Lung Ultrasound After COVID-19: A Pivotal Moment for Clinical Integration—Navigating Challenges and Seizing Opportunities
Abstract
1. Introduction
2. Materials and Methods
3. Lung Ultrasound Basics
4. Interstitial Syndrome and Interstitial Lung Diseases
4.1. Interstitial Lung Diseases: Classification and Diagnostic Criteria
- Idiopathic Interstitial Pneumonias (IIPs): Diseases of unknown etiology.
- Known-Cause ILDs: Specifically associated with identifiable factors or exposures.
- Secondary ILDs: Linked to other, less clearly defined processes or conditions.
4.2. The Crucial Role of LUS in Diagnosing Interstitial Lung Diseases
- Multiple B-Lines: These appear in a diffuse, inhomogeneous distribution and are critical for ILD diagnosis.
- Pleural Line Abnormalities: These may include thickening, irregularities, and fragmentation of the pleural line.
4.3. Impact of LUS on SARS-CoV-2
5. LUS for the Detection of Consolidations
5.1. Limitations
5.2. Differential Diagnosis
5.3. Promising Tools
6. Acute Respiratory Distress Syndrome (ARDS)
6.1. Sonographic Findings
- Anterior subpleural consolidations;
- Absent or reduced lung sliding;
- “Preserved areas” of normal lung parenchyma;
- Abnormalities in the pleural line (e.g., fragmented, thickened, or irregular pleural line);
- Inhomogeneous distribution of B-lines.
6.2. LUS as a Tool for Intervention Assessment and Outcomes
6.3. The LUS-ARDS Score System
6.4. The LUS Score as a First-Line Screening Tool
7. Controversy and Future Perspectives
7.1. Difficulties in Implementation in Daily Practice
7.2. Future Perspectives
7.3. Strengths and Limitations
8. Conclusions
9. Key Messages
- Lung ultrasound (LUS) has emerged as a vital tool in assessing post-COVID-19 lung diseases, enabling rapid, safe, and accessible diagnosis, particularly in resource-limited settings.
- LUS provides notable advantages over other imaging methods, including its repeatability, absence of ionizing radiation, and suitability for vulnerable populations such as children and pregnant women.
- To enhance its integration into everyday clinical practice and optimize medical decision-making, the standardization of protocols and systematic documentation of ultrasound findings are essential.
- Lung ultrasound is crucial for diagnosing and monitoring severe conditions like ARDS, ILDs, and pneumonia, as it can effectively identify B-lines, consolidations, and pleural alterations.
- The future of LUS is being shaped by advancements in artificial intelligence and standardized training, which will improve diagnostic accuracy, reduce variability between operators, and broaden its use across various clinical settings.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LUS | Lung ultrasound |
| US | Ultrasound |
| ARDS | Acute respiratory distress syndrome |
| ILD | Interstitial lung disease |
| IIP | Idiopathic interstitial pneumonia |
| PFT | Pulmonary function tests |
| DLCO | Diffusing capacity for carbon monoxide |
| TLC | Total lung capacity |
| ICU | Intensive care unit |
| NIRS | Non-invasive respiratory support |
| PEEP | Positive end-expiratory pressure |
References
- Vetrugno, L.; Biasucci, D.G.; Deana, C.; Spadaro, S.; Lombardi, F.A.; Longhini, F.; Pisani, L.; Boero, E.; Cereser, L.; Cammarota, G.; et al. Lung ultrasound and supine chest X-ray use in modern adult intensive care: Mapping 30 years of advancement (1993–2023). Ultrasound J. 2024, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Weile, J.; Brix, J.; Moellekaer, A.B. Is point-of-care ultrasound disruptive innovation? Formulating why POCUS is different from conventional comprehensive ultrasound. Crit. Ultrasound J. 2018, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound. 2014, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Boumans, M.M.A.; Aerts, W.; Pisani, L.; Bos, L.D.J.; Smit, M.R.; Tuinman, P.R. Diagnostic accuracy of lung ultrasound in diagnosis of ARDS and identification of focal or non-focal ARDS subphenotypes: A systematic review and meta-analysis. Crit. Care 2024, 28, 224. [Google Scholar] [CrossRef]
- Arbeid, E.; Demi, A.; Brogi, E.; Gori, E.; Giusto, T.; Soldati, G.; Vetrugno, L.; Giunta, F.; Forfori, F. Lung ultrasound pattern is normal during the last gestational weeks: An observational pilot study. Gynecol. Obstet. Invest. 2017, 82, 398–403. [Google Scholar] [CrossRef]
- Vetrugno, L.; Sala, A.; Orso, D.; Meroi, F.; Fabbro, S.; Boero, E.; Valent, F.; Cammarota, G.; Restaino, S.; Vizzielli, G.; et al. Lung ultrasound signs and their correlation with clinical symptoms in covid-19 pregnant women: The “pink-co” observational study. Front. Med. 2022, 8, 768261. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. BLUE-protocol and FALLS-protocol: Two applications of lung ultrasound in the critically ill. Chest 2015, 147, 1659–1670. [Google Scholar] [CrossRef]
- Gargani, L.; Picano, E. The risk of cumulative radiation exposure in chest imaging and the advantage of bedside ultrasound. Crit. Ultrasound J. 2015, 7, 4. [Google Scholar] [CrossRef]
- Cammarota, G.; Vetrugno, L.; Longhini, F. Lung ultrasound monitoring: Impact on economics and outcomes. Curr. Opin. Anaesthesiol. 2023, 36, 234–239. [Google Scholar] [CrossRef]
- Buonsenso, D.; De Rose, C. Implementation of lung ultrasound in low- to middle-income countries: A new challenge global health? Eur. J. Pediatr. 2022, 181, 1–8. [Google Scholar] [CrossRef]
- Stewart, K.A.; Navarro, S.M.; Kambala, S.; Tan, G.; Poondla, R.; Lederman, S.; Barbour, K.; Lavy, C. Trends in ultrasound use in low and middle income countries: A systematic review. Int. J. Matern. Child Health AIDS 2020, 9, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Baad, M.; Lu, Z.F.; Reiser, I.; Paushter, D. Clinical significance of us artifacts. Radiographics 2017, 37, 1408–1423. [Google Scholar] [CrossRef] [PubMed]
- Heitzman, E.R.; Markarian, B.; Berger, I.; Dailey, E. The secondary pulmonary lobule: A practical concept for interpretation of chest radiographs. Radiology 1969, 93, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Mézière, G.; Biderman, P.; Gepner, A.; Barré, O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef]
- Volpicelli, G. Lung ultrasound b-lines in interstitial lung disease: Moving from diagnosis to prognostic stratification. Chest 2020, 158, 1323–1324. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Goldstein, I.; Mourgeon, E.; Cluzel, P.; Grenier, P.; Rouby, J.-J. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004, 100, 9–15. [Google Scholar] [CrossRef]
- Mira-Avendano, I.; Abril, A.; Burger, C.D.; Dellaripa, P.F.; Fischer, A.; Gotway, M.B.; Lee, A.S.; Lee, J.S.; Matteson, E.L.; Yi, E.S.; et al. Interstitial lung disease and other pulmonary manifestations in connective tissue diseases. Mayo Clin. Proc. 2019, 94, 309–325. [Google Scholar] [CrossRef]
- Volpicelli, G.; Mussa, A.; Garofalo, G.; Cardinale, L.; Casoli, G.; Perotto, F.; Fava, C.; Frascisco, M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am. J. Emerg. Med. 2006, 24, 689–696. [Google Scholar] [CrossRef]
- Sesé, L.; Khamis, W.; Jeny, F.; Uzunhan, Y.; Duchemann, B.; Valeyre, D.; Annesi-Maesano, I.; Nunes, H. Adult interstitial lung diseases and their epidemiology. La Presse Med. 2020, 49, 104023. [Google Scholar] [CrossRef]
- American Thoracic Society; European Respiratory Society. American thoracic society/european respiratory society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2002, 165, 277–304. [Google Scholar] [CrossRef]
- Wang, Y.; Gargani, L.; Barskova, T.; Furst, D.E.; Cerinic, M.M. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res. Ther. 2017, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, H.; Hasan, A. B-lines: Transthoracic chest ultrasound signs useful in assessment of interstitial lung diseases. Ann. Thorac. Med. 2014, 9, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mongodi, S.; Chiumello, D.; Mojoli, F. Lung ultrasound score for the assessment of lung aeration in ARDS patients: Comparison of two approaches. Ultrasound Int. Open 2024, 10, a24218709. [Google Scholar] [CrossRef] [PubMed]
- Pinal-Fernandez, I.; Pallisa-Nuñez, E.; Selva-O’Callaghan, A.; Castella-Fierro, E.; SimeOn-Aznar, C.P.; Fonollosa-Pla, V.; Vilardell-Tarres, M. Pleural irregularity, a new ultrasound sign for the study of interstitial lung disease in systemic sclerosis and antisynthetase syndrome. Clin. Exp. Rheumatol. 2015, 33 (Suppl. S91), S136–S141. [Google Scholar]
- Gargani, L.; Bruni, C.; Romei, C.; Frumento, P.; Moreo, A.; Agoston, G.; Guiducci, S.; Bellando-Randone, S.; Lepri, G.; Belloli, L.; et al. Prognostic value of lung ultrasound b-lines in systemic sclerosis. Chest 2020, 158, 1515–1525. [Google Scholar] [CrossRef]
- Laursen, C.B.; Clive, A.; Hallifax, R.; Pietersen, P.I.; Asciak, R.; Davidsen, J.R.; Bhatnagar, R.; Bedawi, E.O.; Jacobsen, N.; Coleman, C.; et al. European Respiratory Society statement on thoracic ultrasound. Eur. Respir. J. 2021, 57, 2001519. [Google Scholar] [CrossRef]
- Guerra, M.G.; Pinto, T.M.; Águeda, A.; Rodrigues, J.; Marona, J.; Violante, A.; Oliveira, M. The role of lung ultrasound in systemic sclerosis: A systematic review. J. Clin. Rheumatol. 2023, 29, e32–e39. [Google Scholar]
- Radić, M.; Đogaš, H.; Gelemanović, A.; Petričević, S.J.; Škopljanac, I.; Radić, J. Pulmonary ultrasonography in systemic sclerosis-induced interstitial lung disease—A systematic review and meta-analysis. Diagnostics 2023, 13, 1429. [Google Scholar] [CrossRef]
- Vetrugno, L.; Bove, T.; Orso, D.; Barbariol, F.; Bassi, F.; Boero, E.; Ferrari, G.; Kong, R.; A Lichtenstein, D. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography 2020, 37, 625–627. [Google Scholar] [CrossRef]
- Boccatonda, A.; Grignaschi, A.; Lanotte, A.M.G.; Cocco, G.; Vidili, G.; Giostra, F.; Schiavone, C. Role of lung ultrasound in the management of patients with suspected sars-cov-2 infection in the emergency department. J. Clin. Med. 2022, 11, 2067. [Google Scholar] [CrossRef]
- D’Ardes, D.; Boccatonda, A.; Schiavone, C.; Santilli, F.; Guagnano, M.T.; Bucci, M.; Cipollone, F. A case of coinfection with sars-cov-2 and cytomegalovirus in the era of covid-19. Eur. J. Case Rep. Intern. Med. 2020, 7, 1652. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Vetrugno, L. Lung ultrasound in adults and children with covid-19: From first discoveries to recent advances. J. Clin. Med. 2022, 11, 4340. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Gargani, L.; Perlini, S.; Spinelli, S.; Barbieri, G.; Lanotte, A.; Casasola, G.G.; Nogué-Bou, R.; Lamorte, A.; Agricola, E.; et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: An international multicenter study. Intensive Care Med. 2021, 47, 444–454. [Google Scholar] [CrossRef] [PubMed]
- D’ardes, D.; Tana, C.; Salzmann, A.; Ricci, F.; Guagnano, M.T.; Giamberardino, M.A.; Cipollone, F. Ultrasound assessment of SARS-CoV-2 pneumonia: A literature review for the primary care physician. Ann. Med. 2022, 54, 1140–1149. [Google Scholar] [CrossRef]
- Boccatonda, A.; Cocco, G.; Ianniello, E.; Montanari, M.; D’ardes, D.; Borghi, C.; Giostra, F.; Copetti, R.; Schiavone, C. One year of SARS-CoV-2 and lung ultrasound: What has been learned and future perspectives. J. Ultrasound 2021, 24, 115–123. [Google Scholar] [CrossRef]
- Buonsenso, D.; Morello, R.; Mariani, F.; De Rose, C.; Cortese, R.; Vetrugno, L.; Valentini, P. Role of lung ultrasound in the follow-up of children with previous sars-cov-2 infection: A case-control assessment of children with long covid or fully recovered. J. Clin. Med. 2023, 12, 3342. [Google Scholar] [CrossRef]
- Barbieri, G.; Gargani, L.; Lepri, V.; Spinelli, S.; Romei, C.; De Liperi, A.; Chimera, D.; Pistelli, F.; Carrozzi, L.; Corradi, F.; et al. Long-term lung ultrasound follow-up in patients after COVID-19 pneumonia hospitalization: A prospective comparative study with chest computed tomography. Eur. J. Intern. Med. 2023, 110, 29–34. [Google Scholar] [CrossRef]
- Vetrugno, L.; Meroi, F.; Orso, D.; D’andrea, N.; Marin, M.; Cammarota, G.; Mattuzzi, L.; Delrio, S.; Furlan, D.; Foschiani, J.; et al. Can lung ultrasound be the ideal monitoring tool to predict the clinical outcome of mechanically ventilated covid-19 patients? An observational study. Healthcare 2022, 10, 568. [Google Scholar] [CrossRef]
- Cammarota, G.; Bruni, A.; Morettini, G.; Vitali, L.; Brunelli, F.; Tinarelli, F.; Simonte, R.; Rossi, E.; Bellucci, M.; De Girolamo, G.; et al. Lung ultrasound to evaluate aeration changes in response to recruitment maneuver and prone positioning in intubated patients with COVID-19 pneumonia: Preliminary study. Ultrasound J. 2023, 15, 3. [Google Scholar] [CrossRef]
- Bouhemad, B.; Dransart-Rayé, O.; Mojoli, F.; Mongodi, S. Lung ultrasound for diagnosis and monitoring of ventilator-associated pneumonia. Ann. Transl. Med. 2018, 6, 418. [Google Scholar] [CrossRef]
- Stevic, N.; Chatelain, E.; Dargent, A.; Argaud, L.; Cour, M.; Guérin, C. Lung recruitability evaluated by recruitment-to-inflation ratio and lung ultrasound in covid-19 acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2021, 203, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sayat, M.; Colaianni-Alfonso, N.; Vetrugno, L.; Olaizola, G.; Benay, C.; Herrera, F.; Saá, Y.; Montiel, G.; Haedo, S.; Previgliano, I.; et al. Lung ultrasound score predicts outcomes in patients with acute respiratory failure secondary to COVID-19 treated with non-invasive respiratory support: A prospective cohort study. Ultrasound J. 2024, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Estrada, M.; Gamero-Rodríguez, M.J.; García-De-Acilu, M.; Roca, O.; Sandoval-Plascencia, L.; Aguirre-Avalos, G.; García-Salcido, R.; Aguirre-Díaz, S.A.; Vines, D.L.; Mirza, S.; et al. Lung ultrasound response to awake prone positioning predicts the need for intubation in patients with COVID-19 induced acute hypoxemic respiratory failure: An observational study. Crit. Care 2022, 26, 189. [Google Scholar] [CrossRef]
- Targhetta, R.; Chavagneux, R.; Bourgeois, J.M.; Dauzat, M.; Balmes, P.; Pourcelot, L. Sonographic approach to diagnosing pulmonary consolidation. J. Ultrasound Med. 1992, 11, 667–672. [Google Scholar] [CrossRef]
- Haaksma, M.E.; Smit, J.M.; Heldeweg, M.L.A.; Nooitgedacht, J.S.B.; de Grooth, H.J.; Jonkman, A.H.M.; Girbes, A.R.J.; Heunks, L.; Tuinman, P.R. Extended lung ultrasound to differentiate between pneumonia and atelectasis in critically ill patients: A diagnostic accuracy study. Crit. Care Med. 2022, 50, 750–759. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Lascols, N.; Mezière, G.; Gepner, A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004, 30, 276–281. [Google Scholar] [CrossRef]
- Inchingolo, R.; Copetti, R.; Smargiassi, A.; Gerardi, R.E.; Conte, E.G.; Corbo, G.M.; Gatto, A.; Pierandrei, C.; Capossela, L.; Lazzareschi, I.; et al. Air bronchogram integrated lung ultrasound score to monitor community-acquired pneumonia in a pilot pediatric population. J. Ultrasound 2021, 24, 191–200. [Google Scholar] [CrossRef]
- Shah, A.; Oliva, C.; Stem, C.; Cummings, E. Application of dynamic air bronchograms on lung ultrasound to diagnose pneumonia in undifferentiated respiratory distress. Respir. Med. Case Rep. 2022, 39, 101706. [Google Scholar] [CrossRef]
- Akutagawa, K. Dynamic air bronchogram and lung hepatization: Ultrasound for early diagnosis of pneumonia. Med. Ultrason. 2021, 23, 238–239. [Google Scholar]
- Görg, C.; Bert, T. Transcutaneous colour Doppler sonography of lung consolidations: Review and pictorial essay. Part 1: Pathophysiologic and colour Doppler sonographic basics of pulmonary vascularity. Ultraschall Med. 2004, 25, 221–226. [Google Scholar] [CrossRef]
- Görg, C.; Bert, T. Transcutaneous colour Doppler sonography of lung consolidations: Review and pictorial essay. Part 2: Colour Doppler sonographic patterns of pulmonary consolidations. Ultraschall Med. 2004, 25, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Mezière, G.; Seitz, J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest 2009, 135, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- D’ardes, D.; Boccatonda, A.; Cocco, G.; Fabiani, S.; Rossi, I.; Bucci, M.; Guagnano, M.T.; Schiavone, C.; Cipollone, F. Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship. World J. Gastroenterol. 2022, 28, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Ianniello, E.; D’Ardes, D.; Cocco, G.; Giostra, F.; Borghi, C.; Schiavone, C. Can lung ultrasound be used to screen for pulmonary embolism in patients with sars-cov-2 pneumonia? Eur. J. Case Rep. Intern. Med. 2020, 7, 1748. [Google Scholar] [CrossRef]
- Sperandeo, M.; Sperandeo, G.; Varriale, A.; Filabozzi, P.; Decuzzi, M.; Dimitri, L.; Vendemiale, G. Contrast-enhanced ultrasound (Ceus) for the study of peripheral lung lesions: A preliminary study. Ultrasound Med. Biol. 2006, 32, 1467–1472. [Google Scholar] [CrossRef]
- Quarato, C.M.; Cipriani, C.; Dimitri, L.; Lacedonia, D.; Graziano, P.; Copetti, M.; De Cosmo, S.; Simeone, A.; Scioscia, G.; Barbaro, M.F.; et al. Assessing value of contrast-enhanced ultrasound vs. conventional transthoracic ultrasound in improving diagnostic yield of percutaneous needle biopsy of peripheral lung lesions. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5781–5789. [Google Scholar]
- Sperandeo, M.; Trovato, F.M.; Dimitri, L.; Catalano, D.; Simeone, A.; Martines, G.F.; Piscitelli, A.P.; Trovato, G.M. Lung transthoracic ultrasound elastography imaging and guided biopsies of subpleural cancer: A preliminary report. Acta Radiol. 2015, 56, 798–805. [Google Scholar] [CrossRef]
- Lemieux, S.; Kim, T.; Pothier-Piccinin, O.; Racine, L.-C.; Firoozi, F.; Drolet, M.; Pasian, S.; Kennedy, K.F.; Provencher, S.; Ugalde, P. Ultrasound-guided transthoracic needle biopsy of the lung: Sensitivity and safety variables. Eur. Radiol. 2021, 31, 8272–8281. [Google Scholar] [CrossRef]
- Kuo, Y.-W.; Chen, Y.-L.; Wu, H.-D.; Chien, Y.-C.; Huang, C.-K.; Wang, H.-C. Application of transthoracic shear-wave ultrasound elastography in lung lesions. Eur. Respir. J. 2021, 57, 2002347. [Google Scholar] [CrossRef]
- Boccatonda, A.; Cocco, G.; Schiavone, C. Ai: A new solution for old issues of carotid atherosclerotic plaque. Curr. Med. Chem. 2024, 31, 5305–5307. [Google Scholar] [CrossRef]
- Boccatonda, A. Emergency ultrasound: Is it time for artificial intelligence? J. Clin. Med. 2022, 11, 3823. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Bouhemad, B.; Brisson, H.; Le-Guen, M.; Arbelot, C.; Lu, Q.; Rouby, J.-J. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am. J. Respir. Crit. Care Med. 2011, 183, 341–347. [Google Scholar] [CrossRef]
- Lazzeri, C.; Peris, A. The Kigali modification of the berlin definition: A new epidemiological tool for ARDS? J. Thorac. Dis. 2016, 8, E443–E445. [Google Scholar] [CrossRef]
- Matthay, M.A.; Arabi, Y.; Arroliga, A.C.; Bernard, G.; Bersten, A.D.; Brochard, L.J.; Calfee, C.S.; Combes, A.; Daniel, B.M.; Ferguson, N.D.; et al. A new global definition of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2024, 209, 37–47. [Google Scholar] [CrossRef]
- Sheard, S.; Rao, P.; Devaraj, A. Imaging of acute respiratory distress syndrome. Respir. Care 2012, 57, 607–612. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. Current misconceptions in lung ultrasound: A short guide for experts. Chest 2019, 156, 21–25. [Google Scholar] [CrossRef]
- Copetti, R.; Soldati, G.; Copetti, P. Chest sonography: A useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc. Ultrasound 2008, 6, 16. [Google Scholar] [CrossRef]
- Weinberg, B.; Diakoumakis, E.; Kass, E.; Seife, B.; Zvi, Z. The air bronchogram: Sonographic demonstration. Am. J. Roentgenol. 1986, 147, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, D.G.; on behalf of the Gemelli Against COVID-19 Group; Buonsenso, D.; Piano, A.; Bonadia, N.; Vargas, J.; Settanni, D.; Bocci, M.G.; Grieco, D.L.; Carnicelli, A.; et al. Lung ultrasound predicts non-invasive ventilation outcome in COVID-19 acute respiratory failure: A pilot study. Minerva Anestesiol. 2021, 87, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.S.; Elmeslmany, K.A.; Elsawy, A.S.; Mohamed, N.A. The validity of quantifying pulmonary contusion extent by lung ultrasound score for predicting ards in blunt thoracic trauma. Crit. Care Res. Pr. 2022, 2022, 3124966. [Google Scholar] [CrossRef]
- Bonadia, N.; Carnicelli, A.; Piano, A.; Buonsenso, D.; Gilardi, E.; Kadhim, C.; Torelli, E.; Petrucci, M.; Di Maurizio, L.; Biasucci, D.G.; et al. Lung ultrasound findings are associated with mortality and need for intensive care admission in covid-19 patients evaluated in the emergency department. Ultrasound Med. Biol. 2020, 46, 2927–2937. [Google Scholar] [CrossRef]
- Lichter, Y.; Topilsky, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Oz, A.G.; Vine, J.; Goren, O.; Cohen, B.; et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020, 46, 1873–1883. [Google Scholar] [CrossRef]
- Smit, M.R.; Hagens, L.A.; Heijnen, N.F.L.; Pisani, L.; Cherpanath, T.G.V.; Dongelmans, D.A.; de Grooth, H.-J.S.; Pierrakos, C.; Tuinman, P.R.; Zimatore, C.; et al. Lung ultrasound prediction model for acute respiratory distress syndrome: A multicenter prospective observational study. Am. J. Respir. Crit. Care Med. 2023, 207, 1591–1601. [Google Scholar] [CrossRef]
- Smit, M.R.; Mayo, P.H.; Mongodi, S. Lung ultrasound for diagnosis and management of ARDS. Intensive Care Med. 2024, 50, 1143–1145. [Google Scholar] [CrossRef]
- Biasucci, D.G.; Loi, B.; Centorrino, R.; Raschetti, R.; Piastra, M.; Pisapia, L.; Consalvo, L.M.; Caricato, A.; Grieco, D.L.; Conti, G.; et al. Ultrasound-assessed lung aeration correlates with respiratory system compliance in adults and neonates with acute hypoxemic restrictive respiratory failure: An observational prospective study. Respir. Res. 2022, 23, 360. [Google Scholar] [CrossRef]
- Pierrakos, C.; Smit, M.R.; Pisani, L.; Paulus, F.; Schultz, M.J.; Constantin, J.-M.; Chiumello, D.; Mojoli, F.; Mongodi, S.; Bos, L.D.J. Lung ultrasound assessment of focal and non-focal lung morphology in patients with acute respiratory distress syndrome. Front. Physiol. 2021, 12, 730857. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, S.; Mou, Z.; Wang, Y.; Li, X. Correlation Analysis between Mechanical Power and Lung Ultrasound Score and Their Evaluation of Severity and Prognosis in ARDS Patients. Biomed Res. Int. 2021, 2021, 4156162. [Google Scholar] [CrossRef]
- Constantin, J.-M.; Jabaudon, M.; Lefrant, J.-Y.; Jaber, S.; Quenot, J.-P.; Langeron, O.; Ferrandière, M.; Grelon, F.; Seguin, P.; Ichai, C.; et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): A multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 2019, 7, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Mongodi, S.; Pozzi, M.; Orlando, A.; Bouhemad, B.; Stella, A.; Tavazzi, G.; Via, G.; Iotti, G.A.; Mojoli, F. Lung ultrasound for daily monitoring of ARDS patients on extracorporeal membrane oxygenation: Preliminary experience. Intensive Care Med. 2018, 44, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Arbelot, C.; Schreiber, A.; Langeron, O.; Monsel, A.; Lu, Q. Ultrasound assessment of lung aeration in subjects supported by venovenous extracorporeal membrane oxygenation. Respir. Care 2019, 64, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- CAR’Echo Collaborative Network; Haddam, M.; AzuRea Collaborative Network; Zieleskiewicz, L.; Perbet, S.; Baldovini, A.; Guervilly, C.; Arbelot, C.; Noel, A.; Vigne, C.; et al. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016, 42, 1546–1556. [Google Scholar] [CrossRef]
- Catozzi, G.; Pozzi, T.; Nocera, D.; Donati, B.; Giovanazzi, S.; Ghidoni, V.; Galizia, M.; D’albo, R.; Busana, M.; Romitti, F.; et al. Rethinking ARDS classification: Oxygenation impairment fails to predict VILI risk. Intensive Care Med. 2024, 51, 62–71. [Google Scholar] [CrossRef]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]
- MARS Consortium; Morales-Quinteros, L.; Schultz, M.J.; Bringué, J.; Calfee, C.S.; Camprubí, M.; Cremer, O.L.; Horn, J.; van der Poll, T.; Sinha, P.; et al. Estimated dead space fraction and the ventilatory ratio are associated with mortality in early ARDS. Ann. Intensive Care 2019, 9, 128. [Google Scholar] [CrossRef]
- Tang, K.-Q.; Yang, S.-L.; Zhang, B.; Liu, H.-X.; Ye, D.-Y.; Zhang, H.-Z.; Ma, S. Ultrasonic monitoring in the assessment of pulmonary recruitment and the best positive end-expiratory pressure. Medicine 2017, 96, e8168. [Google Scholar] [CrossRef]
- Chen, L.; Del Sorbo, L.; Grieco, D.L.; Junhasavasdikul, D.; Rittayamai, N.; Soliman, I.; Sklar, M.C.; Rauseo, M.; Ferguson, N.D.; Fan, E.; et al. Potential for Lung Recruitment Estimated by the Recruitment-to-Inflation Ratio in Acute Respiratory Distress Syndrome. A Clinical Trial. Am. J. Respir Crit. Care Med. 2020, 201, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Tutino, L.; Cianchi, G.; Barbani, F.; Batacchi, S.; Cammelli, R.; Peris, A. Time needed to achieve completeness and accuracy in bedside lung ultrasound reporting in Intensive Care Unit. Scand. J. Trauma Resusc. Emerg. Med. 2010, 18, 44. [Google Scholar] [CrossRef]
- Martin, R.; Lau, H.A.; Morrison, R.; Bhargava, P.; Deiling, K. The rising tide of point-of-care ultrasound (Pocus) in medical education: An essential skillset for undergraduate and graduate medical education. Curr. Probl. Diagn. Radiol. 2023, 52, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Dupriez, F.; Hall, A.; Diop, T.; Collard, A.; de Castro, B.R.; Smets, F.; Penaloza, A.; Vanpee, D. Point-of-Care Ultrasound training in undergraduate education in the European Union: Current situation and perspectives. Ultrasound J. 2024, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Nhat, P.T.H.; Van Hao, N.; Tho, P.V.; Kerdegari, H.; Pisani, L.; Thu, L.N.M.; Phuong, L.T.; Duong, H.T.H.; Thuy, D.B.; McBride, A.; et al. Clinical benefit of AI-assisted lung ultrasound in a resource-limited intensive care unit. Crit. Care 2023, 27, 257. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Piscaglia, F. New perspectives on the use of artificial intelligence in the ultrasound evaluation of lung diseases. J. Ultrasound 2024, 27, 429–431. [Google Scholar] [CrossRef]
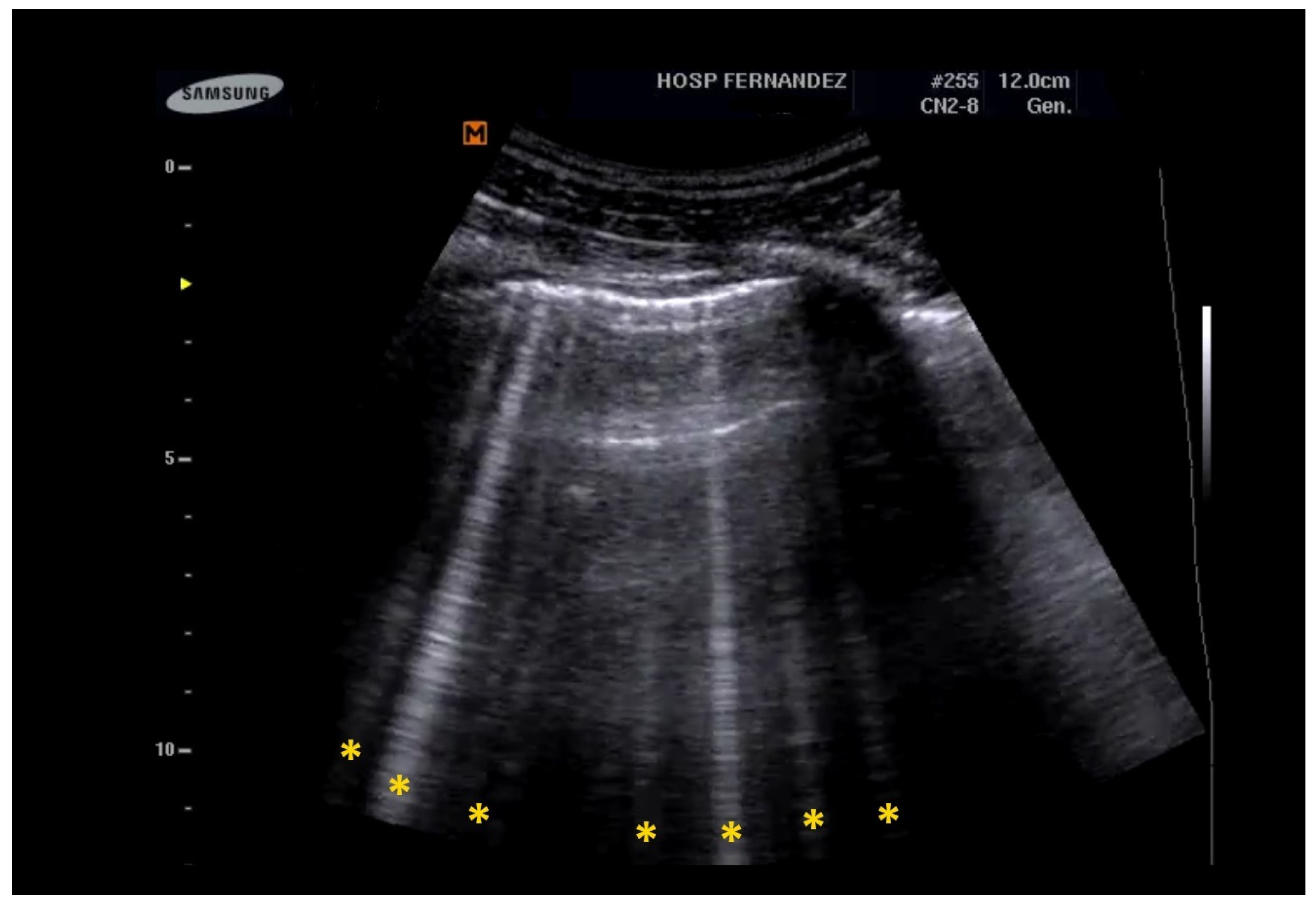
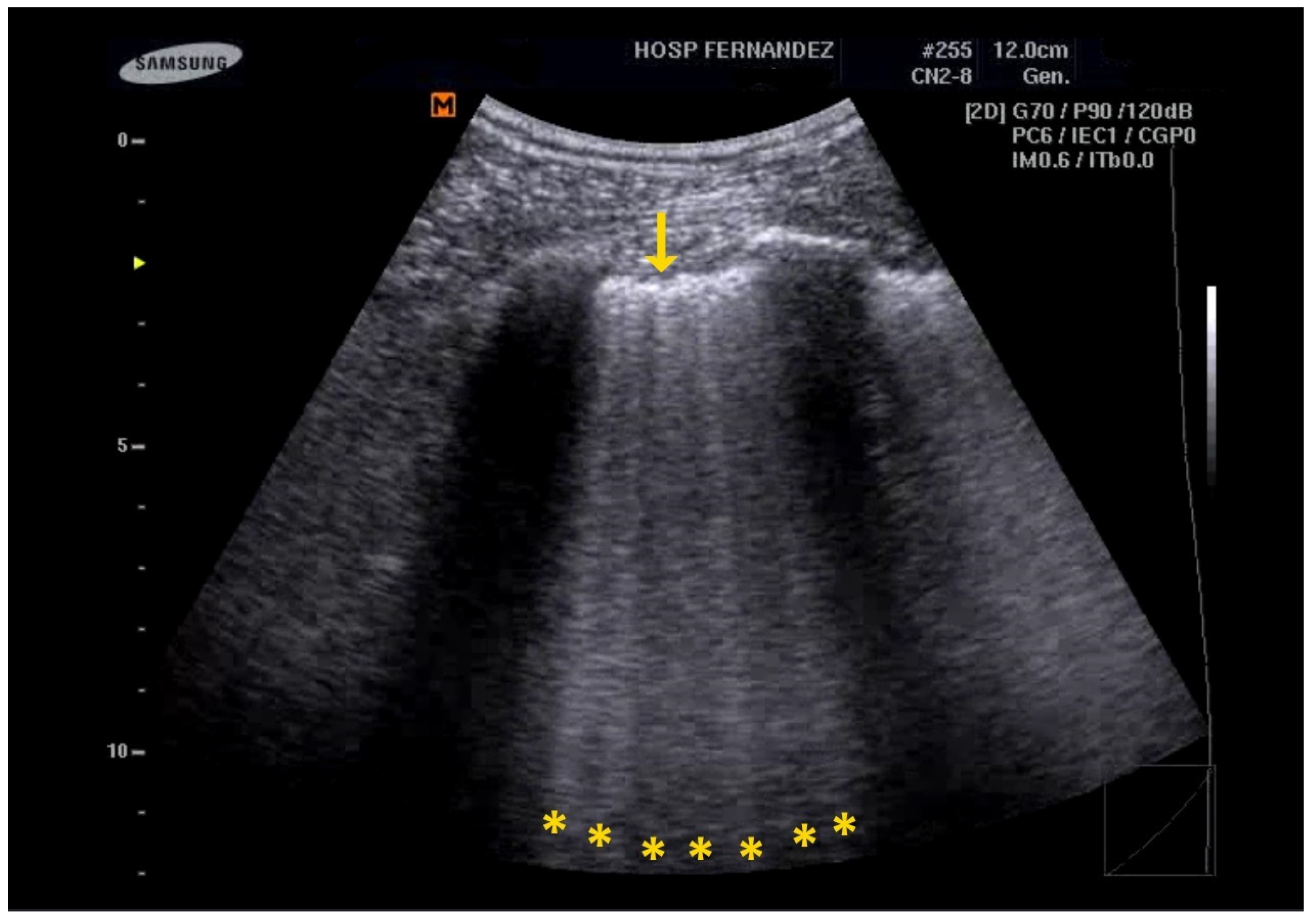
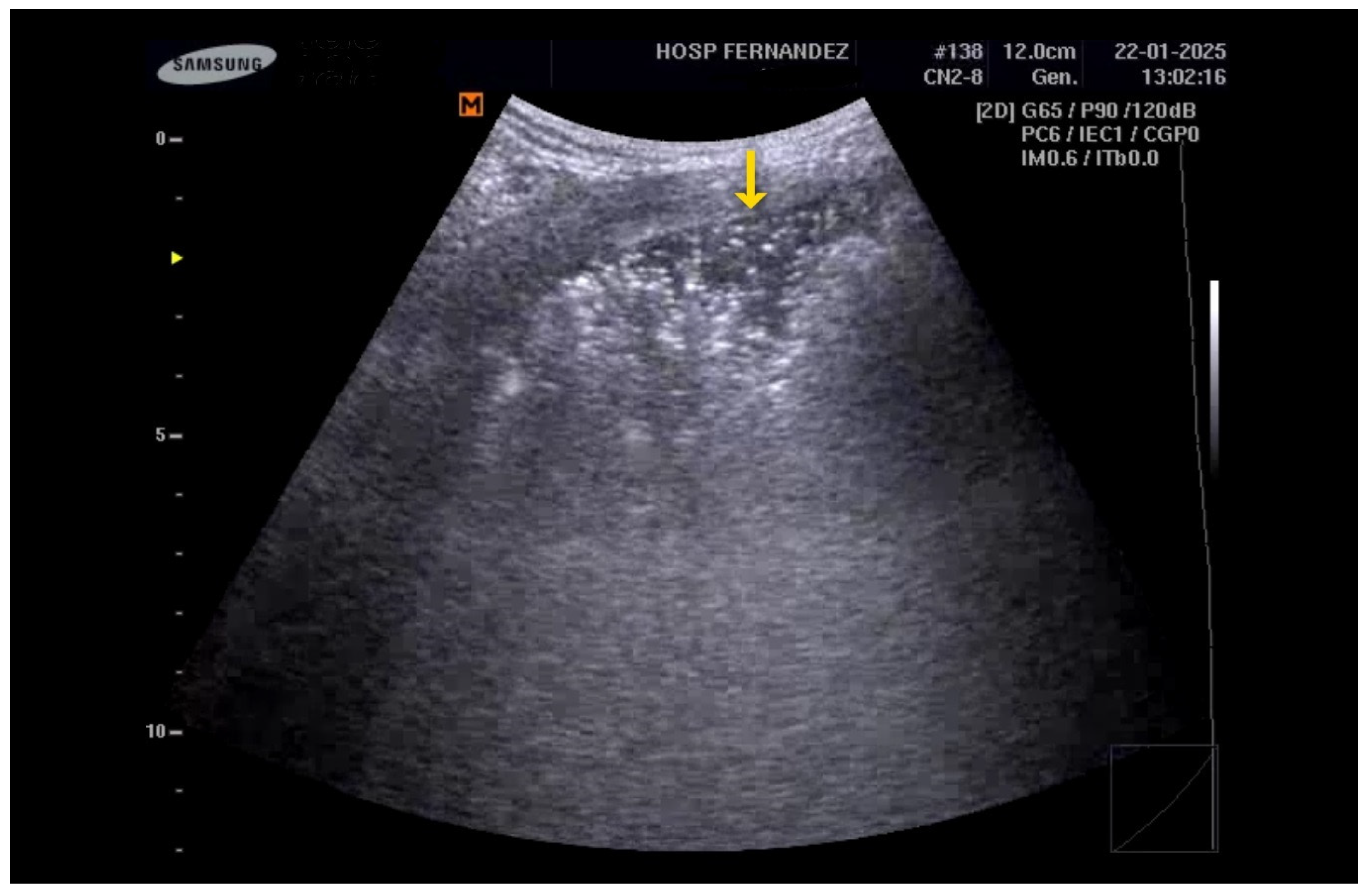
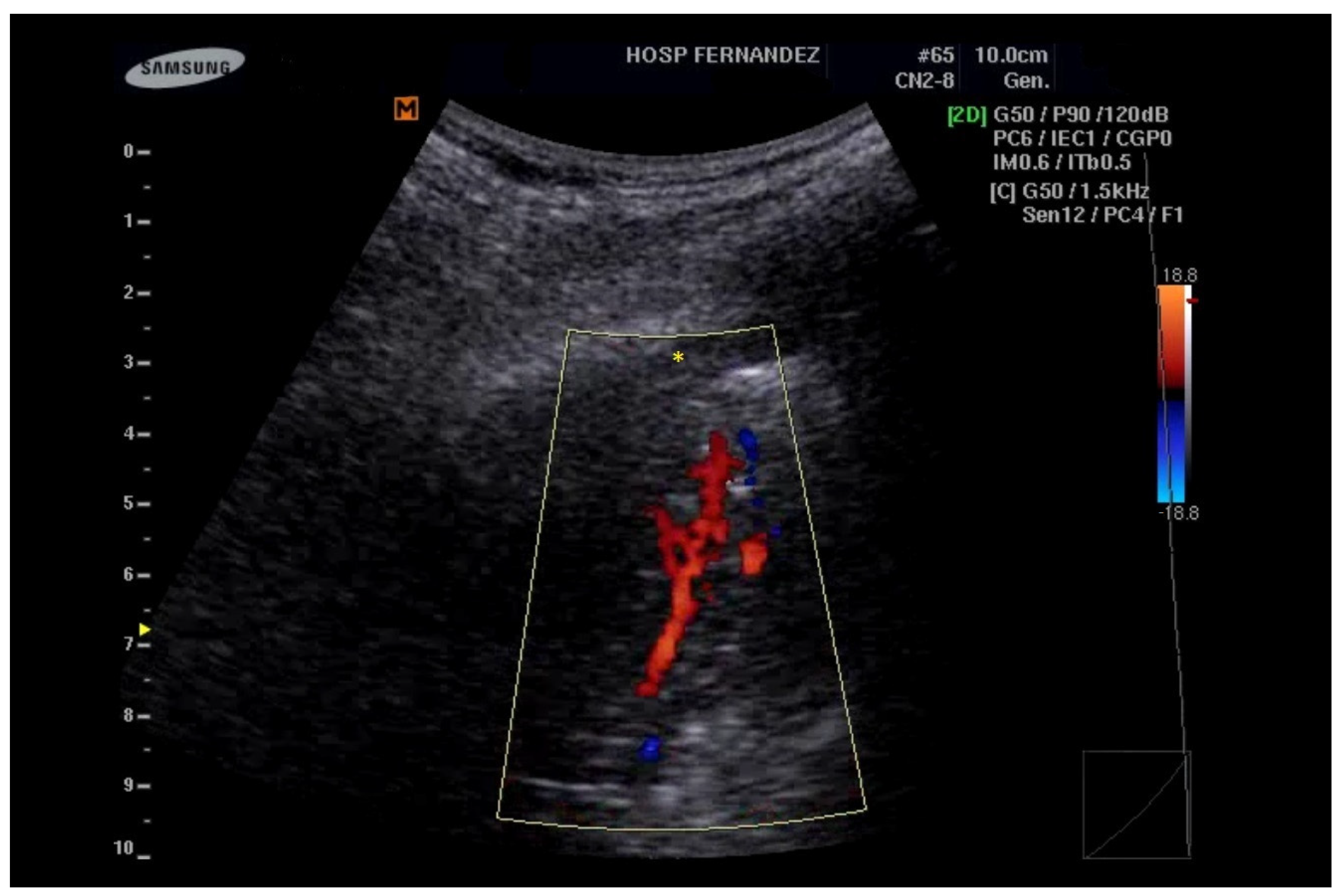
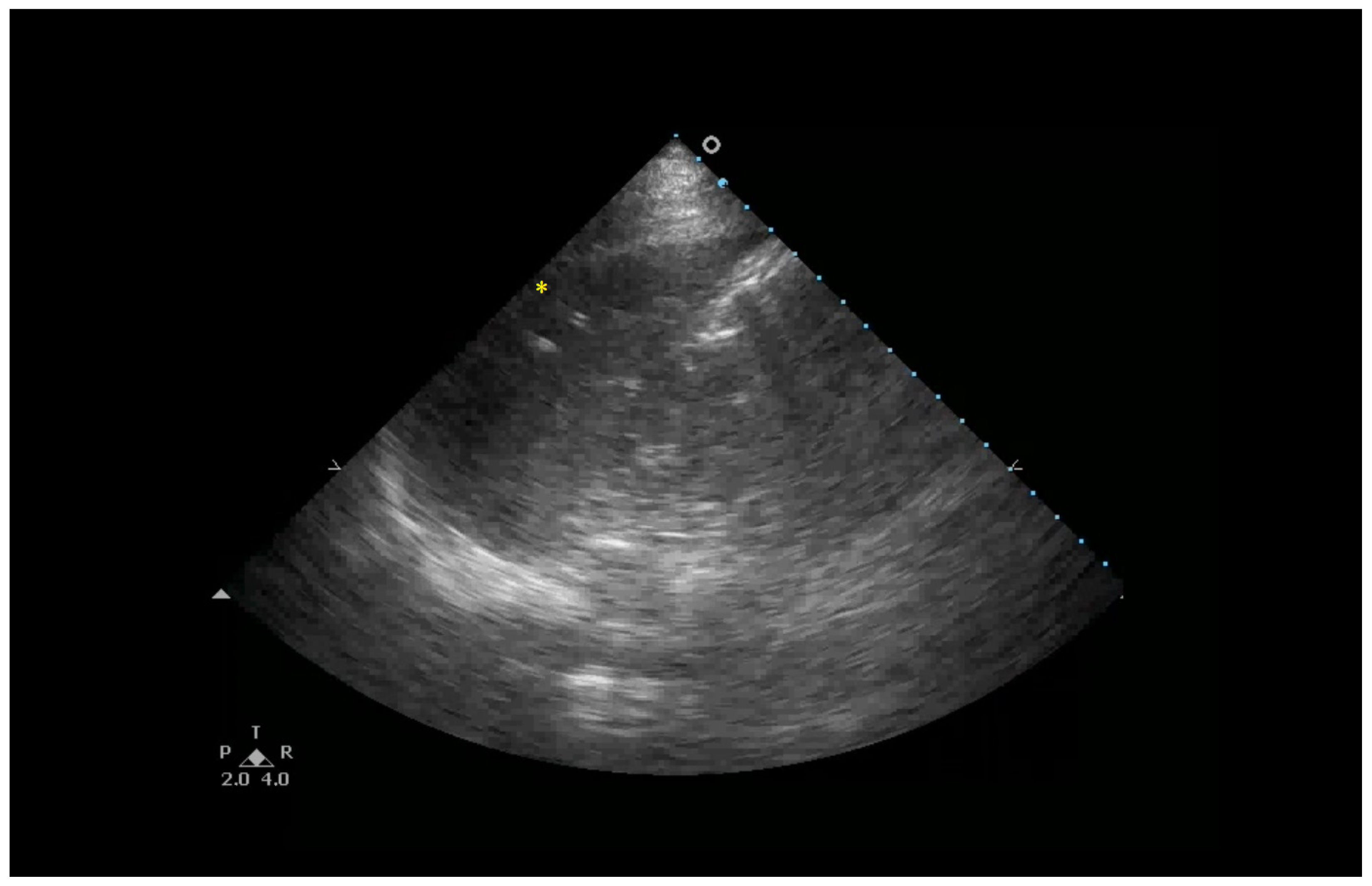
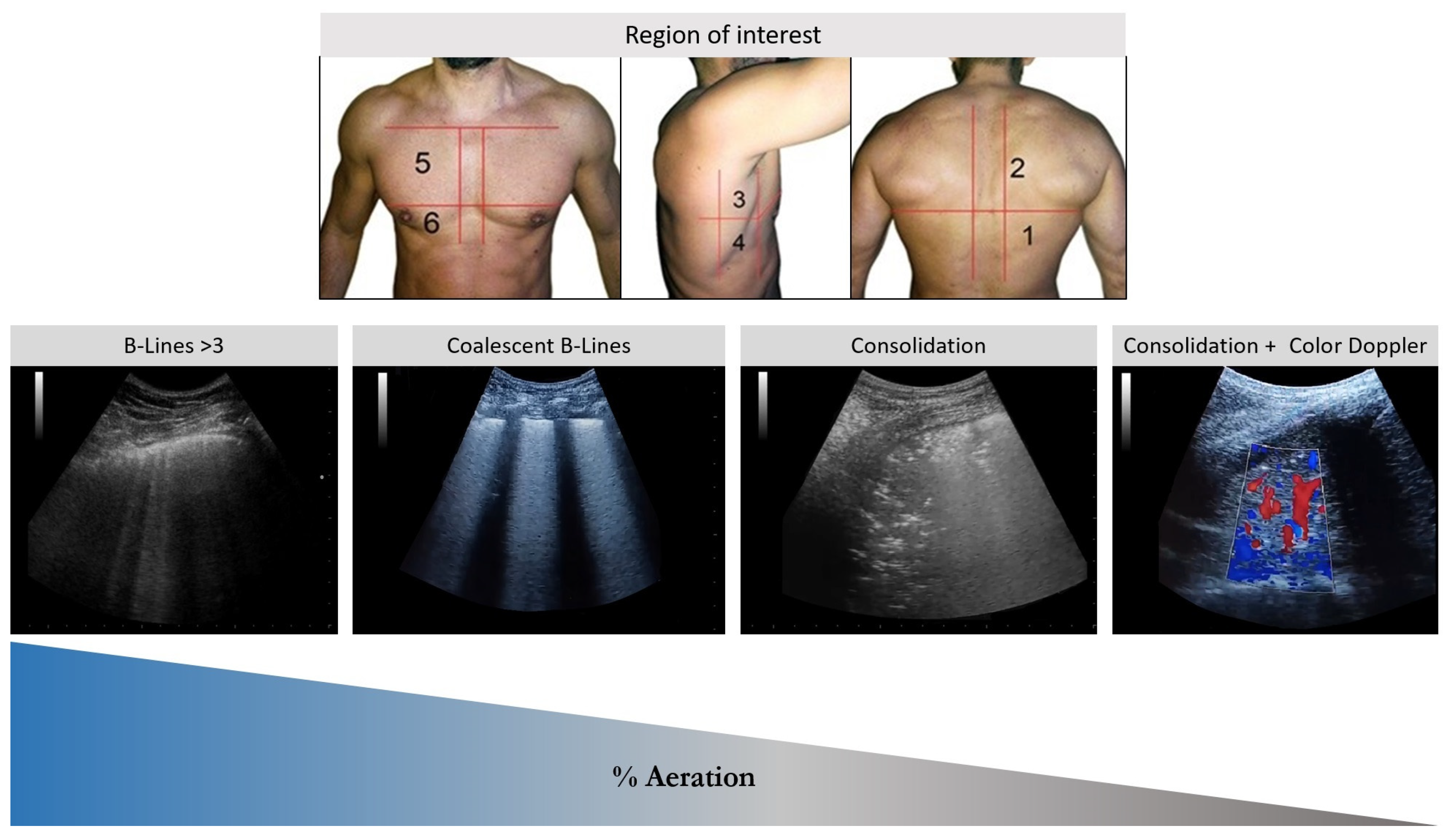
| Clinical Scenarios | B-Lines Distribution | Pleural Line | Consolidation | |||||
|---|---|---|---|---|---|---|---|---|
| Homogeneous | In-homogeneous | Irregular or fragmented | Thickening | Small echo poor aeration areas | Consolidation | Air bronchogram | Visible lung vasculature on Doppler | |
| ILDs | NO | YES | YES | YES | YES | NO | NO | NO |
| Pneumonia | NO | YES, if present | YES | NO | NO | YES | YES | YES |
| ARDS | NO | YES | YES | YES | YES | YES | YES (conditional to severity) | YES (conditional to the severity) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ardes, D.; Deana, C.; Boccatonda, A.; Biasucci, D.G.; Cipollone, F.; Castro-Sayat, M.; Colaianni-Alfonso, N.; Gallardo, A.; Vetrugno, L. Lung Ultrasound After COVID-19: A Pivotal Moment for Clinical Integration—Navigating Challenges and Seizing Opportunities. Healthcare 2025, 13, 1148. https://doi.org/10.3390/healthcare13101148
D’Ardes D, Deana C, Boccatonda A, Biasucci DG, Cipollone F, Castro-Sayat M, Colaianni-Alfonso N, Gallardo A, Vetrugno L. Lung Ultrasound After COVID-19: A Pivotal Moment for Clinical Integration—Navigating Challenges and Seizing Opportunities. Healthcare. 2025; 13(10):1148. https://doi.org/10.3390/healthcare13101148
Chicago/Turabian StyleD’Ardes, Damiano, Cristian Deana, Andrea Boccatonda, Daniele Guerino Biasucci, Francesco Cipollone, Mauro Castro-Sayat, Nicolás Colaianni-Alfonso, Adrián Gallardo, and Luigi Vetrugno. 2025. "Lung Ultrasound After COVID-19: A Pivotal Moment for Clinical Integration—Navigating Challenges and Seizing Opportunities" Healthcare 13, no. 10: 1148. https://doi.org/10.3390/healthcare13101148
APA StyleD’Ardes, D., Deana, C., Boccatonda, A., Biasucci, D. G., Cipollone, F., Castro-Sayat, M., Colaianni-Alfonso, N., Gallardo, A., & Vetrugno, L. (2025). Lung Ultrasound After COVID-19: A Pivotal Moment for Clinical Integration—Navigating Challenges and Seizing Opportunities. Healthcare, 13(10), 1148. https://doi.org/10.3390/healthcare13101148











