervention, whereas the control group demonstrated deterioration, indicating potential preservation of function in advanced disease.
Adherence was generally reported as acceptable to high, ranging from 70.8% to 100%. Several studies documented adherence around 80% (e.g., 76.9%, 80%, 80.5%, and 93.3%). In some cases, exact percentages were not specified, but compliance was described as good. Reported strategies to promote adherence included shorter interventions and regular follow-up, although consistency in reporting was limited.
No study reported outcomes related to catastrophizing. Life expectancy was primarily used for patient characterization, and no findings were presented regarding pharmacological treatment.
3.4. Risk of Bias Assessment
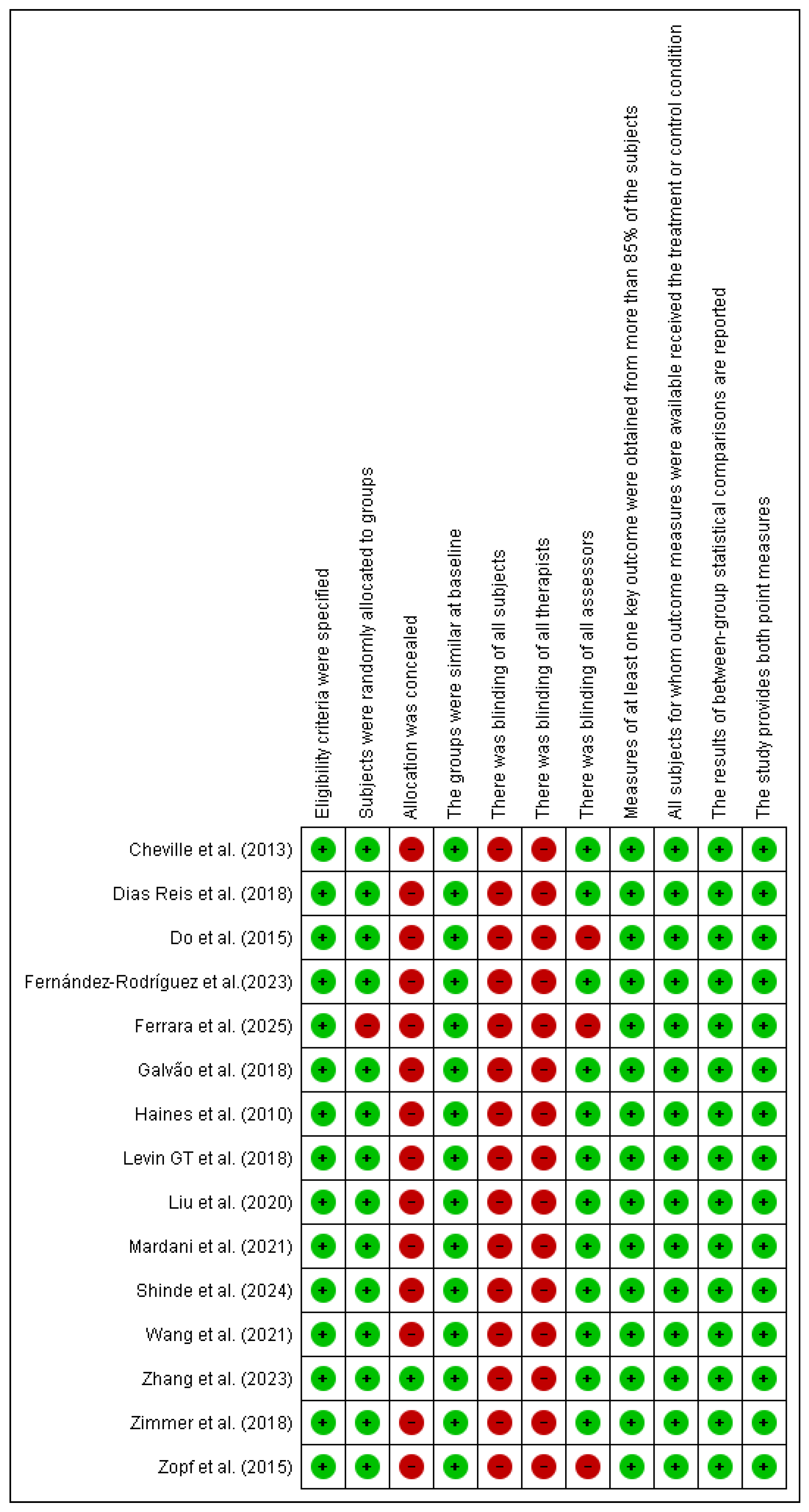
The risk of bias assessment, using the PEDro scale [28] identified the absence of blinding of participants and therapists as the main methodological limitation, which is inherent to exercise-based interventions. In addition, three studies did not implement blinding of outcome assessors, which may have introduced detection bias and affected the reliability of the findings. Nevertheless, other PEDro criteria, including randomization and intention-to-treat analysis, were consistently fulfilled. Overall, the methodological quality of the included studies is best characterized as moderate (see Figure 2).
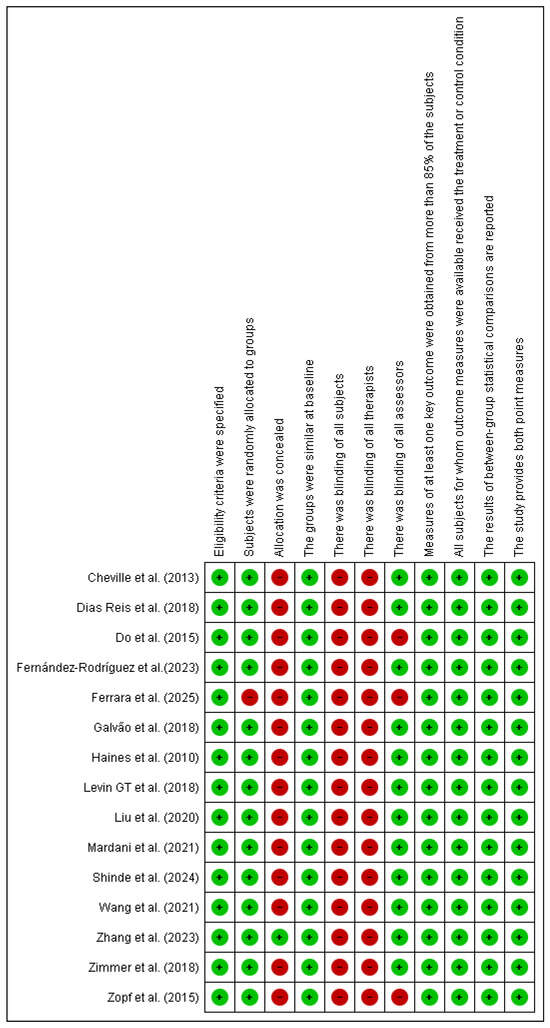
Figure 2.
Risk of bias Pedro scale. Source: Risk of bias using the Pedro scale (developed with Revman 5.3) Abbreviations: Green: Low risk Red: High risk. Cheville et al. (2013) [29], Reis et al. (2018) [30], Mardani et al. (2021) [31], Wang et al. (2021) [32], Zimmer et al. (2018) [33], Levin GT et al. (2018) [34], Liu et al. (2020) [35], Zopf et al. (2015) [36], Do et al. (2015) [37], Shinde et al. (2024) [38,39], Ferrara et al. (2025) [40], Fernández-Rodríguez et al. (2023) [41], Haines et al. (2010) [42], Galvão et al. (2018) [43], Zhang et al. (2023) [44].
4. Discussion
Consistent with global cancer prevalence patterns, the most frequently reported cancer types in the included studies were breast cancer (7 studies) and prostate cancer (3 studies), primarily in advanced stages and under active treatment.
Multicomponent exercise interventions were implemented across various cancer types and stages, with benefits observed in both early and advanced disease. Programs generally combined aerobic, resistance, flexibility, balance, and strength training, tailored to patient characteristics and treatment status. Aerobic exercise was consistently included, most often walking, cycling, or treadmill sessions, performed typically 2–3 times per week for 20–30 min per session, at moderate-to-vigorous intensity (65–90% HRmax or Borg 13–16), with some protocols encouraging the accumulation of 150 min/week of additional aerobic activity. Resistance training was conducted twice weekly, using elastic bands, free weights, or machines, with progressive overload (e.g., starting at 2 sets of 12 repetitions and advancing up to 4 sets of 6 repetitions) and targeting both upper and lower body muscle groups. Balance training was incorporated particularly in older adults and those with functional limitations, including static and dynamic exercises to improve stability, gait, and fall prevention. Flexibility exercises were usually included at the end of each session through stretching of major muscle groups. Additional disease-specific components included pelvic floor training in prostate cancer, aiming to improve continence and functional outcomes. Overall, interventions commonly lasted 8–12 weeks, with supervised 60–90-min sessions delivered two to three times per week, demonstrating the feasibility and effectiveness of structured multicomponent approaches in oncological populations.
For breast cancer, several studies reported consistent improvements in quality of life, fatigue, physical function, and psychological outcomes. Overall, multicomponent exercise interventions were associated with beneficial effects on pain, quality of life, and functional capacity in patients with active cancer and survivors. In particular, stage IV cancers including breast, and lung cancer demonstrated benefits from multicomponent exercise in reducing pain, lowering disability, and improving functional status and quality of life.
These findings align with Zhang et al. (2025) [45], who conducted a systematic review and meta-analysis of 25 randomized controlled trials including 2577 participants. Exercise interventions, ranging from aerobic to resistance training, significantly reduced depression (p < 0.0001) and anxiety (p = 0.0002) in breast cancer survivors. Multicomponent training, examined in 16 studies, was identified as the most effective approach, particularly when performed at least three times per week in sessions lasting ≤60 min. In contrast to Zhang et al. (2025) [45], the present review establishes that multicomponent exercise programs should include at least three modalities out of aerobic, resistance, and either flexibility, balance, or pelvic floor training. This distinction provides a clearer framework for clinical translation and underscores the importance of structured and progressive programs that mirror real-world rehabilitation needs. Furthermore, by synthesizing dosage parameters (frequency, intensity, duration, and progression), the current review offers practical guidance that is often lacking in previous meta-analyses. This broader perspective, encompassing both active cancer patients and survivors, highlights the feasibility and additional benefits of comprehensive multicomponent interventions compared with protocols that incorporate only two exercise modalities.
Other approaches have also been evaluated. Correia et al. (2023) [46], in a meta-analysis of 28 randomized controlled trials with 2424 participants, reported that home-based exercise interventions during active cancer treatment significantly improved cardiorespiratory capacity as measured by the six-minute walk test. However, no significant changes were observed in strength or body composition, underscoring the importance of supervised interventions in this population.
Bowers et al. (2025) [47] reviewed 62 interventions in adults with cancer-associated cachexia and found that 52 percent of the programs included exercise components. These multicomponent interventions focused primarily on preserving physical function, with resistance training being the most used component.
In cancer survivors, Murnaghan et al. (2024) [48] identified exercise as a central component in six studies that implemented supervised aerobic, resistance, or group-based training programs. These interventions were associated with improvements in functional and psychosocial reintegration.
Regarding the feasibility of multicomponent exercise interventions for patients with stage I to III breast cancer undergoing active treatment, one study comparing radiotherapy and chemotherapy groups reported improvements in quality of life, body composition, and physical function, supporting the safety of these interventions [49].
4.1. Clinical Practice Implications
This review supports multicomponent exercise as a safe and effective adjunct therapy for patients with cancer, including those with advanced disease. To optimize outcomes, interventions should be individualized according to cancer type, disease stage, and clinical status. Programs must be structured and supervised, with exercise type and dose clearly defined during design. Integration of exercise into interdisciplinary cancer care has the potential to improve quality of life and functional performance in this population.
4.2. Research Implications
The findings underscore the need for studies with larger, well-defined populations and robust methodological designs to support the generalizability of multicomponent exercise effects in oncology, particularly in advanced or frail populations. Future research should include long-term follow-up to evaluate not only functional outcomes, pain, and quality of life but also disease progression, survival, and sustained adherence to exercise programs. It is also essential to incorporate pharmacological monitoring into study designs, given the potential impact of interactions between medical treatments and exercise interventions. Future studies should incorporate patient-centered outcomes. When assessing pain-related variables, a biopsychosocial framework is required, making constructs such as catastrophizing and kinesiophobia particularly relevant. Additional outcomes, including social participation, perceived well-being, feasibility, cost-effectiveness, and the personalization of exercise programs by cancer type and stage, should also be examined.
4.3. Limitations
This review has several limitations that should be considered when interpreting its findings. First, no firm conclusions can be drawn regarding the effects of multicomponent exercise on survival or pharmacological management. Most included studies did not report medication dosages, highlighting the need to account for concomitant treatments and specify which were used, particularly in patients with active cancer, to enable meaningful comparisons and assess potential treatment–exercise interactions. In addition, most studies lacked long-term follow-up and focused primarily on short-term outcomes such as physical function and quality of life. Although long-term follow-up is challenging given the prognosis of this population, it remains essential for future research.
Second, there was marked heterogeneity in the tools used to assess physical function (e.g., six-minute walk test, Short Physical Performance Battery, dynamometry) and pain. Standardization of pain assessment, for example, using the VAS for intensity and the BPI for impact, would improve comparability across studies. Finally, substantial variability was observed in intervention protocols. Future studies should adopt reporting checklists such as TIDieR [50] to ensure detailed description of exercise interventions. The limited number of trials directly comparing different modalities (e.g., multicomponent vs. aerobic-only exercise) also restricts conclusions about relative effectiveness. These limitations emphasize the need for studies with more homogeneous designs, longer follow-up, and more rigorous reporting. It is possible that, due to the search and information systematization process, some relevant studies were not accessible and therefore are not reflected in this review.
5. Conclusions
Multicomponent exercise shows beneficial effects on quality of life, depression, and physical function in patients with different cancer types, with mixed but promising evidence regarding pain outcomes. The most consistent benefits were observed in mobility, strength, and balance, including in advanced or metastatic disease and in vulnerable populations such as older adults and individuals with bone metastases. Programs combining at least three modalities—typically aerobic, resistance, and flexibility—implemented two to three times per week in 60–90-min sessions with progressive intensity adjustments appear particularly effective compared with passive or educational interventions. Although heterogeneity in study design remains a limitation and long-term effects on survival require further research, current evidence indicates that well-structured multicomponent interventions are safe, feasible, and clinically relevant even in complex oncological populations.
Author Contributions
Conceptualization, L.T.O.-M., I.D.R. and M.M.-B.; methodology, J.L.E.-Z., L.T.O.-M., J.F.G.-G., G.P.R.-T. and L.T.O.-M.; validation, J.L.E.-Z., I.D.R., L.T.O.-M.; formal analysis, J.L.E.-Z., J.F.G.-G., I.D.R. and L.T.O.-M.; Investigation J.F.G.-G., M.M.-B., I.D.R., J.L.E.-Z. and L.T.O.-M.; resources, J.L.E.-Z.; data curation, J.F.G.-G., M.M.-B., I.D.R., G.P.R.-T., J.L.E.-Z. and L.T.O.-M.; writing—original draft preparation, J.F.G.-G., M.M.-B., I.D.R., G.P.R.-T., J.L.E.-Z. and L.T.O.-M.; writing—review and editing, J.L.E.-Z. and L.T.O.-M. visualization, J.L.E.-Z. and L.T.O.-M.; supervision, L.T.O.-M.; project administration L.T.O.-M.; funding acquisition, J.L.E.-Z. and L.T.O.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the General Directorate of Investigations of Universidad Santiago de Cali under call No. DGI 01-2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Search Terms
| Search Combination Terms |
| Exercise therapy AND Cancer |
| Exercise multicomponent AND Cancer pain |
| Exercise multicomponent AND neoplasm |
| Exercise therapy AND Cancer pain |
| physical training AND Cancer Pain |
| Exercise therapy AND neoplasm |
| Exercise multicomponent AND Chemotherapy |
| Exercise multicomponent AND Radiation therapy |
| Exercise multicomponent AND Palliative therapy |
References
- PAHO/WHO Pan American Health Organization. World Cancer Day 2023: Close the Care Gap. Available online: https://www.paho.org/en/campaigns/world-cancer-day-2023-close-care-gap (accessed on 10 June 2025).
- Cancer. Available online: https://www.who.int/es/news-room/fact-sheets/detail/cancer (accessed on 10 June 2025).
- Cancer Today. Available online: https://gco.iarc.fr/today/en (accessed on 10 June 2025).
- American Cancer Society. Available online: https://www.cancer.org/es/cancer/diagnostico-y-etapa-del-cancer/senales-y-sintomas-del-cancer.html (accessed on 10 June 2025).
- VanHoose, L.; Black, L.L.; Doty, K.; Sabata, D.; Twumasi-Ankrah, P.; Taylor, S.; Johnson, R. An analysis of the distress thermometer problem list and distress in patients with cancer. Support. Care Cancer 2015, 23, 1225–1232. [Google Scholar] [CrossRef]
- Expósito Vizcaíno, S. Chronic Pain Associated with Cancer in Adults. Ph.D. Thesis, Universitat Rovira i Virgili, Tarragona, Spain, 26 November 2019. Available online: http://hdl.handle.net/10803/668978 (accessed on 10 March 2025).
- American Cancer Society. Available online: https://www.cancer.org/es/cancer/supervivencia/bienestar-tras-el-tratamiento/actividad-fisica-y-el-paciente-de-cancer.html (accessed on 10 June 2025).
- Traa, M.J.; De Vries, J.; Roukema, J.A.; Rutten, H.J.T.; Den Oudsten, B.L. The sexual health care needs after colorectal cancer: The view of patients, partners, and health care professionals. Support. Care Cancer 2014, 22, 763–772. [Google Scholar] [CrossRef]
- Paraskevi, T. Quality of life outcomes in patients with breast cancer. Oncol. Rev. 2012, 6, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.; Koch, L.; Brenner, H.; Arndt, V. Quality of life among long-term (≥5 years) colorectal cancer survivors—Systematic review. Eur. J. Cancer 2010, 46, 2879–2888. [Google Scholar] [CrossRef]
- Thewes, B.; Butow, P.; Zachariae, R.; Christensen, S.; Simard, S.; Gotay, C. Fear of cancer recurrence: A systematic literature review of self-report measures. Psychooncology 2012, 21, 571–587. [Google Scholar] [CrossRef]
- Kale, H.P.; Carroll, N.V. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer 2016, 122, 1283–1289. [Google Scholar] [CrossRef]
- Keesing, S.; Rosenwax, L.; McNamara, B. The implications of women’s activity limitations and role disruptions during breast cancer survivorship. Womens Health 2018, 14, 1745505718756381. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Miró, J.; de la Vega, R.; Gertz, K.J.; Jensen, M.P.; Engel, J.M. The Role of Perceived Family Social Support and Parental Solicitous Responses in Adjustment to Bothersome Pain in Young People with Physical Disabilities. Disabil. Rehabil. 2017, 41, 641. [Google Scholar] [CrossRef]
- Kuo, J.C.; Graham, D.M.; Salvarrey, A.; Kassam, F.; Le, L.W.; Shepherd, F.A.; Burkes, R.; Hollen, P.J.; Gralla, R.J.; Leighl, N.B. A Randomized Trial of the Electronic Lung Cancer Symptom Scale for Quality-of-Life Assessment in Patients with Advanced Non-small-Cell Lung Cancer. Curr. Oncol. 2020, 27, 156–162. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef]
- Courneya, K.S.; Vardy, J.L.; O’Callaghan, C.J.; Gill, S.; Friedenreich, C.M.; Wong, R.K.S.; Dhillon, H.M.; Coyle, V.; Chua, N.S.; Jonker, D.J.; et al. Structured Exercise after Adjuvant Chemotherapy for Colon Cancer. N. Engl. J. Med. 2025, 393, 13–25. [Google Scholar] [CrossRef]
- Miyamoto, T.; Nagao, A.; Okumura, N.; Hosaka, M. Effect of Post-diagnosis Physical Activity on Breast Cancer Recurrence: A Systematic Review and Meta-analysis. Curr. Oncol. Rep. 2022, 24, 1645–1659. [Google Scholar] [CrossRef]
- Cavalheri, V.; Burtin, C.; Formico, V.R.; Nonoyama, M.L.; Jenkins, S.; Spruit, M.A.; Hill, K. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst. Rev. 2019, 6, CD009955. [Google Scholar] [CrossRef]
- Rosero, I.D.; Ramírez-Vélez, R.; Martínez-Velilla, N.; Cedeño-Veloz, B.A.; Morilla, I.; Izquierdo, M. Effects of a multicomponent exercise program in older adults with non-small-cell lung cancer during adjuvant/palliative treatment: An intervention study. J. Clin. Med. 2020, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Peddle-McIntyre, C.J.; Singh, F.; Thomas, R.; Newton, R.U.; Galvao, D.A.; Cavalheri, V. Exercise training for advanced lung cancer. Cochrane Database Syst. Rev. 2019, 2, CD012685. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2011. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 372, n71. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Casas Herrero, Á.; Cadore, E.L.; Martínez Velilla, N.; Izquierdo Redin, M. El ejercicio físico en el anciano frágil: Una actualización [Physical exercise in the frail elderly: An update]. Rev. Esp. Geriatr. Gerontol. 2015, 50, 74–81. (In Spanish) [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Cheville, A.L.; Kollasch, J.; Vandenberg, J.; Shen, T.; Grothey, A.; Gamble, G.; Basford, J.R. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: A randomized controlled trial. J. Pain Symptom Manag. 2013, 45, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.D.; Pereira, P.T.V.T.; Diniz, R.R.; de Castro Filha, J.G.L.; dos Santos, A.M.; Ramallo, B.T.; Filho, F.A.A.; Navarro, F.; Garcia, J.B.S. Effect of exercise on pain and functional capacity in breast cancer patients. Health Qual. Life Outcomes 2018, 16, 58. [Google Scholar] [CrossRef]
- Mardani, A.; Pedram Razi, S.; Mazaheri, R.; Haghani, S.; Vaismoradi, M. Effect of the exercise programme on the quality of life of prostate cancer survivors: A randomized controlled trial. Int. J. Nurs. Pract. 2021, 27, e12883. [Google Scholar] [CrossRef]
- Wang, L.F.; Eaglehouse, Y.L.; Poppenberg, J.T.; Brufsky, J.W.; Geramita, E.M.; Zhai, S.; Davis, K.K.; Gibbs, B.B.; Metz, J.; van Londen, G.J. Effects of a personal trainer-led exercise intervention on physical activity, physical function, and quality of life of breast cancer survivors. Breast Cancer 2021, 28, 737–745. [Google Scholar] [CrossRef]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support. Care Cancer 2018, 26, 615–624. [Google Scholar] [CrossRef]
- Levin, G.T.; Greenwood, K.M.; Singh, F.; Newton, R.U. Modality of exercise influences rate of decrease in depression for cancer survivors with elevated depressive symptomatology. Support. Care Cancer 2018, 26, 1597–1606. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, T.; Pei, L.; Zhang, Y.; Xu, L.; Cui, Y.; Liang, N.; Li, S.; Chen, W.; Huang, Y. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: A randomized controlled trial. Anesth. Analg. 2020, 131, 840–849. [Google Scholar] [CrossRef]
- Zopf, E.M.; Bloch, W.; Machtens, S.; Zumbé, J.; Rübben, H.; Marschner, S.; Kleinhorst, C.; Schulte-Frei, B.; Herich, L.; Felsch, M.; et al. Effects of a 15-Month Supervised Exercise Program on Physical and Psychological Outcomes in Prostate Cancer Patients Following Prostatectomy: The ProRehab Study. Integr. Cancer Ther. 2015, 14, 409–418. [Google Scholar] [CrossRef]
- Do, J.; Cho, Y.; Jeon, J. Effects of a 4-week multimodal rehabilitation program on quality of life, cardiopulmonary function, and fatigue in breast cancer patients. J. Breast Cancer 2015, 18, 87–96. [Google Scholar] [CrossRef]
- Shinde, S.B.; Jain, P.P.; Gudur, A.; Patil, S.K.; Shinde, R.V. Effect of Multi-component Exercise Program on Body Composition and Physical, Emotional and Social well being in Breast Cancer Survivors. Asian Pac. J. Cancer Prev. 2024, 25, 4397. [Google Scholar] [CrossRef]
- Shinde, S.B.; Jain, P.P.; Gudur, A.; Patil, S.K.; Shinde, R.V. Effect of Multi-component Exercise Program on Functional Performance in Breast Cancer Survivors. Asian Pac. J. Cancer Prev. 2024, 25, 4323–4331. [Google Scholar] [CrossRef]
- Ferrara, M.C.; Zambom-Ferraresi, F.; Castillo, A.; Delgado, M.; Galbete, A.; Arrazubi, V.; Morilla, I.; Zambom-Ferraresi, F.; Fernández González de la Riva, M.L.; Vera Garcìa, R.; et al. Effects of an individualised exercise program in hospitalised older adults with cancer: A randomised clinical trial. J. Nutr. Health Aging 2025, 29, 100424. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, E.J.; Sanchez-Gomez, C.; Mendez-Sanchez, R.; Recio-Rodriguez, J.I.; Puente-Gonzalez, A.S.; Gonzalez-Sanchez, J.; Cruz-Hernandez, J.J.; Rihuete-Galve, M.I. Multimodal Physical Exercise and Functional Rehabilitation Program in Oncological Patients with Cancer-Related Fatigue—A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2023, 20, 4938. [Google Scholar] [CrossRef] [PubMed]
- Haines, T.P.; Sinnamon, P.; Wetzig, N.G.; Lehman, M.; Walpole, E.; Pratt, T.; Smith, A. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Res. Treat. 2010, 124, 163–175. [Google Scholar] [CrossRef]
- Galvão, D.A.; Taaffe, D.R.; Spry, N.; Cormie, P.; Joseph, D.; Chambers, S.K.; Chee, R.; Peddle-McIntyre, C.J.; Hart, N.H.; Baumann, F.T.; et al. Exercise Preserves Physical Function in Prostate Cancer Patients with Bone Metastases. Med. Sci. Sports Exerc. 2018, 50, 393–399. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, Y.; Jiang, R.; Ge, S.; Song, M. Effect of Multimodal Exercise on Cancer-Related Fatigue in Patients Undergoing Simultaneous Radiotherapy and Chemotherapy: A Randomized Trial in Patients with Breast Cancer. Altern. Ther. Health Med. 2023, 29, 233–237. [Google Scholar] [PubMed]
- Zhang, Y.; Li, G.; Zhang, S.; Zhou, Y.; Lv, Y.; Feng, L.; Yu, L. Effects of Exercise on Depression and Anxiety in Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cancer Med. 2025, 14, e70671. [Google Scholar] [CrossRef]
- Correia, I.R.; Cardoso, V.; Cargaleiro, C.; Magalhães, J.P.; Hetherington-Rauth, M.; Rosa, G.B.; Malveiro, C.; de Matos, L.V.; Cardoso, M.J.; Sardinha, L.B. Effects of home-based exercise programs on physical fitness in cancer patients undergoing active treatment: A systematic review and meta-analysis of randomized controlled trials. J. Sci. Med. Sport 2023, 26, 222–231. [Google Scholar] [CrossRef]
- Bowers, M.; Petrasso, C.; McLuskie, A.; Bayly, J.; Laird, B.J.A.; Higginson, I.J.; Maddocks, M. Multicomponent Interventions for Adults With Cancer Cachexia: A Systematic Review. J. Cachexia Sarcopenia Muscle 2025, 16, e13716. [Google Scholar] [CrossRef]
- Murnaghan, S.; Scruton, S.; Urquhart, R. Psychosocial interventions that target adult cancer survivors’ reintegration into daily life after active cancer treatment: A scoping review. JBI Evid. Synth. 2024, 22, 607–656. [Google Scholar] [CrossRef]
- González-Santos, Á.; Lopez-Garzon, M.; Gil-Gutiérrez, R.; del Mar Salinas-Asensio, M.; Postigo-Martin, P.; Cantarero-Villanueva, I. Nonlinear, Multicomponent Physical Exercise With Heart Rate Variability-Guided Prescription in Women With Breast Cancer During Treatment: Feasibility and Preliminary Results (ATOPE Study). Phys. Ther. 2023, 103, pzad070. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).