Hypersensitivity Reactions to Iodinated Contrast Media: A Narrative Review of Current Evidence and Clinical Challenges
Abstract
1. Introduction
2. Methods
3. Discussion
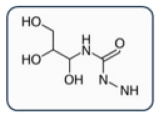
3.1. Pharmacological and Pathogenetic Correlations in ICM HSRs
| Lerondeau et al. [9] | Schrijvers et al. [10] | Srisuwatchari et al. [11] | Seo et al. [12] |
|---|---|---|---|
| Group A: Iohexol, iomeprol, ioversol, iodixanol, and iopamidol [sharing 2 identical N-(2,3-dihydroxypropyl) carbamoyl sidechains] | Group A: Iohexol, iomeprol, ioversol, iodixanol, and iopromide [all sharing a N-(2,3-dihydroxypropyl) carbamoyl sidechain] | Group A: Iohexol, iomeprol, ioversol, iodixanol, iopromide, and iopamidol [with at least 1 N-(2,3-dihydroxypropyl) carbamoyl group] | Iohexol, iomeprol, ioversol, iodixanol, and iopromide [share a benzyl sidechain with an N-(2,3-dihydroxypropyl) carbamoyl group]. Iopromide and iobitridol have an N-(2,3-dihydroxypropyl)-N-methyl-carbamoyl group. |
| Group B: Iobitridol | Group B: Iobitridol and iopamidol | Group B: Iobitridol | Iopamidol does not possess either a N-(2,3-dihydroxypropyl) carbamoyl group or N-(2,3-dihydroxypropyl)-N-methyl-carbamoyl group. |
3.2. Epidemiology, Clinical Manifestations, and Risk Factors of HSRs to ICM
3.3. In Vivo and In Vitro Diagnosis of ICM HSRs
3.4. Risk of Cross-Reactivity (CR) Between ICM
3.5. Protocols of DPTs
- The potential risks of ARs, either IHSRs or NIGHSRs, but also reactions due to toxicity, such as kidney injury, thyrotoxic crisis, and lactic acidosis. The assessment of serum creatinine and calculation of the estimated glomerular filtration rate before DPTs, as well as the monitoring of renal function, should be performed. Contraindications to perform DPTs are pregnant and breastfeeding women, patients with renal failure or patients taking nephrotoxic drugs, hyperthyroidism, and radioactive iodine therapy.
- The lack of standardization: There is no consensus on the optimal dose of ICM for DPTs, as various doses have been used. For IHRs, challenge doses range from 10 mL to 120 mL, with the NPV of STs compared to DPTs varying between 37.5% and 100%. For NIHRs, doses between 10 mL and 100 mL have been used, showing a higher NPV of 66.6% to 100%. Concerning administration rates, some study protocols use increasing intravenous doses, reaching a total administration time of 4 h; others use a rapid full dose, administering 100 mL of ICM in 12 min.
3.6. Approach to Premedication
| Study | Premedication Scheme |
|---|---|
| Lee et al. [54] | For patients with a mild index reaction: 4 mg of intravenous (IV) chlorpheniramine 30 min before ICM administration. |
| For patients with a moderate index reaction: 40 mg of IV methylprednisolone and 4 mg of IV chlorpheniramine 1 h before ICM administration. | |
| For patients with a severe index reaction: 40 mg of IV methylprednisolone 4 h and 1 h before and 4 mg of IV chlorpheniramine 1 h before ICM administration via the IV catheter inserted for ICM injection. | |
| Park et al. [55] | No predefined premedication regimen but decided by physician in charge; histamines and steroids administered 0.5–1 h before exposure to ICM. |
| Mervak et al. [48] | Doses of 50 mg of prednisone administered 13 and 7 h and 1 h before CT (total, 150 mg prednisone), and 50 mg of diphenhydramine administered 1 h before CT. |
| Jung et al. [56] | No predefined premedication regimen but decided by physician in charge; histamines and steroids (usually 40 mg of methylprednisolone IV and 4 mg of chlorpheniramine and/or 20 mg of famotidine) administered 0.5–1 h before exposure to ICM. |
| Kolbe et al. [57] | Diphenhydramine alone (25–50 mg orally or intravenously 1 h prior to imaging); Corticosteroid alone (two doses of methylprednisolone 32 mg orally administered 12 and 2 h prior to imaging); Combined corticosteroid plus diphenhydramine. |
| Jha et al. [58] | IV corticosteroid (methylprednisone 125 mg, hydrocortisone 100 mg, or dexamethasone 16 mg), IV H2-blocker (famotidine 50 mg), and IV antihistamine (diphenhydramine 25 mg). |
| Bae Y et al. [59] | In case of mild reaction: chlorpheniramine, 4 mg alone, IV or intramuscularly 1 h before ICM; In case of severe reactions: IV hydrocortisone, 200 mg, plus chlorpheniramine, 4 mg 1 h before ICM. |
| Hubbard et al. [60] | In case of known previous AEs to ICM: methylprednisolone 80 mg IV, cimetidine 300 mg IV, prochlorperazine 10 mg orally, and montelukast 10 mg orally. |
3.7. Unmet Needs, Evidence Gaps, and Future Directions
4. Conclusions
- Low skin test sensitivity due to heterogeneous pathogenetic mechanisms and the time interval between reaction and allergy evaluation, which is often performed more than 6 months later;
- Potential cross-reactivity among ICM, which can affect up to 50% of skin tests;
- Lack of consensus about the real efficacy and safety of premedication in preventing the recurrence of adverse events;
- Lack of consensus on DPTs, regarding timing, dose, and administration rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICM | Iodinated contrast media |
| ARs | Adverse reactions |
| HSRs | Hypersensitivity reactions |
| ST | Skin test |
| CR | Cross-reactivity |
| DTP | Drug provocation test |
| IHSRs | Immediate HSRs |
| NIHSRs | Non-immediate HSRs |
| MRGPRX2 | Mas-Related G Protein-coupled Receptor X2 |
| SCARs | Severe cutaneous adverse reactions |
| SJS | Stevens–Johnson syndrome |
| AGEP | Acute generalized pustulosis |
| DRESS | Drug reaction with systemic symptoms and eosinophilia |
| SPTs | Skin prick tests |
| IDTs | Intradermal tests |
| PTs | Patch Tests |
| BAT | Basophil activation test |
| LTT | Lymphocyte transformation test |
| NPV | Negative predictive value |
| PPV | Positive predictive value |
| IV | Intravenous |
References
- Rosado Ingelmo, A.; Doña Diaz, I.; Cabañas Moreno, R.; Moya Quesada, M.C.; García-Avilés, C.; García Nuñez, I.; Martínez Tadeo, J.I.; Mielgo Ballesteros, R.; Ortega-Rodríguez, N.; Padial Vilchez, M.A.; et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J. Investig. Allergol. Clin. Immunol. 2016, 26, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Jolliet, P.; Ballereau, F. An Overview of the Clinical Pharmacokinetics of X-Ray Contrast Media. Clin. Pharmacokinet. 1997, 32, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Meurer, K.; Kelsch, B.; Hogstrom, B. The pharmacokinetic profile, tolerability and safety of the iodinated, non-ionic, dimeric contrast medium Iosimenol 340 injection in healthy human subjects. Acta Radiol. 2015, 56, 581–586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saretta, F.; Caimmi, S.; Mori, F.; Bianchi, A.; Bottau, P.; Crisafulli, G.; Franceschini, F.; Liotti, L.; Paglialunga, C.; Ricci, G.; et al. Radiocontrast Media Hypersensitivity Reactions in Children. Medicina 2022, 58, 517. [Google Scholar] [CrossRef]
- Pasternak, J.J.; Williamson, E.E. Clinical Pharmacology, Uses, and Adverse Reactions of Iodinated Contrast Agents: A Primer for the Non-radiologist. Mayo Clin. Proc. 2012, 87, 390–402. [Google Scholar] [CrossRef]
- Costa, N. Understanding Contrast Media. J. Infus. Nurs. 2004, 27, 302–312. [Google Scholar] [CrossRef]
- Rossi, F.; Cuomo, V.; Riccardi, C. Farmacologia: Principi di Base e Applicazioni Terapeutiche, 5th ed.; Minerva Medica: Turin, Italy, 2023. [Google Scholar]
- Vega, F.; van de Ven, A.A.J.M.; van der Molen, A.J. Cross-reactivity in hypersensitivity reactions to contrast agents: New classification and guide for clinical practice. Eur. Radiol. 2024, 34, 7583–7588. [Google Scholar] [CrossRef]
- Lerondeau, B.; Trechot, P.; Waton, J.; Poreaux, C.; Luc, A.; Schmutz, J.L.; Paris, C.; Barbaud, A. Analysis of cross-reactivity among radiocontrast media in 97 hypersensitivity reactions. J. Allergy Clin. Immunol. 2016, 137, 633–635.e4. [Google Scholar] [CrossRef]
- Schrijvers, R.; Breynaert, C.; Bourrain, J.L.; Demoly, P.; Chiriac, A.M. Patient versus allergy specialist interpretation of a negative workup for suspected iodinated contrast media allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 1081–1082. [Google Scholar] [CrossRef]
- Srisuwatchari, W.; Vo, T.; Gauthier, A.; Molinari, N.; Schrijvers, R.; Demoly, P.; Chiriac, A.M. Hypersensitivity reactions to iodinated radiocontrast media: Cluster analysis reveals distinct clinical phenotypes. World Allergy Organ. J. 2022, 15, 100680. [Google Scholar] [CrossRef]
- Sohn, K.H.; Seo Jho Kang, D.Y.; Lee, S.Y.; Kang, H.R. Finding the Optimal Alternative for Immediate Hypersensitivity to Low-Osmolar Iodinated Contrast. Investig. Radiol. 2021, 56, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Trcka, J.; Schmidt, C.; Seitz, C.S.; Bröcker, E.B.; Gross, G.E.; Trautmann, A. Anaphylaxis to Iodinated Contrast Material: Nonallergic Hypersensitivity or IgE-Mediated Allergy? Am. J. Roentgenol. 2008, 190, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K. Allergy to Radiocontrast Dye. Immunol. Allergy Clin. N. Am. 2022, 42, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Feltrin, G.P.; Zandonà, M.; Borile, V.; Rettore, C.; Miotto, D. Fundamentals on iodinated contrast media and adverse reactions. Radiol. Med. 2004, 107, 8–31. [Google Scholar]
- Chiu, T.M.; Chu, S.Y. Hypersensitivity Reactions to Iodinated Contrast Media. Biomedicines 2022, 10, 1036. [Google Scholar] [CrossRef]
- Brockow, K.; Christiansen, C.; Kanny, G.; Clément, O.; Barbaud, A.; Bircher, A.; Dewachter, P.; Guéant, J.L.; Rodriguez Guéant, R.M.; Mouton-Faivre, C.; et al. Management of hypersensitivity reactions to iodinated contrast media. Allergy 2005, 60, 150–158. [Google Scholar] [CrossRef]
- Brockow, K. Diagnosing and Managing Patients with Reactions to Radiocontrast Media. Curr. Treat. Options Allergy 2021, 8, 210–221. [Google Scholar] [CrossRef]
- Böhm, I. Kounis syndrome in a patient with allergy to iodinated contrast media. Int. J. Cardiol. 2011, 151, 102–103. [Google Scholar] [CrossRef]
- Clement, O.; Dewachter, P.; Mouton-Faivre, C.; Nevoret, C.; Guilloux, L.; Bloch Morot, E.; Katsahian, S.; Laroche, D.; Audebert, M.; Benabes-Jezraoui, B.; et al. Immediate Hypersensitivity to Contrast Agents: The French 5-year CIRTACI Study. eClinicalMedicine 2018, 1, 51–61. [Google Scholar] [CrossRef]
- Webb, J.A.; Stacul, F.; Thomsen, H.S.; Morcos, S.K. Members of the *Contrast Media Safe. Late adverse reactions to intravascular iodinated contrast media. Eur. Radiol. 2003, 13, 181–184. [Google Scholar] [CrossRef]
- Yasuda, R.; Munechika, H. Delayed Adverse Reactions to Nonionic Monomeric Contrast-Enhanced Media. Investig. Radiol. 1998, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.; Bernier, C.; Veyrac, G.; Barbaud, A.; Puymirat, E.; Milpied, B. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: A retrospective study. J. Am. Acad. Dermatol. 2020, 82, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Van der Molen, A.J.; van de Ven, A.A.J.M.; Vega, F.; Dekkers, I.A.; Laguna, J.J. Rare delayed hypersensitivity reactions to contrast media: Severe cutaneous adverse reactions. Eur. J. Radiol. 2025, 183, 111908. [Google Scholar] [CrossRef] [PubMed]
- Voltolini, S.; Cofini, V.; Murzilli, F.; Bignardi, D.; Borro, M.; Calamari, M.; Caruso, C.; Cittadini, G.; Contatore, M.; Cortellini, G.; et al. Hypersensitivity reactions to iodinated contrast media in Italy: A retrospective study. Characteristics of patients and risk factors. Eur. Ann. Allergy Clin. Immunol. 2022, 54, 60–67. [Google Scholar] [CrossRef]
- Costantino, M.T.; Romanini, L.; Gaeta, F.; Stacul, F.; Valluzzi, R.L.; Passamonti, M.; Bonadonna, P.; Cerri, G.; Pucci, S.; Ricci, P.; et al. SIRM-SIAAIC consensus, an Italian document on management of patients at risk of hypersensitivity reactions to contrast media. Clin. Mol. Allergy 2020, 18, 13. [Google Scholar] [CrossRef]
- Torres, M.J.; Trautmann, A.; Böhm, I.; Scherer, K.; Barbaud, A.; Bavbek, S.; Bonadonna, P.; Cernadas, J.R.; Chiriac, A.M.; Gaeta, F.; et al. Practice parameters for diagnosing and managing iodinated contrast media hypersensitivity. Allergy 2021, 76, 1325–1339. [Google Scholar] [CrossRef]
- Kang, H.R.; Jeong, J.; Brockow, K. Diagnosis and Prevention of Hypersensitivity Reactions to Iodinated Contrast Media. Allergy Asthma Immunol. Res. 2022, 14, 348. [Google Scholar] [CrossRef]
- Schmid, A.A.; Hungerbühler, M.N.; Lombardo, P.; Boehm, I.B. Intradermal testing of iodinated contrast media: Should we test up to pure or with diluted compounds only? Fundam. Clin. Pharmacol. 2024, 38, 789–798. [Google Scholar] [CrossRef]
- Brockow, K. Immediate and Delayed Reactions to Radiocontrast Media: Is There an Allergic Mechanism? Immunol. Allergy Clin. N. Am. 2009, 29, 453–468. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, S.Y.; Kang, H.R.; Kim, J.Y.; Hahn, S.; Park, C.M.; Chang, Y.S.; Goo, J.M.; Cho, S.H. Skin tests in patients with hypersensitivity reaction to iodinated contrast media: A meta-analysis. Allergy 2015, 70, 625–637. [Google Scholar] [CrossRef]
- Brockow, K.; Romano, A.; Aberer, W.; Bircher, A.J.; Barbaud, A.; Bonadonna, P.; Faria, E.; Kanny, G.; Lerch, M.; Pichler, W.J.; et al. Skin testing in patients with hypersensitivity reactions to iodinated contrast media—A European multicenter study. Allergy 2009, 64, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Çelik, G.E.; Pascal, M.; Hoffmann, H.J.; Eberlein, B.; Torres, M.J.; Brockow, K.; Garvey, L.H.; Barbaud, A.; Madrigal-Burgaleta, R.; et al. Flow-based basophil activation test in immediate drug hypersensitivity. An EAACI task force position paper. Allergy 2024, 79, 580–600. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Ariza, A.; Blanca-López, N.; Torres, M.J. Nonimmediate hypersensitivity reactions to iodinated contrast media. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cabeza, C.; Roa-Medellín, D.; Torrado, I.; De Barrio, M.; Fernández-Álvarez, C.; Montes-Aceñero, J.F.; De La Riva, I.; Prieto-García, A. Immediate reactions to iodinated contrast media. Ann. Allergy Asthma Immunol. 2017, 119, 553–557. [Google Scholar] [CrossRef]
- Torres Jaén, M.J.; Gomez, F.; Doña, I.; Rosado, A.; Mayorga, C.; Garcia, I.; Blanca-Lopez, N.; Canto, G.; Blanca, M. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy 2012, 67, 929–935. [Google Scholar] [CrossRef]
- Ghiordanescu, I.M.; Molinari, N.; Ciocănea-Teodorescu, I.; Schrijvers, R.; Motei, C.; Forsea, A.M.; Demoly, P.; Chiriac, A.M. Skin Test Reactivity Patterns in Patients Allergic to Iodinated Contrast Media: A Refined View. J. Allergy Clin. Immunol. Pract. 2024, 12, 705–713.e6. [Google Scholar] [CrossRef]
- Umakoshi, H.; Nihashi, T.; Takada, A.; Hirasawa, N.; Ishihara, S.; Takehara, Y.; Naganawa, S.; Davenport, M.S.; Terasawa, T. Iodinated Contrast Media Substitution to Prevent Recurrent Hypersensitivity Reactions: A Systematic Review and Meta-Analysis. Radiology 2022, 305, 341–349. [Google Scholar] [CrossRef]
- Prieto-García, A.; Tomás, M.; Pineda, R.; Tornero, P.; Herrero, T.; Fuentes, V.; Zapatero, L.; de Barrio, M. Skin test-positive immediate hypersensitivity reaction to iodinated contrast media: The role of controlled challenge testing. J. Investig. Allergol. Clin. Immunol. 2013, 23, 183–189. [Google Scholar]
- Madrigal-Burgaleta, R. The Path to Optimal Evaluation of Anaphylactic Iodinated Contrast Media Reactions: Are We There Yet? J. Allergy Clin. Immunol. Pract. 2022, 10, 2693–2694. [Google Scholar] [CrossRef]
- Sánchez-Borges, M.; Aberer, W.; Brockow, K.; Celik, G.E.; Cernadas, J.; Greenberger, P.A.; Masse, M.S.; Schrijvers, R.; Trautmann, A. Controversies in Drug Allergy: Radiographic Contrast Media. J. Allergy Clin. Immunol. Pract. 2019, 7, 61–65. [Google Scholar] [CrossRef]
- Trautmann, A.; Brockow, K.; Behle, V.; Stoevesandt, J. Radiocontrast Media Hypersensitivity: Skin Testing Differentiates Allergy From Nonallergic Reactions and Identifies a Safe Alternative as Proven by Intravenous Provocation. J. Allergy Clin. Immunol. Pract. 2019, 7, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.; Gomez, F.; Fernandez, T.D.; Doña, I.; Aranda, A.; Ariza, A.; Blanca-López, N.; Mayorga, C.; Blanca, M.; Torres, M.J. Diagnosis of immediate hypersensitivity reactions to radiocontrast media. Allergy 2013, 68, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Dettwiler, M.; Boehm, I.B. Drug provocation tests (DPTs) of contrast media: Useful or not useful?—A narrative review. World Allergy Organ. J. 2024, 17, 100946. [Google Scholar] [CrossRef] [PubMed]
- Schopp, J.G.; Iyer, R.S.; Wang, C.L.; Petscavage, J.M.; Paladin, A.M.; Bush, W.H.; Dighe, M.K. Allergic reactions to iodinated contrast media: Premedication considerations for patients at risk. Emerg. Radiol. 2013, 20, 299–306. [Google Scholar] [CrossRef]
- Wang, C.L.; Asch, D.; Cavallo, J.; Dillman, J.R.; Ellis, J.H.; Forbes-Amrhein, M.M.; Gilligan, L.A.; Krishnan, P.; McDonald, R.J.; McDonald, J.S.; et al. Statement from the ACR Committee on Drugs and Contrast Media on the Intravenous Iodinated Contrast Media Shortage. J. Am. Coll. Radiol. 2022, 19, 834–835. [Google Scholar] [CrossRef]
- Greenberger, P.; Patterson, R.; Radin, R. Two pretreatment regimens for high-risk patients receiving radiographic contrast media. J. Allergy Clin. Immunol. 1984, 74, 540–543. [Google Scholar] [CrossRef]
- Mervak, B.M.; Davenport, M.S.; Ellis, J.H.; Cohan, R.H. Rates of Breakthrough Reactions in Inpatients at High Risk Receiving Premedication Before Contrast-Enhanced CT. Am. J. Roentgenol. 2015, 205, 77–84. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.H.; Lee, S.M.; Kang, H.R.; Park, H.W.; Kim, S.S.; Cho, S.H.; Min, K.U.; Kim, Y.Y.; Chang, Y.S. Outcomes of premedication for non-ionic radio-contrast media hypersensitivity reactions in Korea. Eur. J. Radiol. 2011, 80, 363–367. [Google Scholar] [CrossRef]
- Freed, K.S.; Leder, R.A.; Alexander, C.; DeLong, D.M.; Kliewer, M.A. Breakthrough Adverse Reactions to Low-Osmolar Contrast Media After Steroid Premedication. Am. J. Roentgenol. 2001, 176, 1389–1392. [Google Scholar] [CrossRef]
- Park, S.J.; Kang, D.Y.; Sohn, K.H.; Yoon, S.H.; Lee, W.; Choi, Y.H.; Cho, S.H.; Kang, H.R. Immediate Mild Reactions to CT with Iodinated Contrast Media: Strategy of Contrast Media Readministration without Corticosteroids. Radiology 2018, 288, 710–716. [Google Scholar] [CrossRef]
- Hsieh, C.; Wu, S.C.; Kosik, R.O.; Huang, Y.C.; Chan, W.P. Pharmacological Prevention of Hypersensitivity Reactions Caused by Iodinated Contrast Media: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1673. [Google Scholar] [CrossRef] [PubMed]
- Amiri, E. Optimizing Premedication Strategies for Iodinated Contrast Media in CT scans: A Literature Review. J. Med. Imaging Radiat. Sci. 2025, 56, 101782. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Yang, M.S.; Choi, Y.H.; Park, C.M.; Park, H.W.; Cho, S.H.; Kang, H.R. Stratified premedication strategy for the prevention of contrast media hypersensitivity in high-risk patients. Ann. Allergy Asthma Immunol. 2017, 118, 339–344.e1. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, J.W.; Yang, M.S.; Kim, M.Y.; Kim, S.H.; Jang, G.C.; Nam, Y.H.; Kim, G.W.; Kim, S.; Park, H.K.; et al. Re-exposure to low osmolar iodinated contrast media in patients with prior moderate-to-severe hypersensitivity reactions: A multicentre retrospective cohort study. Eur. Radiol. 2017, 27, 2886–2893. [Google Scholar] [CrossRef]
- Jung, J.W.; Choi, Y.H.; Park, C.M.; Park, H.W.; Cho, S.H.; Kang, H.R. Outcomes of corticosteroid prophylaxis for hypersensitivity reactions to low osmolar contrast media in high-risk patients. Ann. Allergy Asthma Immunol. 2016, 117, 304–309.e1. [Google Scholar] [CrossRef]
- Kolbe, A.B.; Hartman, R.P.; Hoskin, T.L.; Carter, R.E.; Maddox, D.E.; Hunt, C.H.; Hesley, G.K. Premedication of patients for prior urticarial reaction to iodinated contrast medium. Abdom. Imaging 2014, 39, 432–437. [Google Scholar] [CrossRef]
- Jha, K.K.; El Hajj, M.; Nealy, Z.; Ofoma, U.; Berger, A.; Yost, G.; Green, S.; Agarwal, S.; Scott, T.D.; Thakur, L.; et al. Prognostic implications of prior contrast reaction in patients with emergency premedication before undergoing percutaneous coronary intervention. Int. J. Cardiol. 2021, 330, 30–34. [Google Scholar] [CrossRef]
- Bae, Y.J.; Hwang, Y.W.; Yoon, S.K.; Kim, S.; Lee, T.; Lee, Y.S.; Kwon, H.S.; Cho, Y.S.; Shin, M.J.; Moon, H.B.; et al. The Effectiveness of Automatic Recommending System for Premedication in Reducing Recurrent Radiocontrast Media Hypersensitivity Reactions. PLoS ONE 2013, 8, e66014. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Blankenship, J.C.; Scott, T.D.; Skelding, K.A.; Berger, P.B. Emergency Pretreatment for Contrast Allergy Before Direct Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2008, 102, 1469–1472. [Google Scholar] [CrossRef]
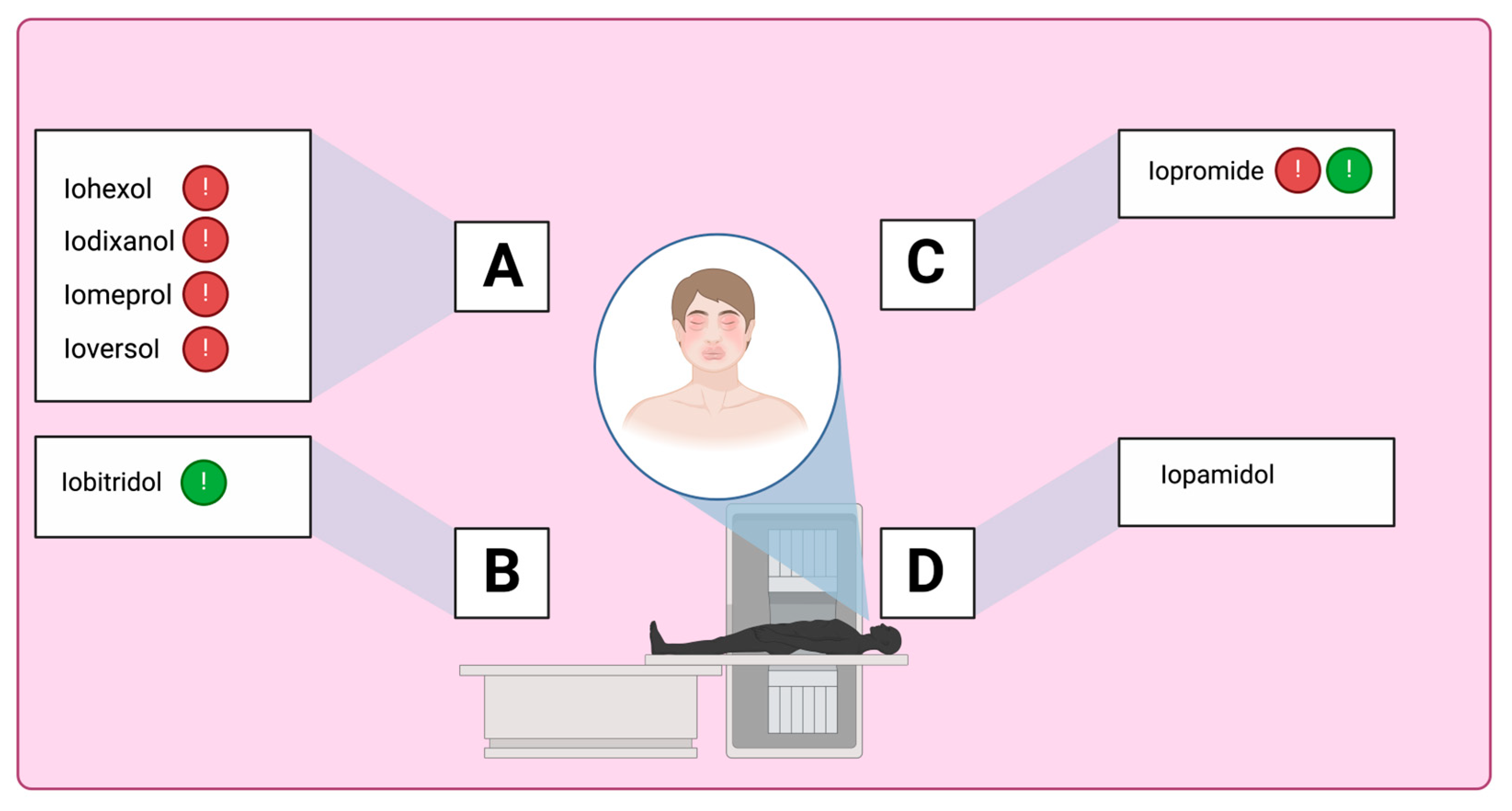
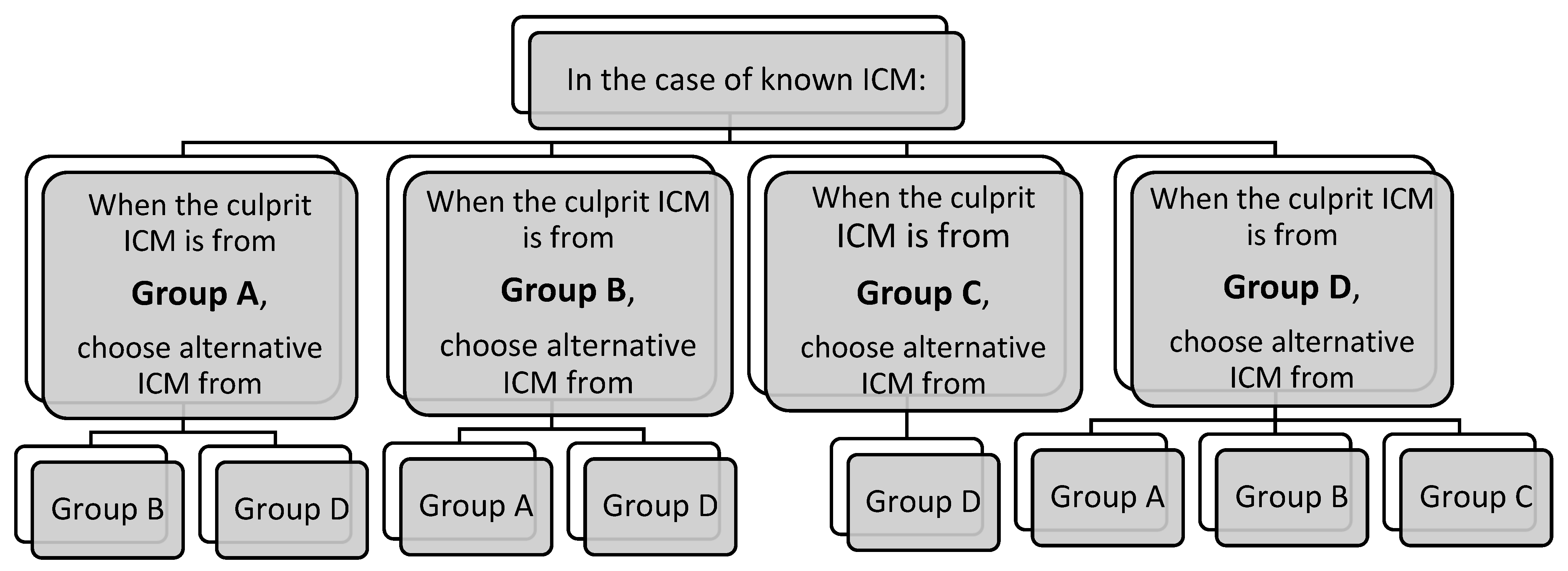
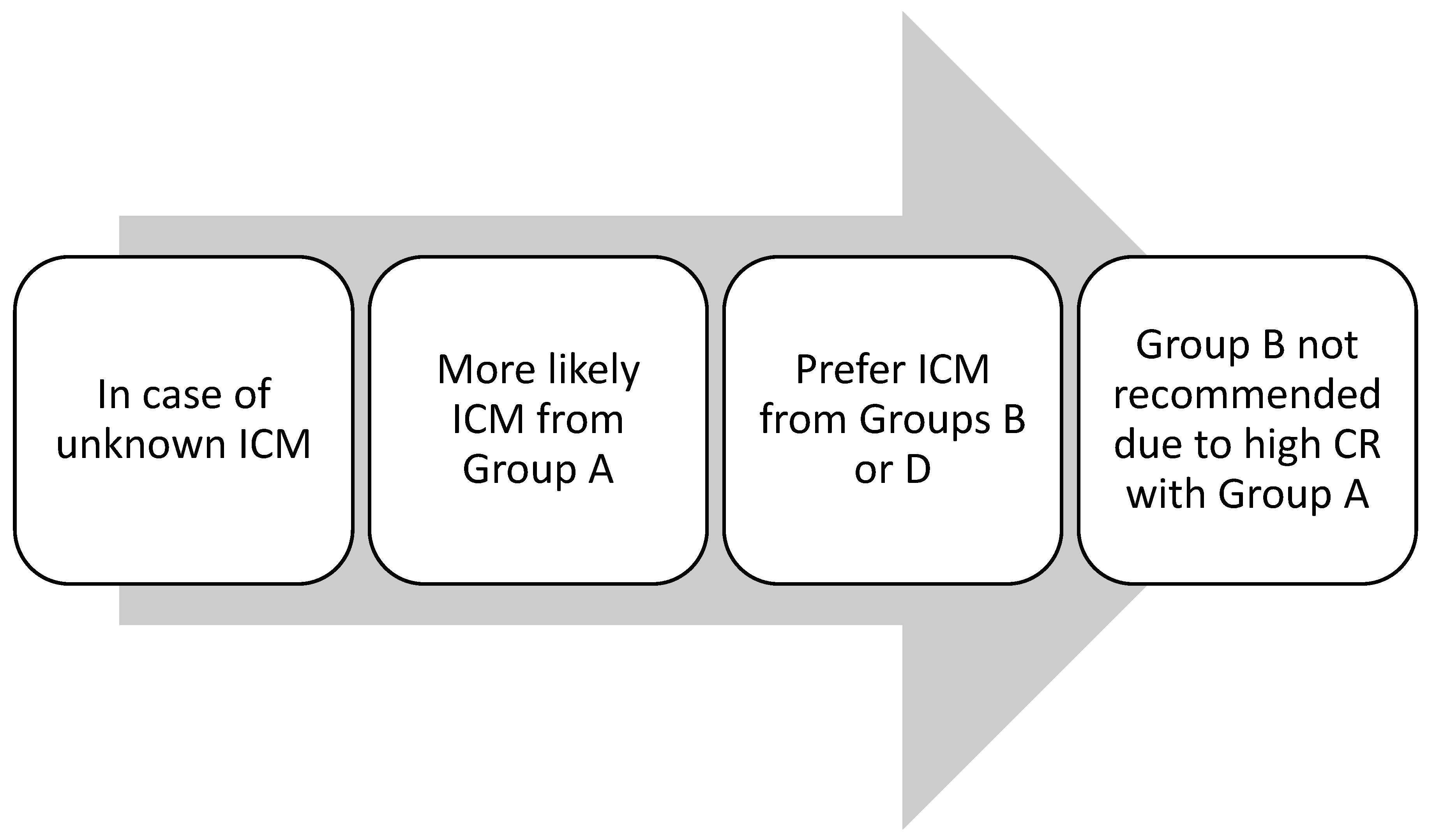
 | Group A: N-(2,3-dihydroxypropyl) carbamoyl side chains
|
 | Group B: N-(2,3-dihydroxypropyl)-N-methyl carbamoyl side chains
|
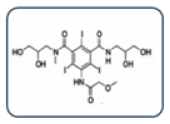 | Group C: N-(2,3-dihydroxypropyl)-carbamoyl AND N(2,3-dihydroxypropyl)-N-methyl-carbamoyl side chains
|
 | Group D: 1-N,3-N-bis(1,3 dihydroxypropan-2-yl) side chains
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losa, F.; Paoletti, G.; Borgonovo, L.; Buta, F.; Merli, S.; Nannipieri, S.; Piantanida, M.; Rossi, C.M.; Sambugaro, G.; Yacoub, M.-R.; et al. Hypersensitivity Reactions to Iodinated Contrast Media: A Narrative Review of Current Evidence and Clinical Challenges. Healthcare 2025, 13, 1308. https://doi.org/10.3390/healthcare13111308
Losa F, Paoletti G, Borgonovo L, Buta F, Merli S, Nannipieri S, Piantanida M, Rossi CM, Sambugaro G, Yacoub M-R, et al. Hypersensitivity Reactions to Iodinated Contrast Media: A Narrative Review of Current Evidence and Clinical Challenges. Healthcare. 2025; 13(11):1308. https://doi.org/10.3390/healthcare13111308
Chicago/Turabian StyleLosa, Francesca, Giovanni Paoletti, Linda Borgonovo, Federica Buta, Stefania Merli, Serena Nannipieri, Marta Piantanida, Carlo Maria Rossi, Giada Sambugaro, Mona-Rita Yacoub, and et al. 2025. "Hypersensitivity Reactions to Iodinated Contrast Media: A Narrative Review of Current Evidence and Clinical Challenges" Healthcare 13, no. 11: 1308. https://doi.org/10.3390/healthcare13111308
APA StyleLosa, F., Paoletti, G., Borgonovo, L., Buta, F., Merli, S., Nannipieri, S., Piantanida, M., Rossi, C. M., Sambugaro, G., Yacoub, M.-R., Patella, V., Canonica, G. W., Heffler, E., & Costantino, M. T. (2025). Hypersensitivity Reactions to Iodinated Contrast Media: A Narrative Review of Current Evidence and Clinical Challenges. Healthcare, 13(11), 1308. https://doi.org/10.3390/healthcare13111308









